Abstract
Objectives
Angiotensin-converting enzyme inhibitors (ACEi), angiotensin II receptor blockers (ARBs), and HMG-CoA reductase inhibitors (“statins”) have been hypothesized to affect COVID-19 severity. However, up to now, no studies investigating this association have been conducted in the most vulnerable and affected population groups (ie, older adults residing in nursing homes). The objective of this study was to explore the association of ACEi/ARB and/or statins with clinical manifestations in COVID-19–infected older adults residing in nursing homes.
Design
We undertook a retrospective multicenter cohort study to analyze the association between ACEi/ARB and/or statin use with clinical outcome of COVID-19. The outcomes were (1) serious COVID-19 defined as long-stay hospital admission or death within 14 days of disease onset, and (2) asymptomatic (ie, no disease symptoms in the whole study period while still being diagnosed by polymerase chain reaction).
Setting and participants
A total of 154 COVID-19–positive subjects were identified, residing in 1 of 2 Belgian nursing homes that experienced similar COVID-19 outbreaks.
Measures
Logistic regression models were applied with age, sex, functional status, diabetes, and hypertension as covariates.
Results
We found a statistically significant association between statin intake and the absence of symptoms during COVID-19 (odds ratio [OR] 2.91; confidence interval [CI] 1.27–6.71), which remained statistically significant after adjusting for covariates (OR 2.65; CI 1.13–6.68). Although the effects of statin intake on serious clinical outcome were in the same beneficial direction, these were not statistically significant (OR 0.75; CI 0.24–1.87). There was also no statistically significant association between ACEi/ARB and asymptomatic status (OR 2.72; CI 0.59–25.1) or serious clinical outcome (OR 0.48; CI 0.10–1.97).
Conclusions and Implications
Our data indicate that statin intake in older, frail adults could be associated with a considerable beneficial effect on COVID-19 clinical symptoms. The role of statins and renin-angiotensin system drugs needs to be further explored in larger observational studies as well as randomized clinical trials.
Keywords: Angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, statins, COVID-19, nursing home residents
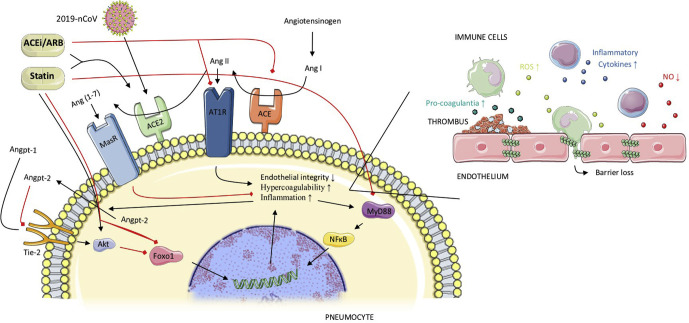
Patients with serious and fatal COVID-19 infections are characterized by pneumonia-associated acute respiratory distress syndrome (ARDS) and sepsis-associated multiorgan failure. The underlying mechanisms are linked to an imbalance between angiotensin-converting enzyme (ACE) and ACE2, as well as endothelial dysfunction1, 2, 3 (Figure 1 ). Animal in vivo experiments have indicated that angiotensin II receptor blockers (ARBs), ACE inhibitors (ACEis), and statins can prevent experimentally induced ARDS.4 , 5 Moreover, these drugs are likely to counteract the effects of sepsis-associated coagulopathy, elevated proinflammatory cytokines (eg, interleukin-6), and sepsis-associated effects on pulmonary vascular permeability (Figure 1).6, 7, 8, 9, 10, 11, 12, 13, 14, 15 A recent in silico docking study has also suggested a possible direct binding and inhibition of COVID-19 viral proteins by statins.16
Fig. 1.
Three mechanisms suggested for the effects of statins and ACEis/ARBs in preventing severe pulmonary disease in COVID-19. (1) Under normal conditions the Tie-2 receptor is continuously activated by Angiopoetin-1 (Angpt-1), which in turn activates Akt-kinase, leading to phosphorylation and hence inhibition of the transcription factor Foxo1. Unphosphorylated or active Foxo1 initiates the transcription of genes leading to increased inflammation, decreased endothelial barrier integrity, and hypercoagulability. Angpt-2 is a partial antagonist of the Tie-2 receptor, stimulating inflammation, endothelial dysfunction and hypercoagulability. COVID-19 infection and ARDS are associated with increased Angpt-2 levels in blood, whereas statins simulate the Angpt-1 pathways. (2) The RAS system activates angiotensin-1 receptors (AT1R), stimulating inflammation, hypercoagulability, and endothelial permeability. The Ang II-ACE2-Ang(1–7)-Mas receptor pathway counteracts the effects of this RAS system. COVID-19 enters the cell through ACE2 receptors, thereby decreasing these membrane-bound receptors, and relatively stimulating the RAS system. ACEis/ARBs inhibit the RAS system, while concomitantly increasing ACE-2 expression, which protects against ARDS. Statins also increase ACE-2 expression. (3) In ARDS, there is an increase in the activation of the MyD88-NFkB inflammatory pathway. Statins preserve MyD88 at normal levels and downregulate NFkB. Black lines = stimulating effects; red lines = inhibiting effects.
