On March 13, 2020, the White House declared a State of Emergency in response to the growing coronavirus disease 2019 (COVID-19) pandemic.1 As health systems across the country fortified themselves to treat and limit infection, they shared a common goal: to test large numbers of patients and swiftly disseminate test results. Discussions of COVID-19 results have added complexity, because they must address patient questions on symptom management, test interpretation, self-isolation, and our evolving understanding of the disease.2 , 3 The scale, infectivity, and public health implications of COVID-19 have required health systems to rapidly develop new workflows and to redeploy physicians to support these operations. Dermatologists are well-suited to manage test results, because patient evaluation and counseling are integral to our practice, and we can redirect these skills to guide patients on COVID-19.
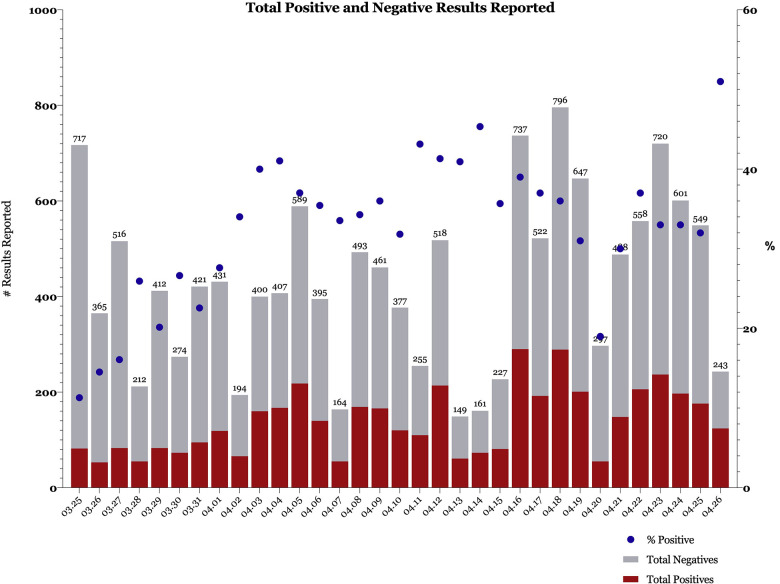
At Penn Medicine, we have rapidly redeployed our dermatology department to create a COVID-19 Results Management team. An Applied Informatics Specialist/Physician Builder within our faculty spearheaded this effort in collaboration with Penn's Information Technology leadership. On March 25, our department began covering all ambulatory COVID-19 results, and in our first week of operation, we processed 2222 results. On March 30, we expanded our efforts to include all COVID-19, respiratory virus panel, and influenza results for all discharged patients seen in our 5 emergency departments and our urgent care center. In our first month, we managed a total of 14,296 results (Fig 1 ).
Fig 1.
Reported positive results, reported negative results, and proportion of total reported results that are positive, by day. Numbers above bars indicate total results reported that day.
Critical to our performance has been the rapid reconfiguration of our electronic health record, EpicCare, to identify all patients with new COVID-19 results from all ambulatory practices across the health system. This report of patients is run 3 times every day. Patients who are positive and negative populate electronic patient lists that are shared within our team of 85 providers, who volunteer for half-day shifts. An early, critical discovery was the need to manually confirm the laboratory result within each patient's medical record, because the EpicCare sorting strategy is not 100% accurate.
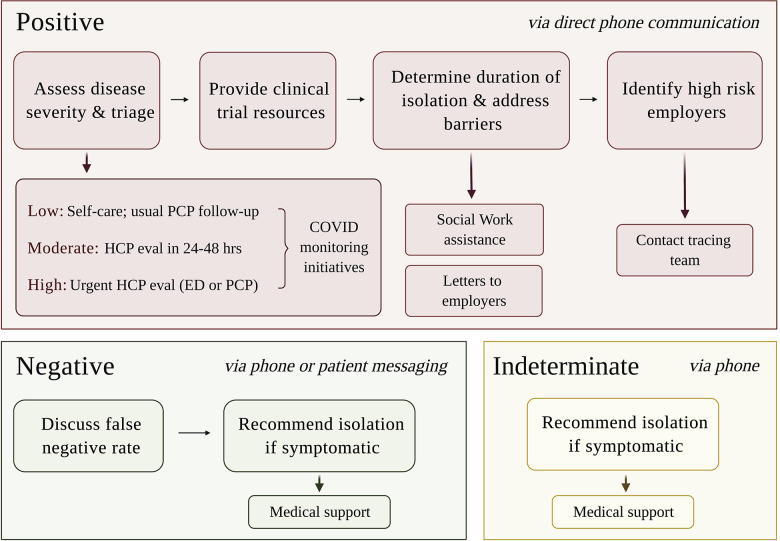
Furthermore, we created department-level documentation tools (SmartPhrases) that allow for standardization of the language and resources provided to patients. The content of these SmartPhrases was developed in collaboration with our internal medicine and infectious disease colleagues and vetted for accuracy and consistency. This standardization allows us to collectively update our recommendations as new COVID-19 guidelines emerge while also enabling providers to give patient-specific advice for positive, negative, and indeterminate results (Fig 2 ). The SmartPhrases also contain background SmartData elements that allow us to track patient severity, outreach, and enrollment in internal COVID-19 monitoring initiatives. All providers use these documentation tools without exception, which ensures fidelity to our tracking system.
Fig 2.
Communication of positive, negative, and indeterminate results. Top row, Results for patients who are positive are directly discussed through phone communication, which serves 4 main purposes. Subsequent rows, Patients are provided additional resources if needed. Patients who are negative are contacted by phone or by direct messaging via Penn Medicine's patient portal. Patients with indeterminate results, typically due to test sample degradation, are called and asked to self-isolate until their symptoms resolve. ED, Emergency department; eval, evaluation; HCP, health care provider; PCP, primary care provider.
We have rapidly redeployed our dermatology workforce to handle all COVID-19, influenza, and respiratory virus panel results from all emergency department and ambulatory test sites within our health care system. We have developed a sustainable, scalable strategy that can incorporate other departments as we resume normal dermatology clinical operations. Through this model, our department has continued to address urgent dermatologic needs while also supporting Penn Medicine's response to the COVID-19 pandemic.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
IRB approval status: Not applicable.
Reprints not available from the authors.
References
- 1.Proclamation No. 9994, 85 F.R. 15337 (March 18, 2020).
- 2.Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases [e-pub ahead of print]. Radiology. https://doi.org/10.1148/radiol.202020064, Accessed May 30, 2020. [DOI] [PMC free article] [PubMed]
- 3.Yang Y, Yang M, Shen C, et al. Evaluating the accuracy of different respiratory specimens in the laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections [preprint]. medRxiv. 10.1101/2020.02.11.20021493. [DOI]