Abstract
Proximal tubule (PT) cells express a single saturable albumin-binding site whose affinity matches the estimated tubular concentration of albumin; however, albumin uptake capacity is greatly increased under nephrotic conditions. Deciphering the individual contributions of megalin and cubilin to the uptake of normal and nephrotic levels of albumin is impossible in vivo, as knockout of megalin in mice globally disrupts PT endocytic uptake. We quantified concentration-dependent albumin uptake in an optimized opossum kidney cell culture model and fit the kinetic profiles to identify albumin-binding affinities and uptake capacities. Mathematical deconvolution fit best to a three-component model that included saturable high- and low-affinity uptake sites for albumin and underlying nonsaturable uptake consistent with passive uptake of albumin in the fluid phase. Knockdown of cubilin or its chaperone amnionless selectively reduced the binding capacity of the high-affinity site, whereas knockdown of megalin impacted the low-affinity site. Knockdown of disabled-2 decreased the capacities of both binding sites. Additionally, knockdown of megalin or disabled-2 profoundly inhibited the uptake of a fluid phase marker, with cubilin knockdown having a more modest effect. We propose a novel model for albumin retrieval along the PT in which cubilin and megalin receptors have different functions in recovering filtered albumin in proximal tubule cells. Cubilin binding to albumin is tuned to capture normally filtered levels of the protein. In contrast, megalin binding to albumin is of lower affinity, and its expression is also essential for enabling the recovery of high concentrations of albumin in the fluid phase.
Keywords: amnionless, cubilin, disabled-2, endocytosis, megalin, proteinuria, proximal tubule
INTRODUCTION
The proximal tubule (PT) of the kidney maintains stable fractional solute and fluid reabsorption over a wide range of glomerular filtration rates to preserve glomerulotubular balance. In addition to reabsorbing the majority of filtered water, Na+, glucose, and other solutes, cells that comprise the S1 and S2 segments of the proximal convoluted tubule internalize essentially all proteins that escape the glomerular filtration barrier to maintain a nearly protein-free urine. The large multiligand receptors megalin [low-density lipoprotein-related protein 2 (LRP2)] and cubilin (CUBN), expressed at the apical surface of these cells, bind to and internalize >50 different plasma proteins (7, 15). Megalin is a ~600-kDa type 1 transmembrane protein related to the low-density lipoprotein receptor. The cytoplasmic domain of megalin contains two NPXY motifs that engage the clathrin adaptor disabled-2 (Dab2) and mediate endocytosis of the protein into apical endocytic compartments. Cubilin (~460 kDa) lacks a transmembrane domain and requires association with amnionless (AMN; ~50 kDa) to reach the apical membrane as a CUBAM complex (9, 10, 46). Like megalin, AMN also contains Dab2-binding motifs that are able to mediate CUBAM internalization in cells and tissues that do not express megalin (12, 22, 38, 39). Loss of either megalin or cubilin expression results in tubular proteinuria (27, 36), and biochemical and morphological studies have suggested that they function as a complex in the PT (1, 30). However, whether direct interaction with megalin is required for cubilin function in the PT is not known.
Plasma albumin levels in adult rats are ~2.6 g/dL (433 µM) (51), and the majority of micropuncture and other studies in the rat have suggested that the slit diaphragm provides an effective barrier to albumin, with only ~23 µg/mL (0.4 µM) albumin present in the filtrate (47). Consistent with this, albumin binds to the apical surface of opossum kidney (OK) PT cells via a saturable high-affinity site (half-maximal binding ~20–30 µg/mL or 0.3–0.45 µM) (17, 44) that seems ideally tuned to capture normally filtered levels of this ligand. The apparent match between tubular levels of albumin and the binding affinity for receptors on the apical surface of PT cells makes physiological sense but raises an underappreciated conundrum: if albumin binding is saturated at this low (normal) concentration, then why are elevated levels of albumin cytotoxic to the PT? Recent experiments have confirmed large increases in albumin accumulation in the PTs of rodents when the glomerular filtration barrier is compromised (35, 48, 49). While knockout (KO) of megalin and cubilin increased urinary albumin levels by ~20-fold, disruption of the glomerular filtration barrier by KO of podocin resulted in an ~3,000-fold increase in urinary albumin. Remarkably, KO of megalin and cubilin in addition to podocin led to an additional 40% increase in albumin excretion, suggesting that the capacity for PT albumin uptake in nephrotic conditions is vastly beyond that needed to maintain a protein-free urine in the normally functioning kidney. The increased albumin uptake capacity in nephrotic PTs does not require increased expression of megalin or cubilin, as mRNA levels of both LRP2 and CUBN were unchanged in podocin KO mice relative to controls (49).
High-capacity albumin uptake has also been demonstrated in isolated perfused rabbit tubules, where increasing uptake of radioiodinated albumin delivered at concentrations up to 2 mg/mL was observed (37). The authors speculated that high-capacity uptake occurred via fluid phase endocytosis. However, fluid phase uptake has generally been considered to have a negligible contribution to the physiological uptake of filtered proteins by PT cells (8, 29, 44).
Understanding the mechanisms and pathways that mediate albumin uptake in the PT is important for designing interventions to treat proteinuric disease. Dissecting the role of megalin and CUBAM receptors in albumin recovery using animal KO models has proven challenging, as KO of megalin or Dab2 in vivo results in a dramatic reduction in PT apical endocytic compartments, with a concomitant increase in urinary protein excretion (27, 33, 34). As an alternative approach, we have modeled concentration-dependent albumin uptake in an optimized cell culture model of the PT. Using siRNA-mediated knockdown, we established the roles of cubilin, megalin, and Dab2 in the uptake of normally filtered and nephrotic levels of albumin. Together with experiments in mouse KO models, the results of our study suggest a new model to explain the remarkable ability of PT cells to recover albumin over the broad range of filtered concentrations in normal and pathological conditions.
MATERIALS AND METHODS
Cell culture and siRNA.
OK cells (RRID:CVCL_0472, female) were cultured on Transwell filters under continuous orbital shear stress at 146 rpm as previously described (28). For RNA inhibition experiments, 1 µg siRNA (Dharmacon) and 1.5 µL Lipofectamine RNAiMAX transfection reagent (ThermoFisher) were added separately to Opti-MEM reduced serum medium (ThermoFisher), mixed together (250 µL total) for 15 min, and then added to the apical chamber of a filter support containing freshly plated OK cells {4 × 105 cells/filter in 250 µL growth medium [DMEM-F-12 (D6421, Sigma) supplemented with 5% FBS (S11150, Atlanta Biologicals) and 5 mM glutaMAX (no. 35050-061, GIBCO), pH 7.2–7.5]}. The siRNA sequences used in this study are shown in Supplemental Table S1 in the Supplemental Material (all Supplemental Material is available at https://doi.org/10.6084/m9.figshare.12169476.v1). The medium was changed after 6 h, and cells were transfected again the following day and shifted to culture under orbital shear stress. The medium was changed after 6 h and then daily for an additional 72 h before experiments began. Where indicated, knockdown was confirmed by indirect immunofluorescence and Western blot analysis as previously described using anti-megalin antibody generously provided by Dr. Daniel Biemesderfer, a new anti-cubilin antibody validated in Supplemental Fig. S1, or anti-Dab-2 antibody (no. 12906, Cell Signaling) (28, 52). Relative quantification (comparative Cq method) for quantitative PCR was performed using iTaq Universal SYBR Green Supermix 500 (no. 1725121, Bio-Rad) on a CFX Connect Real-Time PCR Detection System (Bio-Rad) to assess the effect of each siRNA on transcript levels of CUBN, AMN, and megalin (LRP2). Primers were designed by Primer-Blast (National Center for Biotechnology Information) using default settings and validated by agarose gel electrophoresis. The housekeeping gene β-actin (ACTB) was used as an internal reference.
Albumin uptake assays.
OK cells cultured on filters under orbital shear stress for 72 h as described above were incubated for 15 min at 37°C under continuous orbital shear stress with Alexa Fluor 647-albumin (A34785, Invitrogen) added apically in serum-free growth medium supplemented with 25 mM HEPES (pH 7.2–7.5). Filters were washed three times with cold PBS (with Mg2+ and Ca2+), filters were excised with a razor blade, and cells were solubilized in 300 μL MOPS buffer [20 mM MOPS in 0.1% (vol/vol) Triton X-100, pH 7.4] for 30 min on a shaker at 4°C. Cell-associated Alexa Fluor 647 fluorescence intensity was quantified by spectrofluorimetry.
Modeling albumin uptake.
Albumin uptake data were modeled using the Hill-Langmuir equation and Igor Pro 6.37 (Wavemetrics, Oswego, OR), using custom functions and the program's built-in global fitting routine. We hypothesized that EC50 values (K1, K2, etc.) were intrinsic to specific uptake pathways and that attendant maximum velocities (V1, V2, etc.) reflected the contribution of each pathway to overall uptake. Cooperativity was also considered and treated as intrinsic to specific pathways, giving the following equation:
Accordingly, K values and Hill coefficients (H1, H2, etc.) were common parameters to all experiments (i.e., global parameters), while V values were unique parameters for each experiment (i.e., local parameters). The three-pathway model (n = 3) with no Hill coefficients fit the data significantly better than any of the simpler models (see Supplemental Fig. S2).