Before the COVID-19 pandemic, clinical investigators observed that patients with pneumonia who had been taking statins, ARBs, or ACEis had improved survival.17 , 18 Moreover, recent observational studies reported similar findings for hospitalized patients with COVID-19.19, 20, 21, 22, 23 Recently, randomized controlled clinical trials have begun to evaluate the clinical effects of ARB, ACEi, or statin treatment in hospitalized patients with COVID-19 (eg, NCT04348695, NCT04343001, NCT04351581). However, the estimated completion dates for these trials will be some time in 2021, and most will consider only ARB/ACEi monotherapy, that is, not in combination with statins.
To our knowledge, no ARB/ACEi/statin studies have been or are being conducted among older nursing home residents, the most vulnerable individuals for COVID-19 morbidity and mortality. In Belgium, a country with a well-developed health care system, 3000 residents of nursing homes have died from COVID-19, with still approximately 100 residents a day currently dying.24 Estimates for the United States suggest that almost 20% of all COVID-19 deaths have occurred in long-term care facilities.25 Thus, every day without effective therapy comes at a high human cost.
We aimed to study the proposed association in a population at high risk for developing COVID-19, that is, older adults living in nursing homes. While we wait for the results of prospective clinical trials, our findings allow us to make suggestions about the use of ACEis/ARBs and statins for these patients with COVID-19.
Methods
This retrospective study conformed with all legal guidelines and the protocol was approved by the Ethical Committee of our institute (reference BC-07671).
Study Design
The retrospective study cohort was defined as all (anonymized) residents at 2 care homes with COVID-19 diagnosis based on clinical grounds and/or polymerase chain reaction (PCR) laboratory testing (nasopharyngeal swabs) from March 1 to April 16. Both care homes for older individuals experienced COVID-19 outbreaks during this period. Although several diagnostic tools are currently available, none gives high specificity and sensitivity on its own. However, it is generally accepted that the clinical diagnosis is a key herein.26 Moreover, some techniques like computed tomography are not feasible in the context of nursing homes. To determine the day of disease onset, structured and unstructured diagnostic records were analyzed for symptoms suggesting COVID-19 infection. The first day of suggestive symptoms on 2 of 3 consecutive days was considered as the day of disease onset. For the PCR-diagnosed residents, the suggestive symptoms used for disease onset were cough, shortness of breath (dyspnea), sore throat, runny nose, general weakness, headache, confusion, muscle pain, arthralgia, diarrhea, abdominal pain, vomiting, fever (T° > 37.6°C), increased oxygen need, or low oxygen saturation (SpO2 ≤92%). In cases in which no symptoms were mentioned (while still being PCR COVID-19–positive diagnosed), the date of nasopharyngeal sampling was used as the day of disease onset. For the clinically diagnosed residents without a confirmatory PCR laboratory test, the symptoms used for determining disease onset were defined more strictly, that is, respiratory complaints (cough, shortness of breath, sore throat, runny nose), fever (T° > 37.6°C), increased oxygen need, or low oxygen saturation (SpO2 ≤92%).
The primary outcomes were (1) serious COVID-19, that is, long-stay hospital admission (length of stay ≥7 days) or death (at nursing home or hospital) within 14 days of disease onset, and (2) asymptomatic, that is, no disease symptoms as defined previously throughout the whole study period while still being PCR diagnosed.
Information about daily drug intake was available through the medication administration records at nursing home level. All residents were stratified according to drug exposure to ACEi or ARB within 7 days before the day of disease onset or during the disease (before an outcome being reached). Specifically, we considered as treated all residents taking for ≥2 days an ACEi (ramipril, lisinopril, enalapril, captopril, quinapril, imidapril, fosinopril, trandolapril) or ARB (candesartan, irbesartan, losartan, olmesartan, telmisartan, valsartan) up to 7 days before or 14 days after disease onset. Only 1 day of drug intake (eg, for acute hypertension) was not considered as (chronic) treatment. An identical protocol was used to stratify according to drug exposure to statins (atorvastatin, fluvastatin, pravastatin, rosuvastatin, simvastatin).
As no direct diagnosis codes were available, we developed a mapping table based on clinical drug prescriptions to determine the diabetic and hypertension status of all residents. It was designed by a specialist in care of older individuals and validated by 2 independent physicians, one a general physician and the other a cardiologist.
