Objective
Limited data are available on critically ill pregnant women hospitalized with coronavirus disease 2019 (COVID-19). Although maternal mortality has been reported,1, 2, 3 the frequency with which this devastating outcome occurs is unknown. The objective of this study was to determine the rate of maternal death among pregnant and postpartum women with COVID-19 admitted to the intensive care units (ICUs) in a large integrated health system in the New York metropolitan area. In this study, we described patient demographics, baseline comorbidities, clinical presentation, hospital course, and maternal outcomes.
Study Design
This case series evaluated all consecutively hospitalized pregnant and immediately postpartum women with laboratory-confirmed diagnosis of COVID-19 who were admitted to the ICUs at 10 hospitals within Northwell Health, the largest academic health system in New York, and Maimonides Medical Center, an affiliate of Northwell Health in Brooklyn, NY, from March 1, 2020, to May 6, 2020. Collectively, these hospitals perform approximately 40,000 deliveries per year, representing about 1 in 6 births in the state of New York and 1% of all births in the United States. Respiratory specimens were collected using nasopharyngeal swabs. Symptomatic patients who received positive test results for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by polymerase chain reaction (PCR) assay were included. Admission to the ICU was at the discretion of the consulted critical care attending physician at each site. Patients who had a critical care consultation but were not directly managed by an intensivist were not included. Women who received positive test results for the virus but were admitted to the ICU for indications other than acute or impending hypoxemic respiratory failure were excluded (eg, postpartum hemorrhage). The Northwell Health Institutional Review Board approved this case series as minimal-risk research that used data collected for routine clinical practice and waived the requirement for informed consent. Some women in this study were included in previous publications characterizing COVID-19 hospitalizations within the Northwell Health system,4 , 5 whereas 1 maternal death was previously presented as a case report.1
Clinical data were obtained from the electronic health record system. Subject records were reviewed manually. Data collected included demographics, medical comorbidities, duration of illness before hospitalization, laboratory and imaging results, ICU treatments, and clinical outcomes. No patients were postpartum at the time of hospital admission. The primary outcome was maternal death. Secondary outcomes included length of hospitalization and ICU stay, frequency and duration of invasive mechanical ventilation (ie, requiring endotracheal intubation), frequency of vasopressor administration, urgent or emergent delivery associated with acute respiratory decompensation, and discharge from the hospital. Descriptive statistics were used to characterize the data. Results are presented as means and standard deviations or medians and interquartile ranges, as appropriate. Categorical variables were expressed as numbers and percentages.
Results
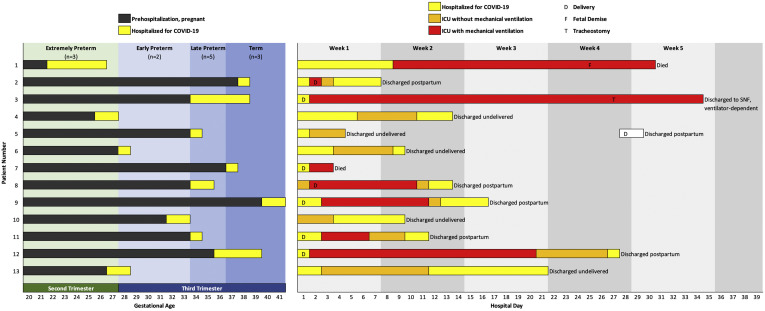
Between March 1, 2020, and May 6, 2020, at the 11 included hospitals, there were 462 pregnant women who received positive test results for SARS-CoV-2, and 70 (15%) patients were classified as having severe or critical COVID-19 per National Institutes of Health (NIH) criteria. Out of these 70 patients, a total of 13 (19%) were admitted to the ICUs for acute or impending hypoxemic respiratory failure (Figure ). Among this group, 2 (15%) died, and 11 (85%) were discharged from the hospital.
Figure.
Individual patient outcomes
A total of 13 pregnant or immediately postpartum women were admitted to the ICUs for COVID-19 and 8 required invasive mechanical ventilation. Although 2 patients (15%) died, 11 (85%) were discharged alive.