Immunofluorescence staining in KO mouse models.
Generation, breeding, and urinary albumin excretion levels of podocin-, megalin-, and cubilin-inducible KO mice have been previously described (32, 49, 50). Mouse breeding and experiments were carried out in a certified animal facility according to the Danish Animal Experiments Inspectorate. Female tamoxifen-inducible UBC-cre/ERT2 transgenic mice (Jackson Laboratory) were used for experiments at ages of 8–12 wk. Podocin KO (Nph2lox/lox;Cre+), cubilin KO (Cubnlox/lox;Cre+), megalin KO (Meglox/lox;Cre+), cubilin/podocin KO (Cubnlox/lox,Nph2lox,lox;Cre+), megalin/podocin KO (Meglox/lox,Nph2lox,lox;Cre+), and control mice (Meglox/lox,Cubnlox/lox,Nph2lox,lox;Cre−), were induced by intraperitoneal injection of tamoxifen (Sigma-Aldrich) at a dose of 33 mg/kg body wt for 5 consecutive days. Knockdown of the target genes in the kidneys was verified by quantitative real-time PCR as previously described (49) and immunohistochemistry at week 5 of the experiment (counting from the first day of tamoxifen induction). At termination, mice were anesthetized using isoflurane and kidneys were fixed by retrograde perfusion through the abdominal aorta with 2% paraformaldehyde in 0.1 M sodium cacodylate buffer (pH 7.4). Afterward, kidneys were dehydrated, embedded in paraffin, and cut into 2-μm sections using standard methods. For immunofluorescence labeling, kidney paraffin sections were incubated with primary antibodies at 4°C overnight followed by incubation with secondary antibodies for 1 h at ambient temperature as previously described (35). Immunofluorescence images were taken using a confocal laser-scanning microscope (LSM 510-META, Carl Zeiss) and processed using Zeiss Zen software (Light Edition). The primary antibodies used were sheep anti-rat megalin (31), Alexa Fluor 488-conjugated rabbit anti-rat cubilin (42), and rabbit anti-human albumin (A0001, DAKO). The secondary antibodies used were donkey anti-rabbit Alexa Fluor 594 (no. 21207, Invitrogen) and donkey anti-sheep Alexa Fluor 680 (no. 21102, Invitrogen).
Quantitation of megalin-to-cubilin transcript ratios by digital PCR.
Droplet digital PCR was performed using cDNA prepared from OK cells and the mouse kidney cortex using a QX100 system (Bio-Rad) according to the manufacturer’s specifications. The sequences used are provided in Supplemental Table S3. Mouse droplet digital PCR (ddPCR) primers and probes were designed and verified by Bio-Rad. Custom probe-based quantitative PCR assays were designed using the Integrated DNA Technologies RealTime qPCR Assay tool with default settings. Consensus sequences for LRP2 and CUBN genes obtained from the de novo transcriptome of a female Didelphis virginiana (opossum) PT cell line (14) were entered into the Integrated DNA Technologies tool as single exons because the location of intron/exon junctions are not clearly defined for this model organism. As a result, it is unclear whether assays span an intron-exon junction. Therefore, downstream experiments included aliquots of each sample for which reverse transcription was omitted to control for contaminating DNA in the RNA preparation. LRP2 and CUBN probes were labeled with HEX and 6-FAM fluorophores, respectively, and assays were optimized by ddPCR amplification across a temperature gradient (49.9–62°C) with 1 ng cDNA to determine the optimum annealing temperature for the assays. PCRs contained 1× ddPCR Supermix for Probes (Bio-Rad), 1× primer/probe mix for both assays, and 3 ng cDNA in a 25-µL reaction volume. Twenty microliters were loaded into the droplet generator to create a digital PCR emulsion. PCR was performed on a MasterCyclerPro (Eppendorf). Following 10 min of enzyme activation, PCR conditions were 40 cycles of (94°C 30 s, 58°C 1 min, and 98°C 10 min). After PCR, droplets were loaded on the QX100 Droplet Reader. Analysis with QuantaSoft Analysis Pro 1.0.596 software (Bio-Rad) returned copies per microliter.
Statistics.
Unless otherwise noted, data were plotted and statistical significance was assessed using Prism software (GraphPad).
RESULTS
Multiple pathways mediate dose-dependent albumin uptake.
We previously observed that OK cells cultured on Transwell supports under continuous orbital shaking develop the uniquely differentiated morphological specializations observed in PT cells in vivo (28, 41). These cells exhibit increased expression of megalin, cubilin, and Dab2 mRNA compared with OK cells maintained under static conditions (unpublished observations) and have an approximately fivefold higher apical endocytic capacity (28). Additionally, cells cultured in this manner develop the robust oxidative metabolism needed to drive PT ion transport and endocytic capacity (41).
Similar to PT cells in vivo, OK cells internalized considerably more apically added albumin when exposed to high concentrations (2 mg/mL) compared with concentrations predicted to be normally filtered by the glomerulus (50 µg/mL; Fig. 1A). To quantitate the dose dependence of albumin uptake, we incubated OK cells with increasing concentrations of Alexa Fluor 647-albumin at 37°C for 15 min and then measured cell-associated albumin in cell lysates by spectrofluorimetry. As shown in the representative experiment in Fig. 1B, albumin uptake increased rapidly with concentration before leveling off, although uptake continued to increase even at very high concentrations (5 mg/mL). Notably, the kinetic profile did not resemble the classic Michaelis-Menten kinetic profile typical of ligand binding to a single site. We reproducibly observed an inflection in the rate of albumin uptake between 350 and 750 µg/mL albumin, suggesting that at least two pathways of differing affinities contributed to the kinetic profile (see Supplemental Fig. S3). Preliminary fits of the data (the final fit determined as described below is shown in Fig. 1B) suggested multiple uptake components with distinct apparent affinities: a high-affinity component (C1) with a ~50 µg/mL affinity constant, a low-affinity component (C2) with a ~300 µg/mL affinity constant, and a nonsaturable component (C3). A semilog plot of the fit is shown in Fig. 1C to better highlight the low-affinity component, which is more difficult to visualize in linear graphs. Control experiments confirmed that C1 and C2 components are saturable, as uptake of Alexa Fluor 647-albumin was virtually abolished in the presence of 100 mg/mL unlabeled albumin (see Supplemental Fig. S3). In contrast, uptake via the C3 component was unaffected by excess unlabeled albumin, as predicted for a fluid phase pathway. We tested the idea of multiple uptake pathways with distinct affinities by knocking down individual components of the uptake machinery as described below.
Fig. 1.
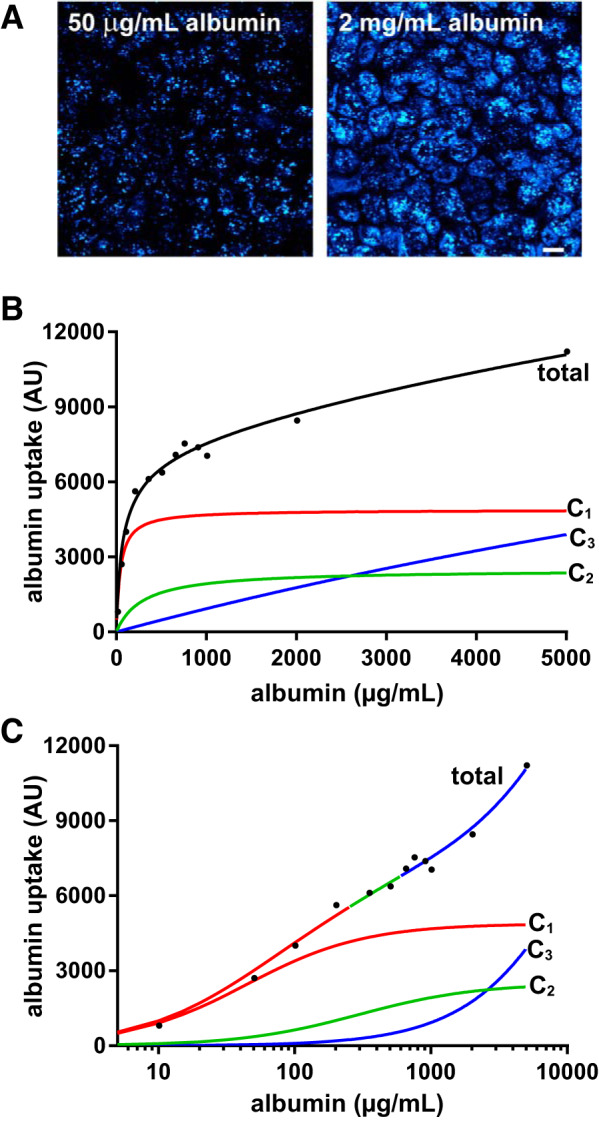
Three pathways mediate dose-dependent albumin uptake by opossum kidney (OK) cells. A: OK cells incubated with 50 µg/mL albumin or 2 mg/mL albumin were fixed and visualized by confocal microscopy. Images were acquired and processed identically, and a maximum projection of a representative field is shown. Scale bar = 10 µm. B: OK cells were incubated with the indicated concentrations of Alexa Fluor 647-albumin at 37°C for 15 min and then rapidly washed and solubilized, and cell-associated albumin was assessed by spectrofluorimetry. Global fitting of >30 experiments was used to resolve the curve into three components: saturable high-affinity (C1; red) and low-affinity (C2; green) uptake components and an underlying nonsaturable component (C3; blue) that we attribute to fluid phase uptake. The half-maximal uptake values for the high-affinity and low-affinity components were 44 and 295 µg/mL albumin, respectively, and the respective maximal uptake capacities in this experiment were 4,883 and 2,494. C: curves shown in B are replotted on a semi-log scale to more easily visualize the three uptake components. The total curve is color coded to show the regions where albumin uptake is dominated by the high-affinity (red), low-affinity (green), or nonsaturable (blue) component. AU, arbitrary units.