The functional status of all residents was a dichotomous variable (high vs low functioning). This definition was based on the available Katz scale for residents before the day of disease onset. The Katz scale is a measure of independent activity of daily living.
Data Collection and Quality Control
Data about residents were available at the level of nursing homes. After anonymization, these data were merged in a relational database for further processing by making use of Extract, Transform, and Load techniques. All received anonymized data were then evaluated on basic data quality attributes such as completeness (ie, the extent of missing data) and accuracy (ie, whether or not suspicious outliers were present in the individual attributes). Data were enriched with Anatomical Therapeutic Chemical codes for the included drugs. Suggestive symptoms were searched for based on biometrical measurements as well as indications in text. For the latter, basic Natural Language Processing (NLP) techniques were used. For the residents still in the hospital at the moment of data extraction, median imputation was used to estimate length of hospital duration. Two independent physicians manually verified all recorded symptoms as well as all data for a random subsample.
Statistical Analysis
We calculated the distributions for dependent and independent variables for the total cohort using appropriate measures of central tendency and dispersion. For our main analysis, we investigated the association between ACEi/ARB and/or statin treatment and (1) serious disease, measured as long-stay hospital admission or death, or (2) asymptomatic disease using a series of logistic regressions applying Firth's correction. Unless otherwise indicated, the logistic analyses were conducted on the whole cohort (n = 154).
This procedure has been used previously by our group and shown to be robust for low-prevalence events and low-dimensional settings.20 , 27 , 28 We first explored the independent association between ACEi/ARB and both outcomes, as well as the association between statins and the same outcomes. Then we adjusted the models stepwise for age, sex, functional status, hypertension, and diabetes mellitus. Also, the method of diagnosis (PCR or clinical) was evaluated as possible confounder. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC) and RStudio 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria).
Sensitivity Analyses
For the statistically significant associations, we also conducted sensitivity analyses to evaluate our modeling assumptions and the extent to which feature allocation and population selection influenced the main results and conclusions. To control for the first (bias from modeling assumption) we conducted exact logistic regressions with models adjusted variable by variable. To control for the bias from feature allocation, we performed Monte Carlo simulations for the features hypertension and functional status. Last, we controlled for the bias from population selection by using the same modeling approach (logistic regression with Firth's correction) to analyze only the PCR-confirmed COVID-19–positive residents.
Results
The study cohort included 154 COVID-19–diagnosed residents aged 86 ± 7 (mean ± SD) years, evenly distributed over the 2 nursing homes (76 and 78 residents, respectively). Baseline characteristics are shown in Table 1 . In our cohort (33% men), 20% were taking ACEis/ARBs (16% ACEi and 4% ARB), and 20% were taking a statin. Eight residents (5%) were taking both an ACEi/ARB and a statin. Importantly, none of the residents stopped ACEi/ARB or statin treatment on the day of disease onset and all continued taking their drugs during the follow-up period unless the clinical situation no longer allowed this. Also, none of the residents was taking other renin-angiotensin system (RAS)-associated drugs such as renin-inhibitors or neprylisine-inhibitors. Clinical symptoms detected by NLP in unstructured texts were all manually verified, with 22% false positives, mostly due to mentioned symptoms with more complex negations in the same sentence. Two physicians also independently evaluated manually all available data from a minimum of 5 random residents each. This resulted in no changes in the results-matrix.
Table 1.