COVID-19, coronavirus disease 2019; ICUs, intensive care units; SNF, skilled nursing facility.
Blitz. Maternal mortality among intensive care unit admissions for coronavirus disease 2019. Am J Obstet Gynecol 2020.
Women admitted to the ICUs had a mean maternal age of 33.8±5.2 years, and 69% were multiparous (Supplemental Table 1). Hispanic women constituted the largest racial or ethnic group (38%). The most common comorbidities were obesity (38%) and pulmonary conditions (23%) such as asthma and obstructive sleep apnea (OSA). However, nearly half of the patients (46%) had no baseline comorbidities. All pregnancies were singleton gestations. Most patients were tachycardic, tachypneic, and hypoxemic on initial evaluation, and few were febrile. Nearly all patients (92%) met the NIH criteria for severe COVID-19 at admission. Lymphopenia, elevated transaminases, and elevated inflammatory markers were common laboratory findings (Supplemental Table 2). The mean gestational age of pregnant women with COVID-19 at hospitalization was 33.3±5.3 weeks, and symptoms started 8±3 days before admission.
The median length of hospitalization and ICU stay were 13 and 8 days, respectively (Supplemental Table 3). The duration of hospitalization before ICU admission and after ICU discharge were 2±2 and 3±3 days, respectively. Invasive mechanical ventilation was required in 8 (62%) cases (at initiation, 6 were postpartum and 2 were pregnant), and the median duration of therapy was 8 days. Among this group, 7 (88%) required vasopressors. One patient was extubated but remained ventilator dependent with a tracheostomy. All patients admitted to the ICUs received either prophylactic or therapeutic dose anticoagulation (100%); there were no known cases of venous thromboembolism. Most patients were administered hydroxychloroquine (85%) and antibiotics for community-acquired pneumonia (92%); some were enrolled in clinical trials for the antiviral drug remdesivir (23%), interleukin-6–receptor inhibitors (38%), or convalescent plasma therapy (15%).
Of the 2 patients who died, 1 had a long, protracted course in the ICU, which was complicated by fetal demise at a periviable gestational age; she developed multiple organ failure and required renal replacement therapy, and after extensive counseling in the setting of a poor maternal prognosis, the family opted for no obstetrical intervention. She had a prepregnancy body mass index (BMI) greater than 40 kg/m2 and OSA. The second patient who died had a rapid clinical deterioration after delivery, which was complicated by severe respiratory distress, multiple organ failure, and cardiopulmonary arrest.1 She had a prepregnancy BMI less than 30 kg/m2 and no baseline comorbidities. Prone positioning to improve oxygenation during mechanical ventilation was used in the first case but not in the second because of the patient’s rapid clinical decline. These cases were critically reviewed by a multidisciplinary team; it was determined that the standard of care was met in both cases and that the outcomes were a consequence of the disease process.
Of the 7 women (54%) who delivered during hospitalization for COVID-19, 5 (71%) were urgent or emergent cesarean deliveries in the setting of acute respiratory decompensation, whereas 1 was an emergent cesarean delivery for cord prolapse during induction of labor for worsening respiratory symptoms, and 1 presented in labor and delivered vaginally. There were 4 (57%) preterm births.
Conclusion
Maternal death occurred in 15% of patients admitted to the ICUs for COVID-19 and in 25% of those who required invasive mechanical ventilation. Delivery occurred in half of the patients with COVID-19 who were admitted to the ICUs and all patients who required invasive mechanical ventilation. Hispanic women constituted the largest racial or ethnic group in the study, which may reflect a disproportionate burden of disease among minority groups.