Knockdown of cubilin, megalin, and Dab2 differentially impair albumin uptake pathways.
Albumin and many other filtered ligands bind to both purified cubilin and megalin; however, the role of these individual receptors in uptake is not well understood (5, 11, 27). We developed siRNA oligonucleotides that efficiently depleted expression of cubilin, megalin, or Dab2 and examined the consequences on protein levels by Western blot analysis. As shown in Fig. 2, A–C (full-length blots in Supplemental Fig. S4), knockdown of each protein was highly efficient. We also developed a siRNA targeting AMN and confirmed its efficacy using quantitative PCR (see Supplemental Fig. S5A). Knockdown of cubilin reduced AMN protein expression and vice versa (Supplemental Figs. S4A and S5B), consistent with prior reports demonstrating that cubilin and AMN are mutually dependent on one another’s expression for efficient maturation and surface delivery (10). In contrast, depletion of megalin or Dab2 did not alter the expression level of the other blotted proteins (Fig. 2, B and C). Indirect immunofluorescence revealed that knockdown of cubilin, megalin, or Dab2 dramatically reduced the uptake of physiological levels of albumin (Fig. 2, D–F). We did notice that the residual albumin staining pattern differed slightly in megalin-deficient cells compared with the other conditions.
Fig. 2.
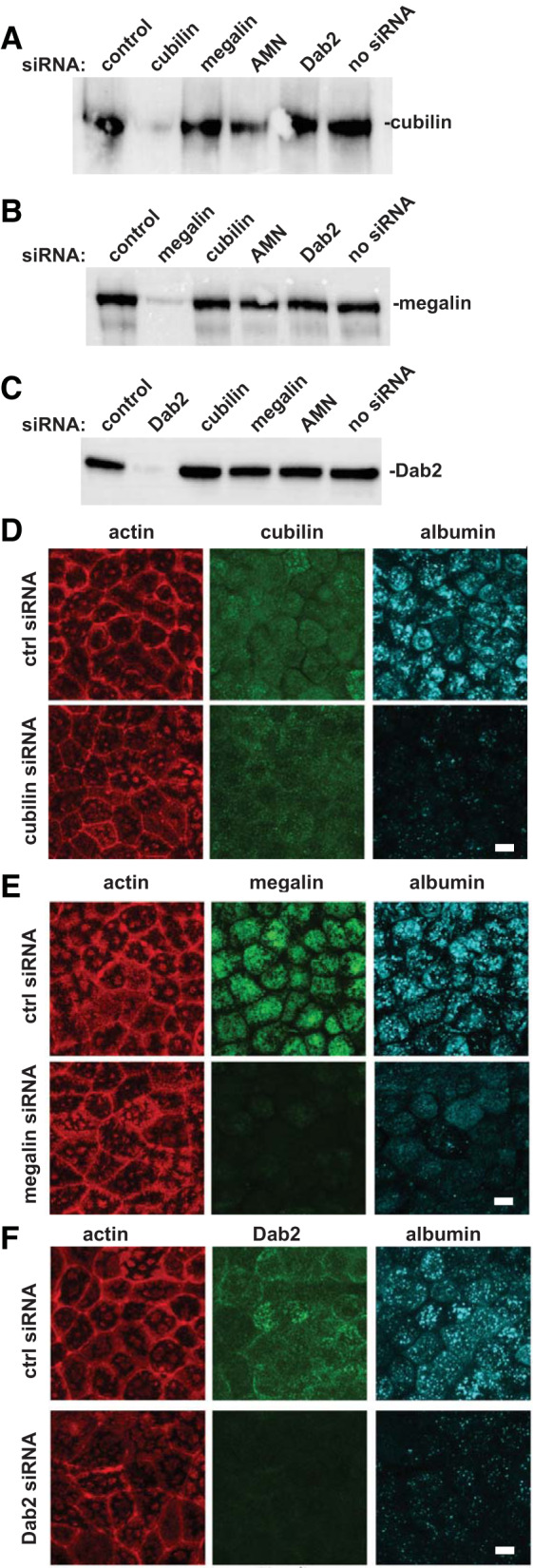
Efficient knockdown of megalin, cubilin, and disabled-2 (Dab2) impairs uptake of physiological concentrations of filtered albumin. Cells were transfected with the indicated siRNAs and cultured on Transwells as described in materials and methods. Samples were solubilized, and equal protein loads were run on SDS-PAGE and Western blotted to detect cubilin (A), megalin (B), or Dab2 (C). Similar results were observed in 2 independent experiments. Opossum kidney cells transfected with the indicated siRNAs were incubated with 50 µg/mL Alexa Fluor 647-albumin for 15 min, fixed, and then processed to detect actin and cubilin (D), megalin (E), or Dab2 (F). Scale bar = 10 µm. ctrl, control.
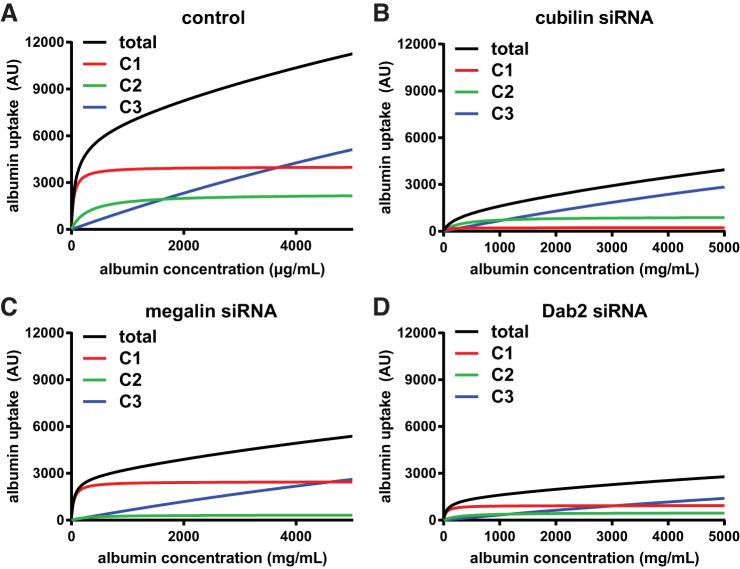
Data from >30 experiments quantifying concentration-dependent uptake of albumin in control and siRNA-transfected cells were fit simultaneously to determine the best model that fit the kinetic profile as described in detail in materials and methods (Supplemental Figs. S2 and S6). The optimal model included saturable high-affinity and low-affinity components with affinity constants K1 and K2 of 44 ± 4 and 295 ± 35 µg/mL, respectively, and a nonsaturable pathway (modeled as a curve with K3 = 20,000 µg/mL) that likely represents fluid phase uptake. The raw data with residuals from all uptake curves are provided in Supplemental Fig. S6, and best-fit curves along with their deconvolved components are shown for control (Fig. 3A), cubilin knockdown (Fig. 3B), megalin knockdown (Fig. 3C), and Dab2 knockdown (Fig. 3D) experiments. The high-affinity (C1) and nonsaturable (C3) components were readily apparent in the fitted curves, whereas the low-affinity component (C2) was more easily visualized in semi-log plots of the data (Supplemental Fig. S6). Notably, the C2 component was more difficult to resolve than the C1 or C3 components, as the latter two are isolated at the low and high ends of the uptake curves. This resulted in relatively greater uncertainty in estimating the contribution of the C2 component. The value we obtained for K1 was very similar to the single saturable ~50 µg/mL Km binding site for albumin previously described in OK cells (17), which we confirmed in our cell culture model (Km = 49 µg/mL; Supplemental Fig. S7), whereas K2 was apparently too weak to measure using surface binding assays.
Fig. 3.
Knockdown of cubilin, megalin, and disabled-2 (Dab2) differentially affect albumin uptake via high- and low-affinity pathways. The average (black) curves and individual components (C1, red; C2, green; and C3, blue) obtained by fitting the average kinetic profiles of albumin uptake in control (A), cubilin siRNA (B), megalin siRNA (C), and Dab2 siRNA (D) data sets are plotted. AU, arbitrary units.