Characteristics of the Study Cohort
| Sample Characteristics | Total (N = 154) | ACEi/ARB (n = 30) | No ACEi/ARB (n = 124) | Statin (n = 31) | No Statin (n = 123) | Symptoms (n = 113) | No Symptoms (n = 41) | Serious COVID (n = 37) | Nonserious COVID (n = 117) |
|---|---|---|---|---|---|---|---|---|---|
| Age | 85.9 (7.2) | 86.2 (6.6) | 85.8 (7.4) | 85.6 (5.3) | 85.9 (7.6) | 86.0 (7.4) | 85.6 (6.6) | 86.8 (6.8) | 85.6 (7.3) |
| Male | 51 (33.1) | 12 (40.0) | 39 (31.5) | 10 (32.3) | 41 (33.3) | 41 (36.3) | 10 (24.4) | 12 (32.4) | 39 (33.3) |
| PCR-based diagnosis | 87 (56.5) | 22 (73.3) | 65 (52.4) | 19 (61.3) | 68 (55.3) | 46 (40.7) | 41 (100) | 5 (13.5) | 82 (70.1) |
| Clinical-based diagnosis | 67 (43.5) | 8 (26.7) | 59 (47.6) | 12 (38.8) | 55 (44.7) | 67 (59.3) | 0 (0) | 32 (86.5) | 35 (29.9) |
| On ACEi/ARB | 30 (19.5) | 30 (100) | 0 (0) | 8 (25.8) | 22 (17.9) | 20 (17.7) | 10 (24.4) | 6 (16.2) | 24 (20.5) |
| On statin | 31 (20.1) | 8 (26.7) | 23 (18.5) | 31 (100) | 0 (0) | 17 (15.0) | 14 (34.1) | 6 (16.2) | 25 (21.4) |
| Low functioning | 137 (89.0) | 23 (76.7) | 114 (91.9) | 26 (83.9) | 111 (90.2) | 106 (93.8) | 31 (75.6) | 35 (94.6) | 102 (87.2) |
| Diabetes mellitus | 28 (18.2) | 6 (20.0) | 22 (17.8) | 10 (32.3) | 18 (14.6) | 18 (15.9) | 10 (24.4) | 7 (18.9) | 21 (17.9) |
| Hypertension | 39 (25.3) | 28 (93.3) | 11 (8.87) | 8 (25.8) | 31 (25.2) | 29 (25.7) | 10 (24.4) | 10 (27.0) | 29 (24.8) |
| Symptoms | 113 (73.4) | 20 (66.7) | 93 (75.0) | 17 (54.8) | 96 (78.0) | 113 (100) | 0 (0) | 36 (97.3) | 77 (65.8) |
| Serious COVID | 37 (24.0) | 6 (20.0) | 31 (25.0) | 6 (19.4) | 31 (25.2) | 36 (31.9) | 1 (2.44) | 37 (100) | 0 (0) |
All variables are shown as n (% of column), except age, which is mean in years (SD).
Of the 154 residents, 41 remained asymptomatic during the study period, that is, 27% of the total cohort and 47% of the PCR-tested COVID-19–positive residents. These numbers are similar to those from another study in a similar population.29 Thirty-seven residents (24%) experienced serious COVID-19. Although this serious outcome number seems high compared with other outpatient population studies, in view of the very vulnerable population, this is not surprising.30 , 31 Among residents treated with ACEis or ARBs, 10 (33%) of 30 remained asymptomatic vs 31 (25%) of 124 of those without such treatment. Residents taking statins remained asymptomatic in 45% of the cases (14 of 31) vs 22% (27 of 123) of those not taking statins. Evaluating COVID-19 severity, 20% (6 of 30) of the residents treated with ACEi/ARBs died or were admitted to hospital for long-stay vs 25% (31 of 124) of those without such treatment. Residents taking statins experienced serious COVID-19 in 19% of the cases (6 of 31) vs 25% (31 of 123) of those not taking statins. Interestingly, 6 of 8 residents (75%) taking the ACEi/ARB and statin combination remained asymptomatic throughout the study period. Only one of them (13%) experienced serious COVID-19.
Although not reaching statistical significance, findings from unadjusted logistic regression suggested a potential beneficial effect on COVID-19 symptoms among residents taking ACEis or ARBs [odd ratio (OR) 1.52; confidence interval (CI) 0.62–3.50; P = .339]. ORs adjusted for age, sex, functional status, diabetes, and hypertension were of similar magnitude (Table 2 , adjusted OR 2.72; CI 0.59–25.1; P = .242). The results for the statins were most interesting, as we observed a clear and statistically significant association between statin intake and asymptomatic status (unadjusted OR 2.91; CI 1.27–6.71; P = .011). This association was partially attenuated but remained statistically significant when adjusted for sex, age, functional status, diabetes, and hypertension (Table 2, adjusted OR 2.65; CI 1.13–6.68; P = .028). Correcting for method of diagnosis (PCR or clinical based), did not change conclusions for both ACEis/ARBs and statins (Table 2).
Table 2.
Summary of ORs for the Asymptomatic COVID-19 Infection Using Logistic Regression With Firth's Correction
| Drug Treatment | Adjustments | OR (95% CI) on Drug vs. No Drug | P Value |
|---|---|---|---|
| ACEi/ARB | - | 1.52 (0.62–3.50) | .339 |
| Age, sex | 1.61 (0.65–3.80) | .283 | |
| Age, sex, functional status | 1.35 (0.51–3.31) | .521 | |
| Age, sex, functional status, diabetes mellitus, hypertension | 2.72 (0.59–25.1) | .242 | |
| Age, sex, functional status, diabetes mellitus, hypertension, diagnosis∗ | 1.41 (0.27–12.4) | .704 | |
| Statins | - | 2.91 (1.27–6.71) | .011 |
| Age, sex | 2.88 (1.26–6.83) | .013 | |
| Age, sex, functional status | 2.87 (1.23–7.07) | .016 | |
| Age, sex, functional status, diabetes mellitus, hypertension | 2.65 (1.13–6.68) | .028 | |
| Age, sex, functional status, diabetes mellitus, hypertension, diagnosis∗ | 3.52 (1.11–16.2) | .040 |
Diagnosis = diagnosis method (PCR-based or clinical-based).