Few studies have evaluated the characteristics and outcomes of critically ill pregnant women with COVID-19. Interestingly, and in contrast with our findings, no cases of maternal mortality were observed in the recent multicenter cohort study by Pierce-Williams et al6 among pregnant women hospitalized with severe or critical COVID-19. At present, that study represents the largest report of such patients, but it is limited by the fact that half of the critically ill patients (11/20) were still hospitalized at the completion of data collection. In our study, approximately half of the women admitted to the ICUs had no baseline comorbidities, making it difficult to identify those who were at highest risk of respiratory failure and death. The patients were generally older, multiparous, and racial or ethnic minorities, which may reflect underlying disease prevalence (a product of various factors, including household size and ability to social distance) rather than intrinsic susceptibility to adverse outcomes. Larger studies are needed to determine which laboratory and imaging findings are most predictive of rapidly progressive respiratory failure in pregnancy. In our study, most patients were delivered in the setting of worsening disease; it is not known how autotransfusion and other physiological and immunologic changes immediately after delivery affect maternal outcomes.
The strengths of this study include consecutive patient enrollment over a well-defined time interval, explicit inclusion and exclusion criteria, utilization of data from a single medical record system, and evaluation of clinically relevant outcomes. In addition, no patients remained hospitalized at study completion, allowing all in-hospital outcomes to be fully evaluated, without omission, further reducing the risk of bias. This study also has limitations. First, our sample size remains small because of the rarity of ICU admissions of pregnant women with COVID-19.5 During the study, ICU bed availability was limited, and patients requiring considerable noninvasive respiratory support (eg, oxygen delivery by using a nasal cannula or face mask) were often managed on lower acuity units. Second, laboratory testing and radiologic imaging were not uniform. Third, treatment algorithms changed throughout the study period and were not identical for all patients. Finally, the true prevalence of COVID-19 among pregnant women in these communities is unknown; a SARS-CoV-2 testing strategy that only includes patients admitted to the hospital, predominantly for delivery, does no’t reflect this number. Universal testing for SARS-CoV-2 was implemented in the middle of the study period on our obstetrical units. Thus, determining an accurate risk of ICU admission or death among pregnant women infected with the virus is not possible.
In summary, pregnant and postpartum women with COVID-19 admitted to the ICUs are at risk for maternal death, which may occur even in the absence of substantial baseline comorbidities. Longitudinal population-based cohort studies may offer more insights into mechanisms determining which patients infected with the virus are at highest risk.
In addition, in the Figure, patients 1 to 7 and 9 were included in Richardson et al.4 Patient 7 was included in Vallejo et al.1 Patients 1 to 4, 6, and 8 to 10 were included in Blitz et al.5 Patients 12 to 13 were included in London et al7 and McLaren et al.8 Patients 3, 4, 6, 8, and 10 were included in Gulersen et al.9
Acknowledgments
We would like to acknowledge the contributions of the Northwell Health COVID-19 Research Consortium.
Footnotes
The authors report no conflict of interest.
This communication has been published in the middle of the COVID-19 pandemic and is available via expedited publication to assist patients and healthcare providers.
Appendix
Supplemental Table 1.
Clinical characteristics of the patients
| Characteristic | Patients (n=13) |
|---|---|
| Demographics | |
| Maternal age, y | 33.8±5.2 |
| ≥35 | 6 (46) |
| Race or ethnicity | |
| Hispanic or Latino | 5 (38) |
| Non-Hispanic white | 4 (31) |
| Non-Hispanic black | 1 (8) |
| Asian | 3 (23) |
| Multiparous | 9 (69) |
| Parity of 3 or more | 6 (46) |
| BMI prepregnancy, kg/m2 | 30.2±6.7 |
| ≥30 | 5 (38) |
| Medical comorbidities | |
| Hypertension | 0 (0) |
| Diabetes | 1 (8) |
| Asthma | 2 (15) |
| Obstructive sleep apnea | 1 (8) |
| Pregnancy complications | |
| Gestational diabetes | 1 (8) |
| Gestational hypertension or preeclampsia | 3 (23) |
| COVID-19 | |
| Duration of illness before hospitalization, d | 8±3 |
| Duration of hospitalization before ICU admission, d | 2±2 |
| Previous hospital visit for respiratory symptoms | 6 (46) |
| Gestational age at positive PCR result for SARS-CoV-2, wk | 32.5±5.2 |
| Gestational age at hospitalization, wk | 33.3±5.3 |
| On admission | |
| Reported symptoms | |
| Fever, subjective or measured | 12 (92) |
| Cough | 13 (100) |
| Dyspnea | 10 (77) |
| Myalgia | 6 (46) |
| Fatigue or malaise | 3 (23) |
| Obstetrical complaints | |
| Contractions | 2 (15) |
| Decreased fetal movement | 3 (23) |
| Vital signs | |
| Temperature, ≥100.4°F or 38°C | 2 (15) |
| Heart rate, >100 beats per min | 10 (77) |
| Respiratory rate, breaths per min | 27±12 |
| >30 | 3 (23) |
| Systolic blood pressure, mm Hg | 122±18 |
| Oxygen saturation, % | 92±5 |
| ≤93 | 9 (69) |
Data are presented as n (%) and mean±standard deviation unless otherwise specified.