Cubilin knockdown caused a significant reduction in overall uptake (average 39% of control uptake at 5 mg/mL albumin, n = 8, P ≤ 0.0005 by one-way ANOVA with Dunnett’s post hoc test; Fig. 3B). In particular, we noted a shift to the right at low albumin concentrations, suggesting selective reduction of a high-affinity uptake pathway. At 50 µg/mL, albumin uptake in cubilin knockdown cells was only 11.5% of control uptake (P ≤ 0.0002). As expected, knockdown of AMN essentially phenocopied the effect of cubilin siRNA on albumin uptake (Supplemental Fig. S5C).
In contrast to cells treated with cubilin or AMN siRNA, albumin uptake in cells treated with megalin siRNA rose steeply at low concentrations, suggesting that the high-affinity component was not impacted by megalin knockdown. Albumin uptake at 50 µg/mL in cells treated with megalin siRNA was 55% of control levels (n = 6, P = 0.075), in contrast to the nearly 90% reduction we measured in cubilin-depleted cells. At high concentrations of albumin (5 mg/mL), uptake in megalin knockdown cells was reduced to 47.0% of control (P ≤ 0.005).
Dab2 binds to NPXY motifs on the cytoplasmic tails of proteins and links these to the endocytic machinery. Knockdown of Dab2 is thus expected to inhibit endocytic uptake of CUBAM and megalin to a similar extent. Additionally, knockdown of Dab2 has been demonstrated to dramatically reduce the number of clathrin-coated pits and apical endocytic vesicles in the PT (33). Consistent with this, the global fit of data from multiple experiments revealed a dose-dependent curve for albumin uptake that was very similar to that of control cells, albeit with profoundly reduced capacities for all components (Fig. 3D).
Figure 4 shows the effect of cubilin, AMN, megalin, and Dab2 knockdown on the maximal uptake capacities of the high-affinity and low-affinity components (V1 and V2, respectively). Whereas cubilin or AMN knockdown caused a significant reduction in V1, megalin knockdown did not significantly affect the capacity of the high-affinity site (Fig. 4A). This suggests that CUBAM receptors are capable of internalizing albumin in PT cells independently of megalin. In contrast, megalin knockdown selectively impaired the maximal uptake capacity of the low-affinity component (V2; Fig. 4B). As predicted, Dab2 knockdown reduced both V1 and V2, consistent with its effect on both cubilin- and megalin-mediated uptake (Fig. 4, A and B).
Fig. 4.
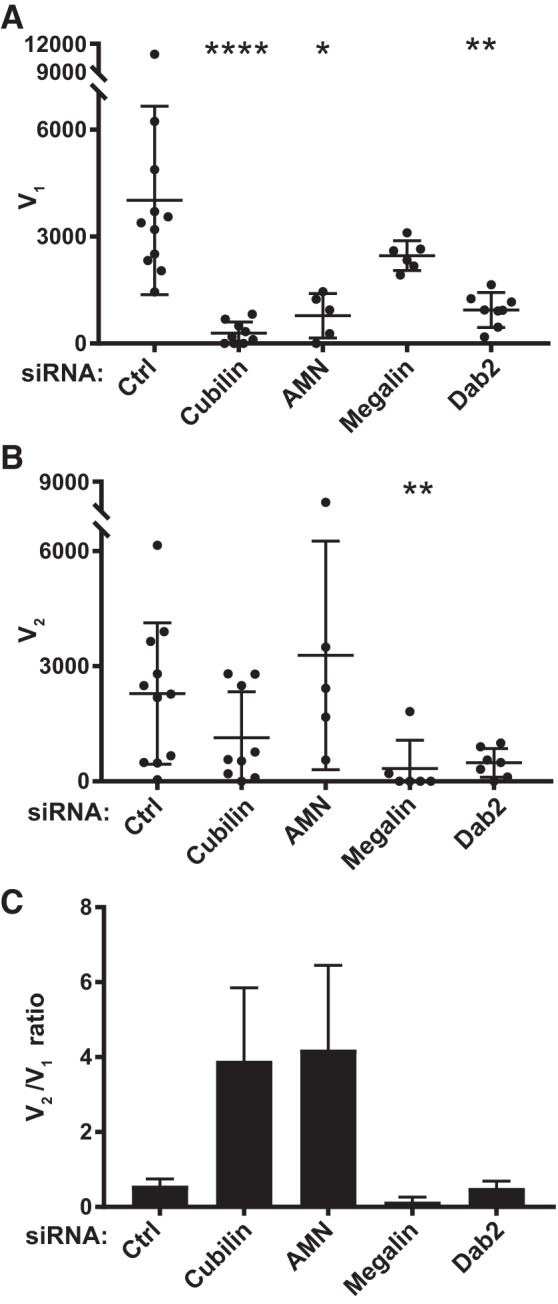
Effect of cubilin, amnionless (AMN), megalin, and disabled-2 (Dab2) knockdown on high- and low-affinity albumin uptake capacity. The albumin uptake profile from each experiment was deconvolved, and high-affinity (V1; A) and low-affinity (V2; B) uptake capacities calculated for each condition are plotted as individual points with the mean and SD noted. Statistical significance was assessed by a Kruskal-Wallis test (one-way ANOVA with Dunn’s multiple comparisons test. *P ≤ 0.01, **P < 0.05, and ****P < 0.0001 vs. control (Ctrl). C: ratio of V2 to V1 obtained from the mean V2 and V1 values from each group is plotted (means ± SE).
Since knockdown of CUBAM or megalin had selective effects on V1 and V2, the relative uptake capacities of each component changed accordingly. The average V2-to-V1 ratio (V2/V1) in control OK cells was 0.57 and increased upon knockdown of cubilin to 3.9 (Fig. 4C). Knockdown of AMN, which should phenocopy the effect of cubilin depletion, resulted in a similar increase in V2/V1 to 4.1. In contrast, knockdown of megalin reduced V2/V1 to 0.14 (Fig. 4C). Consistent with its role in capturing CUBAM and megalin receptors into clathrin-coated pits, the average V2/V1 in Dab2 knockdown cells (0.51) was essentially unchanged compared with control cells (Fig. 4C).
Cubilin is dispensable for PT accumulation of albumin in a mouse model of nephrosis.
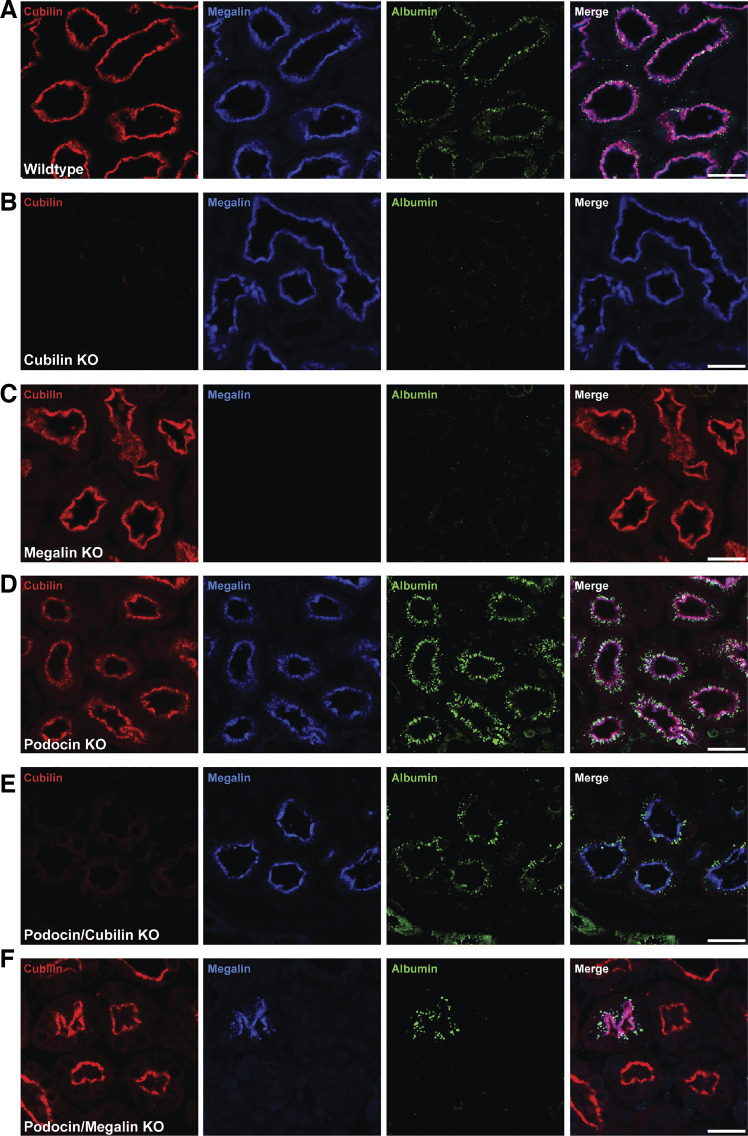
Our data suggest that CUBAM and megalin albumin binding affinities are differentially tuned to mediate uptake of normally filtered versus pathological levels of albumin, respectively. Because KO of megalin in vivo ablates cubilin function in the kidney, it is not possible to determine whether CUBAM receptors can independently mediate uptake of normal albumin concentrations. However, a testable prediction of our hypothesis is that megalin (but not CUBAM) receptors can function independently to retrieve high concentrations of albumin filtered under nephrotic conditions. To test this hypothesis, we examined the requirement for megalin and cubilin in PT albumin uptake in mouse kidney sections from inducible KO models variously targeting cubilin, megalin, and/or podocin (49, 50). Urinary albumin measurements in nephrotic megalin/cubilin KO mice confirmed a significant increase in albuminuria when the receptors are deleted; however, data for nephrotic mice with only megalin or cubilin KO are not available (49). In wild-type mice, low levels of albumin were clearly discernible within megalin- and cubilin-positive PT cells (Fig. 5A). No albumin was observed in PT cells in either megalin- or cubilin-KO mice (Fig. 5, B and C). As expected, PT cells in podocin KO mice accumulated much higher levels of albumin than wild-type mice (Fig. 5D) (49). Strikingly, albumin was efficiently internalized in PT cells of podocin/cubilin double-KO mice (Fig. 5E), suggesting that megalin receptor expression was sufficient to enable albumin retrieval of high [but not normal (Fig. 5B)] levels of albumin from the filtrate. Similarly, albumin uptake was only visible in PTs that expressed residual megalin after induction of podocin and megalin KO (Fig. 5F). Together, these data support the conclusion that CUBAM and megalin are both required for retrieval of normal levels of albumin, but that megalin can mediate cubilin-independent uptake of nephrotic levels of albumin.