We also examined associations between ACEis/ARBs and statins, and serious COVID-19. Although the available data failed to reach statistical significance, the directionality of the ORs suggested a potential beneficial clinical effect of both ACEi/ARB and statins on serious COVID-19 outcome. All ORs (unadjusted as well as adjusted for covariates) were between 0.48 (CI 0.10–1.97; P = .316) and 0.86 (CI 0.25–2.50; P = .788) (Table 3 ). If only considering death as serious outcome (ie, not taking hospitalizations into account), the effect sizes of the ORs became even larger: 0.35 (CI 0.05–1.70; P = .204) and 0.61 (CI 0.15–1.71; P = .380) for adjusted ACEi/ARB and statin treatment, respectively (Supplementary Material 1). Explorative, we also widened the time window of 14 days after day of symptom onset for death as serious outcome. If considering all deaths in the period of 2 months for which data were collected, the adjusted ORs were 0.19 (CI 0.03–0.84; P = .038) and 0.51 (CI 0.14–1.35; P = .209) for ACEi/ARB and statin treatment, respectively (Supplementary Material 1).
Table 3.
Summary of ORs for the Serious COVID-19 Infection Using Logistic Regression With Firth's Correction
| Drug Treatment | Adjustments | OR (95% CI) on Drug vs Not Drug | P Value |
|---|---|---|---|
| ACEi/ARB | - | 0.79 (0.26–1.95) | .629 |
| Age, sex | 0.78 (0.25–1.93) | .610 | |
| Age, sex, functional status | 0.84 (0.27–2.14) | .736 | |
| Age, sex, functional status, diabetes mellitus, hypertension | 0.48 (0.10–1.97) | .316 | |
| Age, sex, functional status, diabetes mellitus, hypertension, diagnosis∗ | 0.72 (0.10–4.56) | .718 | |
| Statins | - | 0.75 (0.25–1.85) | .556 |
| Age, sex | 0.75 (0.25–1.86) | .564 | |
| Age, sex, functional status | 0.77 (0.25–1.91) | .597 | |
| Age, sex, functional status, diabetes mellitus, hypertension | 0.75 (0.24–1.87) | .559 | |
| Age, sex, functional status, diabetes mellitus, hypertension, diagnosis∗ | 0.86 (0.25–2.50) | .788 |
Diagnosis = diagnosis method (PCR-based or clinical-based).
We did not undertake regression analyses on the combined ACEi/ARB + statin group, as there were only 8 residents in our cohort; nor did we undertake separate analyses for the ACEi or ARB groups; only 6 residents were treated with an ARB.
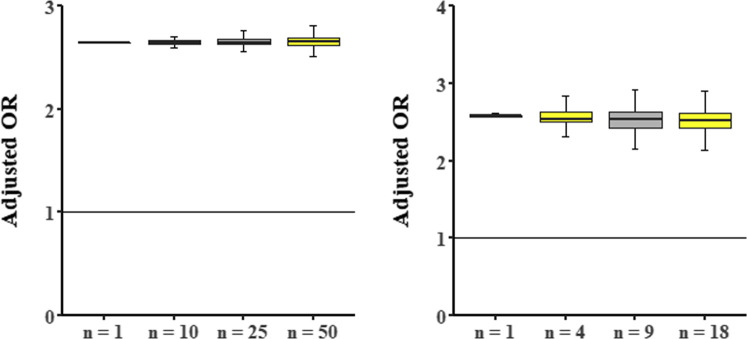
Sensitivity analyses were conducted on the statistically significant association between statins and symptoms. We found that estimates of the impact of statin treatment on asymptomatic status were consistently of the same magnitude and statistically significant as the original analyses (Supplementary Tables 2 and 3, Supplementary Figure 1).
Supplementary Fig. 1.
(A) Monte Carlo simulations (10,000 simulations) for the association statin-asymptomatic status with feature hypertension as changing variable. Random samples of the total cohort (n = 154) were taken with change of the hypertension status for all subjects of this sample. For each sample, the OR for statin intake and asymptomatic status adjusted for age, gender, functional status, diabetes mellitus, and hypertension was calculated. The y-axis shows these ORs: boxplots represent median (midline), 25% and 75% percentiles (upper and lower perimeters) and 1.5× interquartile range (whiskers). The x-axis indicates the chosen sample size. A horizontal line at y = 1 represents the line of no effect. (B) Monte Carlo simulations (10,000 simulations) for the association statin-asymptomatic status with feature functional status as changing variable. Random samples of the residents with a Katz scale indicating a small decrease in functional status or unknown status (n = 56) were taken with change of the functional status for all subjects of this sample. For each sample, the OR for statin intake and asymptomatic status adjusted for age, gender, functional status, diabetes mellitus, and hypertension was calculated. The y-axis shows these ORs: boxplots represent median (midline), 25% and 75% percentiles (upper and lower perimeters), and 1.5× interquartile range (whiskers). The x-axis indicates the chosen sample size. A horizontal line at y = 1 represents the line of no effect.