BMI, body mass index; COVID-19, coronavirus disease 2019; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Blitz. Maternal mortality among intensive care unit admissions for coronavirus disease 2019. Am J Obstet Gynecol 2020.
Supplemental Table 2.
Laboratory results and imaging findings
| Variable | Patients (n=13) | Reference rangesa |
|---|---|---|
| On admissionb | ||
| White blood cell count, ×109/L | 7.9 (5.6–9.9) | 3.8–10.5 |
| >10 | 3 (23) | — |
| Lymphocyte count, ×109/L | 0.8 (0.6–1.0) | 1.0–3.3 |
| Platelet count, ×103/μL | 204 (166–246) | 150–420 |
| <150 | 2 (15) | — |
| Aspartate aminotransferase, U/L | 81 (49–98) | 10–40 |
| >40 | 10 (77) | — |
| Alanine aminotransferase, U/L | 44 (21–67) | 10–45 |
| >40 | 8 (62) | — |
| Venous lactate, mmol/L | 1.0 (0.8–1.4) | 0.7–2.0 |
| >1.5 | 3 (23) | — |
| Serum creatinine, mg/dL | 0.6 (0.5–0.7) | 0.5–1.30 |
| >1.1 | 2 (15) | — |
| Ferritin, ng/mL | 112 (95–246) | 15–400 |
| D-dimer, ng/mL | 613 (383–1169) | 0–229 |
| >1000 | 4 (31) | — |
| C-reactive protein, mg/dL | 20.1 (11.3–33.1) | 0.0–0.40 |
| Procalcitonin, ng/mL | 0.8 (0.3–1.5) | 0.02–0.10 |
| Creatine kinase, U/Lc | 33 (33–128) | 25–200 |
| Troponin above test-specific upper limit of normal | 3/10 (30) | — |
| Chest radiography | ||
| Bilateral infiltrates | 11 (85) | — |
| Pleural effusion | 0 (0) | — |
| During ICU stay | ||
| Highest serum creatinine, mg/dL | 0.7 (0.6–1.1) | — |
| >2.5 | 2 (15) | — |
| Lowest platelet count, ×103/μL | 152 (127–235) | — |
| <100 | 3 (23) | — |
| Highest aspartate aminotransferase, U/L | 103 (81–141) | — |
| Highest alanine aminotransferase, U/L | 97 (77–215) | — |
| Aminotransferase, >1000 U/L | 3 (23) | — |
| Highest D-dimer, ng/mL | ||
| >3000 | 5 (38) | — |
Data are presented as n (%), mean±standard deviation, and median (interquartile range).
ICU, intensive care unit.
Blitz. Maternal mortality among intensive care unit admissions for coronavirus disease 2019. Am J Obstet Gynecol 2020.
Reference range established for nonpregnant patients; many of these laboratory tests change by trimester
Defined as first test results available, typically within the first 24 hours of presentation
Data available for 9 patients.
Supplemental Table 3.