Fig. 5.
Megalin takes up albumin independently of cubilin in nephrotic podocin knockout (KO) mice. A−F: immunostaining for cubilin (red), megalin (blue), and albumin (green) in kidney sections from control mice (A), inducible cubilin KO mice (B), inducible megalin KO mice (C), inducible podocin KO mice (D), inducible podocin/cubilin KO mice (E), and inducible podocin/megalin KO mice (F). Confocal images were taken at ×20 magnification. Scale bars = 40 μm. Only megalin- and cubilin-positive cells take up albumin in normal mice; under proteinuria, cubilin expression is dispensable for albumin uptake (but megalin is not).
Megalin-to-cubilin expression in OK cells and mouse kidneys.
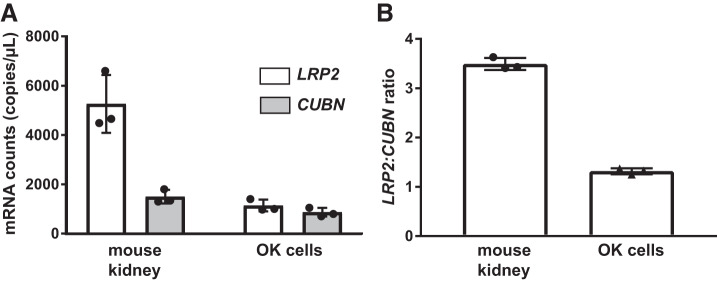
Albumin uptake measured in freshly isolated perfused rabbit PTs suggests that uptake capacity at high concentrations of albumin (≥2 mg/mL) is roughly 50-fold greater than at physiological concentrations (50 µg/mL) (37). In contrast, we measured only an approximately threefold increase in albumin uptake over this concentration range in our OK cells. It is possible that OK cells express less megalin and hence have fewer low-affinity albumin binding sites compared with PT cells in vivo. To test this possibility, we compared the relative expression of LRP2 versus CUBN transcripts and megalin versus cubilin protein in the mouse kidney cortex versus OK cells. Because the primer efficiencies for each reaction may be different, we used ddPCR to obtain accurate values of mRNA transcript levels in each sample. As shown in Fig. 6, the ratio of LRP2 to CUBN transcripts was 1.3-fold in OK cells and 3.5-fold in mice. This modest difference in ratios may reflect the preferential expression of megalin over cubilin in the PT S2 segment, where low levels of CUBN transcripts and cubilin protein levels have been reported (26, 43). Regardless, our data suggest that mouse kidney cortex and OK cells have comparable ratios of low-affinity to high-affinity albumin uptake sites and that differences in relative megalin expression likely do not account for the divergence in albumin uptake profiles between perfused PTs and OK cells.
Fig. 6.
Megalin [low-density lipoprotein-related protein 2 (LRP2)] to cubilin (CUBN) mRNA transcript ratios in opossum kidney (OK) cells and the mouse kidney. Digital droplet PCR was performed using validated primers for LRP2 and CUBN to enable direct quantitation of mRNA transcript ratios in OK cells and in the mouse kidney cortex. Data from RNA isolated from 3 independent experiments are plotted. A: absolute mRNA transcript counts for LRP2 and CUBN in the mouse kidney and OK cells are plotted. B: plot of the LRP2-to-CUBN transcript ratio in the mouse kidney and OK cells. The mean fold difference in LRP2 over CUBN expression was 3.5 ± 0.12 and 1.3 ± 0.06 for the mouse kidney and OK cells, respectively.
Dab2, megalin, and cubilin knockdown inhibit fluid phase uptake in PT cells.
At concentrations that saturate the low- and high-affinity uptake pathways, excess albumin will behave as a fluid phase marker. While PT cells do not apparently possess a clathrin-independent fluid phase uptake pathway, the robust endocytic activity in this nephron segment suggests that a substantial volume of PT filtrate is internalized within clathrin-coated vesicles (4). In vivo, loss of either Dab2 or megalin expression dramatically curtails formation of endocytic vesicles, suggesting that passive uptake of fluid phase markers will also be inhibited. Global fits of the data in Fig. 3 show that knockdown of megalin, cubilin, and especially Dab2 reduced the nonsaturable component of albumin uptake (C3) compared with control. To examine fluid phase uptake independently, we compared the effects of receptor protein and Dab2 knockdown on the uptake of the fluid phase marker Alexa Fluor 647-dextran. Dextran uptake was roughly linear with increasing concentration as expected for uptake of a fluid phase marker (Fig. 7). Knockdown of Dab2 or megalin profoundly inhibited dextran uptake, whereas knockdown of cubilin had a more modest effect. Together, our data are consistent with a model in which passive uptake of albumin in the fluid phase contributes to retrieval of high concentrations of albumin.
Fig. 7.
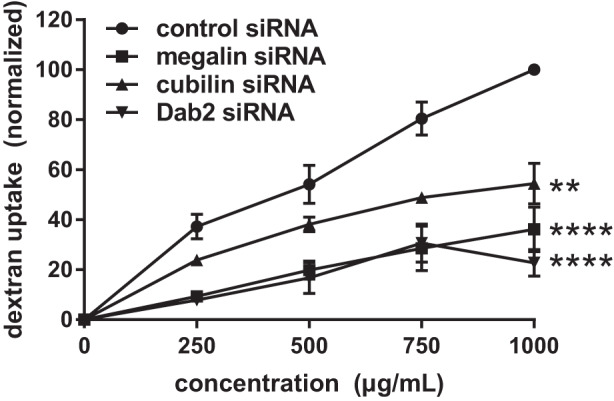
siRNA knockdown of cubilin (CUBN), megalin [low-density lipoprotein-related protein 2 (LRP2)], or disabled-2 (Dab2) inhibits uptake of a fluid phase marker. Opossum kidney (OK) cells transfected with the indicated siRNAs were incubated with the 250–1000 µg/mL Alexa Fluor 647-dextran for 15 min at 37°C and then rapidly washed and solubilized, and cell-associated fluorescence was quantified. Mean uptake ± SD from 3 independent experiments are plotted, with maximal uptake in control cells normalized to 100%. Uptake at each time point for every condition was significantly different from control by two-way ANOVA (**P > 0.002; ****P < 0.0001).
DISCUSSION
The present study demonstrates that cubilin and megalin receptors are differentially involved in the reclamation of normal versus pathological levels of filtered albumin by PT cells. We observed a dose-dependent profile of albumin uptake consistent with the presence of a saturable low-affinity binding site in addition to the single high-affinity site for albumin binding observed in surface binding studies. The best global fit using data from multiple experiments also includes a component for nonsaturable uptake that likely reflects passive incorporation of fluid phase albumin into endocytic vesicles. Knockdown of cubilin or AMN selectively ablated uptake via the high-affinity site, confirming that recovery of normally filtered albumin is dependent on CUBAM receptor expression, as previously observed in mice (2, 50). In contrast, knockdown of megalin impaired the lower-affinity site for albumin uptake. Knockdown of Dab2 impaired uptake via both sites. Additionally, we observed effects on nonsaturable uptake. When we directly examined fluid phase uptake using labeled dextran, we found a dramatic reduction in cells depleted of Dab2 or megalin and a more modest effect of cubilin knockdown. Based on our results in cultured cells and studies in rodents, we propose a new model to explain how the PT accommodates efficient uptake across the wide range of tubular albumin concentrations in normal versus disease states.