Discussion
ACEi/ARBs and statins were not statistically significantly associated with the outcome of serious COVID-19 in older adults who live in nursing homes. However, overall both ACEi/ARBs and statins did show clinical beneficial ORs for this outcome (Table 3). If considering only death as serious COVID-19, the effect sizes become even larger and reach statistical significance (Supplementary Table 1). These results are in line with a very recent large meta-analysis studying the effects of ACEi/ARBs in hospital patients.32
The results for statins and symptoms are most convincing, that is, large effect sizes that are statistically significant. The fact that statin intake is more strongly associated with asymptomatic status than serious COVID-19 suggests that the potential therapeutic effects of statins are more outspoken in the initial stages of COVID-19. Statins are most frequently used to prevent cardiovascular diseases. The safety profile of statins is well known and excellent, even in the population of older persons. Moreover, these drugs are relatively inexpensive and widespread, some even as food supplements such as red yeast rice, making them easily available throughout the world. Although this observational study does not have the power of a randomized controlled clinical trial, in the current absence of other valuable therapies and considering the benefit-risk balance, an older person living in a nursing home could consider taking a statin if at high COVID-19 infection risk. Currently, therapeutic decisions for patients with COVID-19 are driven by well-conducted observational studies.33 , 34 In any case, based on our results, we recommend against stopping statins in patients who are COVID-19 infected.
The combination of ACEi/ARB and statin treatment seemed to have additive beneficial effects on symptoms and serious disease outcome: 6 of 8 residents taking the combination remained asymptomatic and only one of them developed serious COVID-19. Although this result is promising, our sample size was too small to allow us to draw firm conclusions.
One strength of this study is the specific population, that is, older adults (mean age >85 years) residing in nursing homes. Although they are considered highly vulnerable to COVID-19 clinical outcomes, no study has yet reported on the effects of ARB/ACEi and/or statin treatment on COVID-19 in this population. Extracting reliable data from nursing homes with COVID-19 outbreaks is far more cumbersome than extracting data from hospitals. Another strength is that drug treatment was based on real intake, in contrast to most hospital-based studies that use prescriptions as proxies for drug treatment. Last, in contrast to most hospital studies, asymptomatic patients with COVID-19 were included in the study. People admitted to hospitals are evidently always symptomatic.
One limitation of our study is its relatively small cohort size. Consequently, absence of statistical significance should be interpreted with caution. However, the consistency in the observed effect sizes, even without statistical significance due to small sample size, should be considered in the overall evaluation. As number of cases increase, further analyses will be undertaken to better understand our findings and confirm these associations. Also, another limitation was the lack of other potential confounders, including chronic kidney injury and body mass index. For this study, we decided to only include variables as possible confounders that are currently suggested to be associated with COVID-19, and that we could reliable quantitate from the available care home registries. Finally, our results apply to a very specific population (older adults living in nursing homes) and cannot be generalized to other groups, such as young people or hospitalized people.
There are currently no licensed antiviral treatments for COVID-19 approved. Also, the development of COVID-19 vaccines will take time. Moreover, there is no information on when sufficient vaccine supplies will become widely available. Recently, the World Health Organization communicated a Solidarity “megatrial” evaluating 4 broad-spectrum antiviral agents. Among them, the broad-spectrum experimental antiviral drug remdesivir was shown to have low efficacy against Ebola and dropped from further study, although a recent report of its compassionate use in serious COVID-19 was favorable.35 Lopinavir and ritonavir (a protease inhibitor combination used to treat patients with human immunodeficiency virus) were ineffective in a Chinese clinical trial.36 Much attention has gone to chloroquine and hydroxychloroquine. Unfortunately, prospects for their success against COVID-19 are not good.37 Convalescent sera, obtained from patients who have recovered from COVID-19, might be an option to treat acute COVID-19 infections,38 but its implementation will be cumbersome and unlikely to become widely available. The first clinical trials of ACEi/ARB and statin treatments in hospital settings have been initiated within the past month. While we await the results of these trials, which are expected in 2021, this retrospective study should be regarded as both timely and complementary, as it has focused on a frail, nonhospitalized population and demonstrated clinical findings on the use of ACEi/ARB/statins using real world data.