Clinical management and outcomes
| Variable | Patients (n=13) |
|---|---|
| ICU management | |
| Invasive mechanical ventilation | 8 (62) |
| Duration, d | 8 (2–19) |
| Extubateda | 6/8 (75) |
| Prone positioning utilized | 4/8 (50) |
| Extracorporeal membrane oxygenation | 0 (0) |
| Vasopressors | 7 (54) |
| Renal replacement therapy | 1 (8) |
| Antiviral agent | |
| Hydroxychloroquine | 11 (85) |
| Remdesivir | 3 (23) |
| Antibiotics for community-acquired pneumonia | 12 (92) |
| Anticoagulation, prophylactic or therapeutic | 13 (100) |
| Immunomodulatory agent | 9 (69) |
| Corticosteroid (for maternal indication) | 7 (54) |
| Interleukin-1 inhibitor | 2 (15) |
| Interleukin-6 inhibitor | 5 (38) |
| Convalescent plasma | 2 (15) |
| Maternal outcomes | |
| Length of stay, d | |
| In hospital | 13 (9–23) |
| In ICU | 8 (4–15) |
| Delivery during hospitalization | 7/13 (54) |
| Cesarean delivery for acute respiratory decompensation | 5/7 (71) |
| Cesarean delivery for obstetrical indication | 1/7 (14) |
| Vaginal delivery | 1/7 (14) |
| Died in hospital | 2 (15) |
| Discharged from hospital | 11 (85) |
| To home | 10 (77) |
| To long-term care facility | 1 (8) |
| Remained hospitalized at study completion | 0 (0) |
| Neonatal outcomes | |
| Gestational age at delivery, wk | 36.9±2.0 |
| Birthweight, g | 2994±569 |
| Apgar scores | |
| 1 min≤7 | 3/8 (38) |
| 5 min≤7 | 0/8 (0) |
| Positive PCR result for SARS-CoV-2 on first day of lifeb | 0/7 (0) |
| Received antenatal corticosteroids | 5 (50) |
| Gestational age at administration | 29.1±4.2 |
Data are presented as n (%), mean±standard deviation, and median (interquartile range). Denominator is number of patients diagnosed as having COVID-19 at less than 37 weeks’ gestation.
COVID-19, coronavirus disease 2019; ICU, intensive care unit; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Blitz. Maternal mortality among intensive care unit admissions for coronavirus disease 2019. Am J Obstet Gynecol 2020.
One patient was extubated but remained ventilator dependent with a tracheostomy
Results not available for 1 newborn.
References
- 1.Vallejo V, Ilagan JG. A postpartum death due to coronavirus disease 2019 (COVID-19) in the United States. Obstet Gynecol 2020 [Epub ahead of print]. [DOI] [PubMed]
- 2.Hantoushzadeh S., Shamshirsaz A.A., Aleyasin A. Maternal death due to COVID-19. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.04.030. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karami P., Naghavi M., Feyzi A. WITHDRAWN: mortality of a pregnant patient diagnosed with COVID-19: a case report with clinical, radiological, and histopathological findings. Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101665. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020 doi: 10.1001/jama.2020.6775. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blitz M.J., Grünebaum A., Tekbali A. Intensive care unit admissions for pregnant and nonpregnant women with coronavirus disease 2019. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.05.004. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierce-Williams R.A.M., Burd J., Felder L. Clinical course of severe and critical COVID-19 in hospitalized pregnancies: a US cohort study. Am J Obstet Gynecol MFM 2020:100134. Am J Obstet Gynecol MFM. 2020 doi: 10.1016/j.ajogmf.2020.100134. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.London V., McLaren R., Jr., Atallah F. The relationship between status at presentation and outcomes among pregnant women with COVID-19. Am J Perinatol. 2020 doi: 10.1055/s-0040-1712164. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLaren R.A., Jr., London V., Atallah F. Delivery for respiratory compromise among pregnant women with COVID-19. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.05.035. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gulersen M, Blitz MJ, Rochelson B, et al. Clinical implications of SARS-CoV-2 infection in the viable preterm period. Am J Perinatol 2020 (in press). [DOI] [PMC free article] [PubMed]