Our conclusion that binding to cubilin is primarily responsible for the uptake of normally filtered albumin is bolstered by a recent study in humans demonstrating an association of albuminuria with mutations in CUBN (3). Patients with biallelic CUBN variants excreted roughly 20-fold more albumin than normal per day, consistent with observations in cubilin KO mice (50). This suggests that megalin in the PT has neither the affinity nor the capacity to reclaim significant levels of even normally filtered albumin, let alone nephrotic concentrations. Rather, these data support our model in which a primary function of megalin is to maintain cubilin-dependent uptake under normal conditions and to enable fluid phase uptake of albumin in nephrotic states. Additionally, megalin binds directly and with high affinity to many other filtered ligands and is required for their retrieval (6, 15, 16, 18–21, 23, 45).
We hypothesize that passive uptake of albumin via its fluid phase incorporation into endocytic vesicles contributes to the enormous reserve capacity for albumin uptake under nephrotic conditions. Uptake via this pathway in vivo is absolutely dependent on the expression of megalin, as we observed cubilin- but not megalin-independent accumulation of albumin in PTs of nephrotic mice. KO of megalin or Dab2 in mice has been previously observed to impair the elaboration of apical endocytic compartments (27, 33, 34). Consistent with this, we found that the nonsaturable component of albumin uptake and internalization of the fluid phase marker dextran were dramatically reduced in OK cells transfected with megalin or Dab2 siRNA. At first glance, it is surprising that megalin knockdown impaired fluid phase uptake without apparently impacting cubilin-mediated uptake of albumin. It is possible that CUBAM receptors are directed more efficiently into coated pits in the absence of megalin expression. Alternatively, megalin knockdown might impair endocytic pathway organization to redistribute more high-affinity (CUBAM) albumin binding sites to the plasma membrane. Finally, megalin expression may affect the size of endocytic vesicles, which would discoordinately affect uptake of membrane-bound versus fluid phase components.
An elegant recent study by Hall and colleagues (40, 43) demonstrated complete reabsorption of fluorescent albumin injected into the circulation by cells of the S1 segment of the PT. By contrast, uptake of fluid phase markers was low in the S1 segment but robust in S2 (43). Interestingly, whereas cubilin expression in S2 cells was highly variable, megalin was consistently expressed in the S2 segment. Connecting these results with our data here, we propose a novel model for the uptake of normal and nephrotic concentrations of albumin (Fig. 8). In our model, normally filtered albumin is retrieved exclusively in the S1 segment by CUBAM and/or megalin receptors. Under nephrotic conditions where receptor-dependent uptake pathways are saturated, water reabsorption via paracellular and transcellular transport will result in the delivery of progressively concentrated albumin into more distal segments of the proximal tubule. While some megalin-dependent uptake will occur via direct binding, we hypothesize that the primary role of megalin under these conditions is to maintain fluid phase uptake of increasingly concentrated albumin in the S2 and S3 segments. Preferential uptake of albumin in these later segments of the PT could account for the dramatic capacity of the “low-affinity” uptake pathway previously described in perfused rabbit tubules in the landmark studies of Park and Maack (37).
Fig. 8.
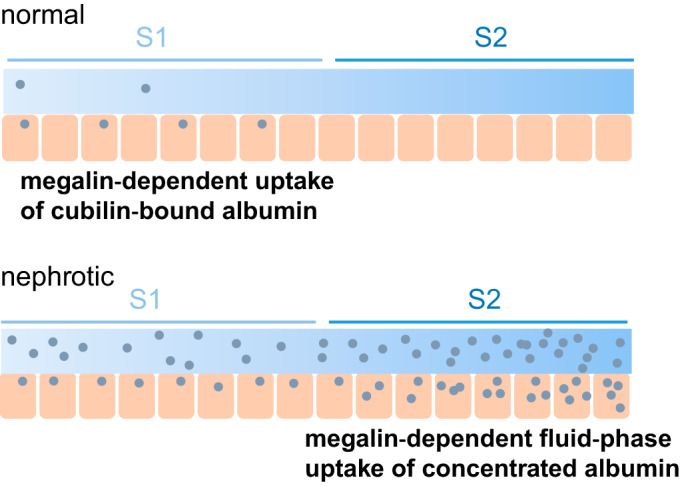
Model for uptake of normal and nephrotic levels of albumin along the proximal tubule. Based on our data and published results by others, we hypothesize that normally filtered albumin is retrieved exclusively in the S1 segment by high-affinity binding to cubilin in association with amnionless (AMN) and/or megalin. Under nephrotic conditions where cubilin-mediated uptake is saturated, the concentration of albumin will increase progressively along the tubule as fluid is reabsorbed via paracellular and transcellular transport mechanisms. We hypothesize that passive uptake of albumin via its incorporation into endocytic vesicles whose formation requires the expression of megalin [and disabled-2 (Dab2)] accounts for the enormous reserve capacity for albumin uptake under nephrotic conditions. See the discussion for more details.
The availability of our optimized OK cell culture model was instrumental in being able to segregate the contributions of megalin and cubilin to PT albumin uptake. OK cells cultured under our conditions have an approximately fivefold higher endocytic capacity compared with OK cells cultured under static conditions, which provided sufficient signal to deconvolve individual components from endocytic uptake curves. However, our data from OK cells also raise important questions that remain to be resolved. Our model is predicated on the assumption that the relative amounts (if not the absolute levels) of megalin and cubilin receptors in OK cells and the S1 segment of the PT are comparable. At present, we cannot quantitatively determine the levels of megalin and cubilin protein or their ratios in OK cells versus the mouse kidney. Even though antibodies exist that recognize similar regions of the proteins, the sequences in these regions differ between species and the affinities of these antibodies for mouse or opossum protein are likely different. An additional complication is that the expression of megalin and cubilin transcripts and proteins has been shown to vary along the length of the PT. That said, the approximately two- to fivefold difference in megalin and cubilin transcript abundance between mouse and opossum that we measured using ddPCR suggests that expression of these receptors is not hugely reduced in our cell culture model compared with PT cells in vivo.
An additional question raised by our study is why did knockdown of megalin in OK cells not impair high-affinity uptake of albumin, given that uptake of normally filtered albumin in vivo is clearly dependent on megalin expression? Although megalin knockdown was very efficient based on Western blot analysis and immunofluorescence, it is formally possible that a small amount of residual megalin is sufficient to maintain CUBAM function in OK cells. It is also possible, as noted above, that the endocytic pathway is impaired in megalin knockdown cells resulting in an increased number of high-affinity albumin surface binding sites. Alternatively, CUBAM receptors may function independently in OK cells, similar to their ability of CUBAM to internalize ligands in other cell types that do not express megalin (22, 38, 39). A recent high-resolution structure reveals a tight assembly of CUBAM consisting of three cubilin fragments bound to AMN, consistent with stable association of the complex after initial delivery to the cell surface (25). Additional studies are needed to illuminate how loss of megalin affects endocytic pathway organization and how interaction with megalin affects cubilin endocytosis. Current initiatives using CRISPR/Cas9 technology to knock out megalin and cubilin in OK cells will resolve this issue.
Our data have implications for the design of therapeutics to treat nephrotic syndrome proteinuria and also point to the need for further studies to clarify the role of fluid phase uptake in albumin uptake under nephrotic conditions. Uptake of excessive levels of filtered proteins in many nephrotic conditions is known to result in PT injury, mediated at least in part due to toxic molecules ferried by albumin (13). Increased uptake of fatty acid-bound albumin in S2 may also explain why this nephron segment is particularly sensitive to megalin-dependent toxicity in mice fed a high-fat diet (24). Our study suggests that cubilin expression is unlikely to contribute significantly to this cytotoxicity. Furthermore, our results suggest that maneuvers to limit water reabsorption or dampen Dab2 expression may be more beneficial in prolonging PT function during proteinuric assault compared with compounds that selectively target receptor-mediated uptake of albumin.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants R01-DK-101484, DK-100357, and DK-118726 (to O. A. Weisz) and DK-098204 (to O. B. Kashlan). Q. Ren was supported by the China Scholarship Council. K. Weyer, R. Nielsen, and E. I. Christensen were supported by the Danish Medical Research Council (K. Weyer: DFF 12-132499 and E. I. Christensen: DFF 4183-00014B) and the Aarhus University Research Foundation (K. Weyer: AUFF-E-2017-7-25). We are grateful to access to the cores of the Pittsburgh Center for Kidney Research funded by NIDDK Grant P30-DK-079307.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Q.R., K.W., O.B.K., and O.A.W. conceived and designed research; Q.R., K.W., Y.R., K.R.L., and C.J.B. performed experiments; Q.R., K.W., K.R.L., R.J.T., R.N., E.I.C., C.J.B., O.B.K., and O.A.W. analyzed data; Q.R., K.W., C.J.B., O.B.K., and O.A.W. interpreted results of experiments; Q.R., K.W., and O.A.W. prepared figures; O.B.K. and O.A.W. drafted manuscript; Q.R., K.W., Y.R., K.R.L., R.J.T., R.N., E.I.C., C.J.B., O.B.K., and O.A.W. edited and revised manuscript; Q.R., K.W., Y.R., K.R.L., R.J.T., R.N., E.I.C., C.J.B., O.B.K., and O.A.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to the University of Pittsburgh Health Sciences Genomics Research Core for assistance with droplet digital PCR. We thank I. Blenker Kristoffersen, P. Kamuk Nielsen, H. Salling Gittens, and H. Sidelmann from the Department of Biomedicine, Aarhus University, for excellent technical assistance and G. Mollet and C. Antignac at INSERM for the podocin knockout mice.