Conclusions and Implications
Our study, based on available data, indicates that in older nursing home residents, statin treatment is associated with beneficial effects on COVID-19–related clinical symptoms. Although not statistically significant, our findings also suggest that statin treatment in combination with an ACEi or ARB is associated with less severe clinical outcomes. In light of these findings, a prudent recommendation is to continue or initiate statin treatment for older adults residing in nursing homes and at high risk for COVID-19 infection.
Acknowledgments
We thank all of the staff of VZW Zorg-Saam Zusters Kindsheid Jesu for their daily care of older people and for their collaboration on this study during these difficult times. We also thank the staff of the Corilus Health IT Center who helped with the data extraction.
Footnotes
A.D.S. and A.B. contributed equally as first authors.
This paper is dedicated to the memory of our wonderful colleague, Prof. Dr. Anton Carl Stolz (University of Pretoria), who recently passed away.
The authors declare no conflicts of interest.
A.D.S. is supported by a grant of Research Foundation Flanders(FWO) (grant number 1158818N).
Supplementary Material 1
Mortality as Outcome
Supplementary Table 1.
Summary of Adjusted∗ ORs for Drug Treatment as Predictor and Death as Serious COVID-19 Outcome Using Logistic Regression With Firth's Correction
| Drug Treatment | Time Frame | OR (95% CI) on Drug vs. Not Drug | P Value |
|---|---|---|---|
| ACEi/ARB | 2 wk | 0.35 (0.05–1.70) | .204 |
| Until end of follow-up (April 29, 2020) | 0.19 (0.03–0.84) | .038 | |
| Statins | 2 wk | 0.61 (0.15–1.71) | .380 |
| Until end of follow-up (April 29, 2020) | 0.51 (0.14–1.35) | .209 |
Age, gender, functional status, diabetes mellitus, hypertension.
Supplementary Material 2. Sensitivity Analyses
Bias From Modeling Assumption
Supplementary Table 2.
Summary of ORs for the Association of Statin Intake and Asymptomatic COVID-19 Infection Using Exact Logistic Regression (Cohort Size = 154)
| Adjustments | OR (95% CI) on Drug vs. No Drug | P Value |
|---|---|---|
| - | 2.91 (1.17–7.20) | .021 |
| Age, sex | 2.87 (1.15–7.15) | .022 |
| Age, sex, functional status | 2.86 (1.11–7.31) | .028 |
| Age, sex, functional status, diabetes mellitus, hypertension | 2.63 (1.02–6.76) | .045 |
Bias From Feature Allocation
Bias From Population Selection
Supplementary Table 3.
Summary of ORs for the Association Statin of Intake and Asymptomatic COVID-19 Infection in Polymerase Chain Reaction–Positive Residents, Using Logistic Regression With Firth Correction (Cohort Size = 87)
| Adjustments | OR (95% CI) on Drug vs No Drug | P Value |
|---|---|---|
| - | 3.98 (1.39–14.4) | .016 |
| Age, sex | 4.20 (1.44–16.5) | .014 |
| Age, sex, functional status | 4.15 (1.38–17.2) | .018 |
| Age, sex, functional status, diabetes mellitus, hypertension | 3.51 (1.11–16.2) | .046 |
References
- 1.Kuba K., Imai Y., Rao S.A. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imai Y., Kuba K., Rao S. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wosten-van Asperen R.M., Bos A.P., Bem R.A. Imbalance between pulmonary angiotensin-converting enzyme and angiotensin-converting enzyme 2 activity in acute respiratory distress syndrome. Pediatr Crit Care Med. 2013;14:E438–E441. doi: 10.1097/PCC.0b013e3182a55735. [DOI] [PubMed] [Google Scholar]
- 4.Woesten-van Asperen R.M., Lutter R., Specht P.A. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1–7) or an angiotensin II receptor antagonist. J Pathol. 2011;225:618–627. doi: 10.1002/path.2987. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson J.R., Barnard J.W., Grigoryev D.N., Ma S.F., Tuder R.M., Garcia J.G. Simvastatin attenuates vascular leak and inflammation in murine inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1026–L1032. doi: 10.1152/ajplung.00354.2004. [DOI] [PubMed] [Google Scholar]
- 6.Fedson D.S. Treating the host response to emerging virus diseases: Lessons learned from sepsis, pneumonia, influenza and Ebola. Ann Transl Med. 2016;4:421. doi: 10.21037/atm.2016.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGonagle D., Sharif K., O'Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19:102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGonagle D., O'Donnell J., Sharif K. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheum. 2020 doi: 10.1016/S2665-9913(20)30121-1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemostas. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin S., Huang M., Li D., Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J Thromb Thrombolysis. 2020 Apr 3 doi: 10.1007/s11239-020-02105-8. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van de Veerdonk F., Netea M.G., van Deuren M. 2020 Apr 3. Kinins and cytokines in COVID-19: A comprehensive pathophysiological approach, Preprints. [Epub ahead of print] [Google Scholar]