REFERENCES
- 1.Ahuja R, Yammani R, Bauer JA, Kalra S, Seetharam S, Seetharam B. Interactions of cubilin with megalin and the product of the amnionless gene (AMN): effect on its stability. Biochem J 410: 301–308, 2008. doi: 10.1042/BJ20070919. [DOI] [PubMed] [Google Scholar]
- 2.Amsellem S, Gburek J, Hamard G, Nielsen R, Willnow TE, Devuyst O, Nexo E, Verroust PJ, Christensen EI, Kozyraki R. Cubilin is essential for albumin reabsorption in the renal proximal tubule. J Am Soc Nephrol 21: 1859–1867, 2010. doi: 10.1681/ASN.2010050492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bedin M, Boyer O, Servais A, Li Y, Villoing-Gaudé L, Tête M-J, Cambier A, Hogan J, Baudouin V, Krid S, Bensman A, Lammens F, Louillet F, Ranchin B, Vigneau C, Bouteau I, Isnard-Bagnis C, Mache CJ, Schäfer T, Pape L, Gödel M, Huber TB, Benz M, Klaus G, Hansen M, Latta K, Gribouval O, Morinière V, Tournant C, Grohmann M, Kuhn E, Wagner T, Bole-Feysot C, Jabot-Hanin F, Nitschké P, Ahluwalia TS, Köttgen A, Andersen CBF, Bergmann C, Antignac C, Simons M. Human C-terminal CUBN variants associate with chronic proteinuria and normal renal function. J Clin Invest 130: 335–344, 2019. doi: 10.1172/JCI129937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birn H, Christensen EI, Nielsen S. Kinetics of endocytosis in renal proximal tubule studied with ruthenium red as membrane marker. Am J Physiol Renal Physiol 264: F239–F250, 1993. doi: 10.1152/ajprenal.1993.264.2.F239. [DOI] [PubMed] [Google Scholar]
- 5.Birn H, Fyfe JC, Jacobsen C, Mounier F, Verroust PJ, Orskov H, Willnow TE, Moestrup SK, Christensen EI. Cubilin is an albumin binding protein important for renal tubular albumin reabsorption. J Clin Invest 105: 1353–1361, 2000. doi: 10.1172/JCI8862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birn H, Zhai X, Holm J, Hansen SI, Jacobsen C, Christensen EI, Moestrup SK. Megalin binds and mediates cellular internalization of folate binding protein. FEBS J 272: 4423–4430, 2005. doi: 10.1111/j.1742-4658.2005.04857.x. [DOI] [PubMed] [Google Scholar]
- 7.Christensen EI, Birn H, Storm T, Weyer K, Nielsen R. Endocytic receptors in the renal proximal tubule. Physiology (Bethesda) 27: 223–236, 2012. doi: 10.1152/physiol.00022.2012. [DOI] [PubMed] [Google Scholar]
- 8.Christensen EI, Maunsbach AB. Effects of dextran on lysosomal ultrastructure and protein digestion in renal proximal tubule. Kidney Int 16: 301–311, 1979. doi: 10.1038/ki.1979.132. [DOI] [PubMed] [Google Scholar]
- 9.Christensen EI, Nielsen R, Birn H. From bowel to kidneys: the role of cubilin in physiology and disease. Nephrol Dial Transplant 28: 274–281, 2013. doi: 10.1093/ndt/gfs565. [DOI] [PubMed] [Google Scholar]
- 10.Coudroy G, Gburek J, Kozyraki R, Madsen M, Trugnan G, Moestrup SK, Verroust PJ, Maurice M. Contribution of cubilin and amnionless to processing and membrane targeting of cubilin-amnionless complex. J Am Soc Nephrol 16: 2330–2337, 2005. doi: 10.1681/ASN.2004110925. [DOI] [PubMed] [Google Scholar]
- 11.Cui S, Verroust PJ, Moestrup SK, Christensen EI. Megalin/gp330 mediates uptake of albumin in renal proximal tubule. Am J Physiol Renal Physiol 271: F900–F907, 1996. doi: 10.1152/ajprenal.1996.271.4.F900. [DOI] [PubMed] [Google Scholar]
- 12.Drake CJ, Fleming PA, Larue AC, Barth JL, Chintalapudi MR, Argraves WS. Differential distribution of cubilin and megalin expression in the mouse embryo. Anat Rec A Discov Mol Cell Evol Biol 277A: 163–170, 2004. doi: 10.1002/ar.a.10123. [DOI] [PubMed] [Google Scholar]
- 13.Erkan E. Proteinuria and progression of glomerular diseases. Pediatr Nephrol 28: 1049–1058, 2013. doi: 10.1007/s00467-012-2335-1. [DOI] [PubMed] [Google Scholar]
- 14.Eshbach ML, Sethi R, Avula R, Lamb J, Hollingshead DJ, Finegold DN, Locker JD, Chandran UR, Weisz OA. The transcriptome of the Didelphis virginiana opossum kidney OK proximal tubule cell line. Am J Physiol Renal Physiol 313: F585–F595, 2017. doi: 10.1152/ajprenal.00228.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eshbach ML, Weisz OA. Receptor-mediated endocytosis in the proximal tubule. Annu Rev Physiol 79: 425–448, 2017. doi: 10.1146/annurev-physiol-022516-034234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faber K, Hvidberg V, Moestrup SK, Dahlbäck B, Nielsen LB. Megalin is a receptor for apolipoprotein M, and kidney-specific megalin-deficiency confers urinary excretion of apolipoprotein M. Mol Endocrinol 20: 212–218, 2006. doi: 10.1210/me.2005-0209. [DOI] [PubMed] [Google Scholar]
- 17.Gekle M, Mildenberger S, Freudinger R, Silbernagl S. Functional characterization of albumin binding to the apical membrane of OK cells. Am J Physiol Renal Physiol 271: F286–F291, 1996. doi: 10.1152/ajprenal.1996.271.2.F286. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Villalobos R, Klassen RB, Allen PL, Johanson K, Baker CB, Kobori H, Navar LG, Hammond TG. Megalin binds and internalizes angiotensin-(1-7). Am J Physiol Renal Physiol 290: F1270–F1275, 2006. doi: 10.1152/ajprenal.00164.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez-Villalobos R, Klassen RB, Allen PL, Navar LG, Hammond TG. Megalin binds and internalizes angiotensin II. Am J Physiol Renal Physiol 288: F420–F427, 2005. doi: 10.1152/ajprenal.00243.2004. [DOI] [PubMed] [Google Scholar]
- 20.Hvidberg V, Jacobsen C, Strong RK, Cowland JB, Moestrup SK, Borregaard N. The endocytic receptor megalin binds the iron transporting neutrophil-gelatinase-associated lipocalin with high affinity and mediates its cellular uptake. FEBS Lett 579: 773–777, 2005. doi: 10.1016/j.febslet.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 21.Jensen D, Kierulf-Lassen C, Kristensen MLV, Nørregaard R, Weyer K, Nielsen R, Christensen EI, Birn H. Megalin dependent urinary cystatin C excretion in ischemic kidney injury in rats. PLoS One 12: e0178796, 2017. doi: 10.1371/journal.pone.0178796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen LL, Andersen RK, Hager H, Madsen M. Lack of megalin expression in adult human terminal ileum suggests megalin-independent cubilin/amnionless activity during vitamin B12 absorption. Physiol Rep 2: e12086, 2014. doi: 10.14814/phy2.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klassen RBS, Allen PL, Batuman V, Crenshaw K, Hammond TG. Light chains are a ligand for megalin. J Appl Physiol 98: 257–263, 2005. doi: 10.1152/japplphysiol.01090.2003. [DOI] [PubMed] [Google Scholar]
- 24.Kuwahara S, Hosojima M, Kaneko R, Aoki H, Nakano D, Sasagawa T, Kabasawa H, Kaseda R, Yasukawa R, Ishikawa T, Suzuki A, Sato H, Kageyama S, Tanaka T, Kitamura N, Narita I, Komatsu M, Nishiyama A, Saito A. Megalin-mediated tubuloglomerular alterations in high-fat diet-induced kidney disease. J Am Soc Nephrol 27: 1996–2008, 2016. doi: 10.1681/ASN.2015020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen C, Etzerodt A, Madsen M, Skjødt K, Moestrup SK, Andersen CBF. Structural assembly of the megadalton-sized receptor for intestinal vitamin B12 uptake and kidney protein reabsorption. Nat Commun 9: 5204, 2018. doi: 10.1038/s41467-018-07468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JW, Chou C-L, Knepper MA. Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J Am Soc Nephrol 26: 2669–2677, 2015. doi: 10.1681/ASN.2014111067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leheste JR, Rolinski B, Vorum H, Hilpert J, Nykjaer A, Jacobsen C, Aucouturier P, Moskaug JO, Otto A, Christensen EI, Willnow TE. Megalin knockout mice as an animal model of low molecular weight proteinuria. Am J Pathol 155: 1361–1370, 1999. doi: 10.1016/S0002-9440(10)65238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Long KR, Shipman KE, Rbaibi Y, Menshikova EV, Ritov VB, Eshbach ML, Jiang Y, Jackson EK, Baty CJ, Weisz OA. Proximal tubule apical endocytosis is modulated by fluid shear stress via an mTOR-dependent pathway. Mol Biol Cell 28: 2508–2517, 2017. doi: 10.1091/mbc.e17-04-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maack T, Johnson V, Kau ST, Figueiredo J, Sigulem D. Renal filtration, transport, and metabolism of low-molecular-weight proteins: a review. Kidney Int 16: 251–270, 1979. doi: 10.1038/ki.1979.128. [DOI] [PubMed] [Google Scholar]
- 30.Moestrup SK, Kozyraki R, Kristiansen M, Kaysen JH, Rasmussen HH, Brault D, Pontillon F, Goda FO, Christensen EI, Hammond TG, Verroust PJ. The intrinsic factor-vitamin B12 receptor and target of teratogenic antibodies is a megalin-binding peripheral membrane protein with homology to developmental proteins. J Biol Chem 273: 5235–5242, 1998. doi: 10.1074/jbc.273.9.5235. [DOI] [PubMed] [Google Scholar]
- 31.Moestrup SK, Nielsen S, Andreasen P, Jørgensen KE, Nykjaer A, Røigaard H, Gliemann J, Christensen EI. Epithelial glycoprotein-330 mediates endocytosis of plasminogen activator-plasminogen activator inhibitor type-1 complexes. J Biol Chem 268: 16564–16570, 1993. [PubMed] [Google Scholar]
- 32.Mollet G, Ratelade J, Boyer O, Muda AO, Morisset L, Lavin TA, Kitzis D, Dallman MJ, Bugeon L, Hubner N, Gubler M-C, Antignac C, Esquivel EL. Podocin inactivation in mature kidneys causes focal segmental glomerulosclerosis and nephrotic syndrome. J Am Soc Nephrol 20: 2181–2189, 2009. doi: 10.1681/ASN.2009040379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris SM, Tallquist MD, Rock CO, Cooper JA. Dual roles for the Dab2 adaptor protein in embryonic development and kidney transport. EMBO J 21: 1555–1564, 2002. doi: 10.1093/emboj/21.7.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagai J, Christensen EI, Morris SM, Willnow TE, Cooper JA, Nielsen R. Mutually dependent localization of megalin and Dab2 in the renal proximal tubule. Am J Physiol Renal Physiol 289: F569–F576, 2005. doi: 10.1152/ajprenal.00292.2004. [DOI] [PubMed] [Google Scholar]
- 35.Nielsen R, Mollet G, Esquivel EL, Weyer K, Nielsen PK, Antignac C, Christensen EI. Increased lysosomal proteolysis counteracts protein accumulation in the proximal tubule during focal segmental glomerulosclerosis. Kidney Int 84: 902–910, 2013. doi: 10.1038/ki.2013.218. [DOI] [PubMed] [Google Scholar]
- 36.Nykjaer A, Fyfe JC, Kozyraki R, Leheste JR, Jacobsen C, Nielsen MS, Verroust PJ, Aminoff M, de la Chapelle A, Moestrup SK, Ray R, Gliemann J, Willnow TE, Christensen EI. Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D(3). Proc Natl Acad Sci USA 98: 13895–13900, 2001. doi: 10.1073/pnas.241516998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park CH, Maack T. Albumin absorption and catabolism by isolated perfused proximal convoluted tubules of the rabbit. J Clin Invest 73: 767–777, 1984. doi: 10.1172/JCI111270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park J, Levic DS, Sumigray KD, Bagwell J, Eroglu O, Block CL, Eroglu C, Barry R, Lickwar CR, Rawls JF, Watts SA, Lechler T, Bagnat M. Lysosome-rich enterocytes mediate protein absorption in the vertebrate gut. Dev Cell 51: 7–20.e6, 2019. doi: 10.1016/j.devcel.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pedersen GA, Chakraborty S, Steinhauser AL, Traub LM, Madsen M. AMN directs endocytosis of the intrinsic factor-vitamin B12 receptor cubam by engaging ARH or Dab2. Traffic 11: 706–720, 2010. doi: 10.1111/j.1600-0854.2010.01042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polesel M, Hall AM. Axial differences in endocytosis along the kidney proximal tubule. Am J Physiol Renal Physiol 317: F1526–F1530, 2019. doi: 10.1152/ajprenal.00459.2019. [DOI] [PubMed] [Google Scholar]
- 41.Ren Q, Gliozzi ML, Rittenhouse NL, Edmunds LR, Rbaibi Y, Locker JD, Poholek AC, Jurczak MJ, Baty CJ, Weisz OA. Shear stress and oxygen availability drive differential changes in opossum kidney proximal tubule cell metabolism and endocytosis. Traffic 20: 448–459, 2019. doi: 10.1111/tra.12648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sahali D, Mulliez N, Chatelet F, Laurent-Winter C, Citadelle D, Sabourin JC, Roux C, Ronco P, Verroust P. Comparative immunochemistry and ontogeny of two closely related coated pit proteins. The 280-kd target of teratogenic antibodies and the 330-kd target of nephritogenic antibodies. Am J Pathol 142: 1654–1667, 1993. [PMC free article] [PubMed] [Google Scholar]
- 43.Schuh CD, Polesel M, Platonova E, Haenni D, Gassama A, Tokonami N, Ghazi S, Bugarski M, Devuyst O, Ziegler U, Hall AM. Combined structural and functional imaging of the kidney reveals major axial differences in proximal tubule endocytosis. J Am Soc Nephrol 29: 2696–2712, 2018. doi: 10.1681/ASN.2018050522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwegler JS, Heppelmann B, Mildenberger S, Silbernagl S. Receptor-mediated endocytosis of albumin in cultured opossum kidney cells: a model for proximal tubular protein reabsorption. Pflügers Arch 418: 383–392, 1991. doi: 10.1007/BF00550876. [DOI] [PubMed] [Google Scholar]
- 45.Seested T, Appa RS, Jacobsen C, Christensen EI. Recombinant activated factor VII is reabsorbed in renal proximal tubules and is a ligand to megalin and cubilin. Nephron Exp Nephrol 117: e82–e92, 2011. doi: 10.1159/000321161. [DOI] [PubMed] [Google Scholar]
- 46.Strope S, Rivi R, Metzger T, Manova K, Lacy E. Mouse amnionless, which is required for primitive streak assembly, mediates cell-surface localization and endocytic function of cubilin on visceral endoderm and kidney proximal tubules. Development 131: 4787–4795, 2004. doi: 10.1242/dev.01341. [DOI] [PubMed] [Google Scholar]
- 47.Tojo A, Endou H. Intrarenal handling of proteins in rats using fractional micropuncture technique. Am J Physiol Renal Physiol 263: F601–F606, 1992. doi: 10.1152/ajprenal.1992.263.4.F601. [DOI] [PubMed] [Google Scholar]
- 48.Wagner MC, Campos-Bilderback SB, Chowdhury M, Flores B, Lai X, Myslinski J, Pandit S, Sandoval RM, Wean SE, Wei Y, Satlin LM, Wiggins RC, Witzmann FA, Molitoris BA. Proximal tubules have the capacity to regulate uptake of albumin. J Am Soc Nephrol 27: 482–494, 2016. doi: 10.1681/ASN.2014111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weyer K, Andersen PK, Schmidt K, Mollet G, Antignac C, Birn H, Nielsen R, Christensen EI. Abolishment of proximal tubule albumin endocytosis does not affect plasma albumin during nephrotic syndrome in mice. Kidney Int 93: 335–342, 2018. doi: 10.1016/j.kint.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 50.Weyer K, Storm T, Shan J, Vainio S, Kozyraki R, Verroust PJ, Christensen EI, Nielsen R. Mouse model of proximal tubule endocytic dysfunction. Nephrol Dial Transplant 26: 3446–3451, 2011. doi: 10.1093/ndt/gfr525. [DOI] [PubMed] [Google Scholar]
- 51.Zaias J, Mineau M, Cray C, Yoon D, Altman NH. Reference values for serum proteins of common laboratory rodent strains. J Am Assoc Lab Anim Sci 48: 387–390, 2009. [PMC free article] [PubMed] [Google Scholar]
- 52.Zou Z, Chung B, Nguyen T, Mentone S, Thomson B, Biemesderfer D. Linking receptor-mediated endocytosis and cell signaling: evidence for regulated intramembrane proteolysis of megalin in proximal tubule. J Biol Chem 279: 34302–34310, 2004. doi: 10.1074/jbc.M405608200. [DOI] [PubMed] [Google Scholar]