- 12.Schol-Gelok S., Morelli F., Arends L.R. A revised systematic review and meta-analysis on the effect of statins on D-dimer levels. Eur J Clin Invest. 2019;49:e13130. doi: 10.1111/eci.13130. [DOI] [PubMed] [Google Scholar]
- 13.Aktas S., Ucak S., Kurt F. Evaluation of protein C and protein S levels in patients with diabetes mellitus receiving therapy with statins and ACE inhibitors or angiotensin II receptor blockers. Diabetes Res Clin Pract. 2018;135:88–92. doi: 10.1016/j.diabres.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Teuwen L.-A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fedson D.S., Opal S.M., Rordam O.M. Hiding in plain sight: An approach to treating patients with severe COVID-19 infection. Mbio. 2020;11:e00398. doi: 10.1128/mBio.00398-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reiner Z., Hatamipour M., Banach M. Statins and the COVID-19 main protease: In silico evidence on direct interaction. Arch Med Sci. 2020;16:490–496. doi: 10.5114/aoms.2020.94655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mortensen E., Anzueto A. Prior use of both a statin and ARB is associated with lower mortality for patients hospitalized with pneumonia. Eur Respir J. 2016;48:OA3329. [Google Scholar]
- 18.Mortensen E.M., Nakashima B., Cornell J. Population-based study of statins, angiotensin II receptor blockers, and angiotensin-converting enzyme inhibitors on pneumonia-related outcomes. Clin Infect Dis. 2012;55:1466–1473. doi: 10.1093/cid/cis733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng J., Xiao G., Zhang J. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9:757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bean D., Kraljevic Z., Searle T. 2020. Treatment with ACE-inhibitors is associated with less severe SARS-Covid-19 infection in a multi-site UK acute Hospital Trust. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guang Y., Zihu T., Ling Z. 2020. Angiotensin II receptor blockers and angiotensin-converting enzyme inhibitors usage is associated with improved inflammatory status and clinical outcomes in COVID-19 patients with hypertension, medRxiv. [Google Scholar]
- 22.Yingxia L., Fengming H., Jun X. 2020. Anti-hypertensive angiotensin II receptor blockers associated to mitigation of disease severity in elderly COVID-19 patients, medRxiv. [Google Scholar]
- 23.Zhang P., Zhu L., Cai J. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126:1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.COVID-19 – Epidemiologisch bulletin van 29 april 2020. https://covid-19.sciensano.be/nl/covid-19-epidemiologische-situatie Available at:
- 25.7,000 of US coronavirus deaths happened in association with nursing homes. That's almost 20% of all US deaths. https://www.businessinsider.com/us-coronavirus-deaths-at-nursing-homes-2020-4 Available at:
- 26.Lovato A., de Filippis C. Clinical presentation of COVID-19: A systematic review focusing on upper airway symptoms. Ear Nose Throat J. 2020 Apr 13 doi: 10.1177/0145561320920762. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Zhang Q.Y. Firth logistic regression for rare variant association tests. Front Genet. 2014;5:187. doi: 10.3389/fgene.2014.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Firth D. Bias reduction of maximum-likelihood-estimates. Biometrika. 1993;80:27–38. [Google Scholar]
- 29.McMichael T.M., Currie D.W., Clark S. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382:2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 Mar 23 doi: 10.1001/jama.2020.4683. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.O'Dowd A. Covid-19: Deaths in care home deaths in England and Wales rise sharply. BMJ. 2020;369:m1727. doi: 10.1136/bmj.m1727. [DOI] [PubMed] [Google Scholar]
- 32.Abdulhak A.A.B., Kashour T., Noman A. Angiotensin converting enzyme inhibitors and angiotensin receptor blockers and outcome of COVID-19: A systematic review and meta-analysis. Perit Dial Int. 2009;29:554–561. [PubMed] [Google Scholar]
- 33.Paranjpe I., Fuster V., Lala A. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76:122–124. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cox C.E. Anticoagulants may aid COVID-19 patients, NYC data suggest. https://www.tctmd.com/news/anticoagulants-may-aid-covid-19-patients-nyc-data-suggest Available at:
- 35.Grein J., Ohmagari N., Shin D. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao B., Wang Y., Wen D. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cortegiani A., Ingoglia G., Ippolito M. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279–283. doi: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casadevall A., Pirofski L.-A. The convalescent sera option for containing COVID-19. J Clin Invest. 2020;130:1545–1548. doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]