Abstract
Recently, research has redirected its interests in uric acid (UA) from gout, an inflammatory disease in joints, to groups of closely interrelated pathologies associated with cardiovascular and kidney dysfunction. Many epidemiological, clinical, and experimental studies have shown that UA may play a role in the pathophysiology of the cardiorenal syndrome continuum; however, it is still unclear if it is a risk factor or a causal role. Hyperuricemia has been well studied in the past two decades, revealing mechanistic insights into UA homeostasis. Likewise, some epidemiological and experimental evidence suggests that hypouricemia can lead to cardiorenal pathologies. The goal of this review is to highlight why studying both hyperuricemia and hypouricemia is warranted as well as to summarize the relevance of UA to kidney function.
Keywords: hyperuricemia, hypouricemia, uric acid, xanthine dehydrogenase, xanthine oxidase, xanthine oxidoreductase
INTRODUCTION
Uric acid (UA) was historically known as a waste product of purine breakdown, with its foremost clinical relevance being gout. Nevertheless, research in the last few decades has shown that both increased and decreased levels of serum UA (hyperuricemia and hypouricemia, respectively) are associated with a plethora of chronic conditions including chronic kidney disease (CKD) (4, 12, 14, 24, 31). Hyperuricemia, in particular, has been linked to cardiovascular disease (CVD) and CKD in both hypertension (HTN) and normotensive populations (18, 40, 44). Clinically more frequent of the two conditions is hyperuricemia (~20% prevalence; Ref. 49). It may be associated with impaired renal UA homeostasis triggered by the reduction in glomerular filtration rate (GFR), diuretic usage, increased renal vascular resistance, and coexistent insulin resistance in diabetic nephropathy (25, 30). The opposite condition, hypouricemia, although less common (prevalence 0.2–0.58% in the general population; Ref. 29), leads to nephrolithiasis and may occur as a result of overtreatment of hyperuricemia, defective renal tubular reabsorption of UA, and genetic mutation affecting purine metabolism (37).
The xanthine dehydrogenase (XDH) gene encodes for the enzyme group responsible for converting xanthine and hypoxanthine to UA, which is the end product of purine catabolism in humans. This enzyme group is known as xanthine oxidoreductase (XOR) and includes both XDH and xanthine oxidase (XO). Clinical (3, 36) and animal (6, 35, 39) studies have shown that reducing UA by inhibiting XOR blunts the progression of HTN, CVD, and CKD. In addition, recent clinical study-based perspectives suggest that UA-lowering therapy could be potentially used in hyperuricemic CKD patients without gout or nephrolithiasis (33).
Both current and impending UA-lowering therapies necessitate investigation into the effects of hypouricemia as the reduction of UA below the normal level with therapeutic management poses additional pathogenic risks. There are large-cohort studies to support the idea that both increased and decreased levels of UA are pathogenic (15, 17, 40, 44). In one such study, levels of circulating UA exhibited a J-shaped association with all-cause mortality of CKD, showing the lowest risk in the middle quintiles (40). Additionally, a prospective cohort study in healthy (based on GFR) people proposed that hypouricemia is a candidate predictor of kidney function decline (15). This indicates that not only hyperuricemia but also hypouricemia may impact CVD, renal function, and consequent mortalities.
UA EVOLUTION AND GENERATION
Humans have higher UA levels (3.5–7.0 and 2.6–5.7 mg/dL for men and women, respectively; Ref. 4) compared with nonprimate mammals (0.5–1.5 mg/dL; Ref. 46) due to an evolutionary mutation they have in the gene encoding uricase enzyme (UOX). In rodents, this enzyme further converts UA into allantoin (47). There are different speculations as to why this mutation persisted in humans. One theory states that increased UA provided an evolutionary advantage as a potent antioxidant (8) and may compensate for the loss of the ability to produce endogenous ascorbic acid (13, 46). UA acts as an antioxidant by scavenging carbon-centered radicals and peroxynitrite in the extracellular hydrophilic environment (4). Another hypothesis is that UA was needed to regulate blood pressure and prevent hypotension at a time of low-salt consumption (46). This hypothesis further highlights the potential importance of UA for CVD and HTN.
Nucleotides can be synthesized via a de novo process or by recycling of free purine and pyrimidine bases through the salvage pathway. The salvage pathway helps conserve energy by reusing the breakdown products to produce nucleotide precursors (22). Changes in the enzymes involved in this pathway cause diseases associated with purine metabolism and/or UA homeostasis. In purine metabolism, UA is produced in the liver from xanthine and hypoxanthine catalyzed by XOR. Purines, such as xanthine, are derived from the degradation of endogenous nucleic acids and dietary sources of purines, such as red meat. Both XDH and XO participate in this pathway, but they require different coenzymes for catalysis and, therefore, result in different byproducts. XDH uses NAD+ and produces NADH, while XO uses H2O2 and produces superoxide radicals (Fig. 1A). XDH can be converted into XO reversibly through oxidation of the sulfhydryl group or irreversibly by proteolytic conversions under various conditions, including tissue ischemia (31).
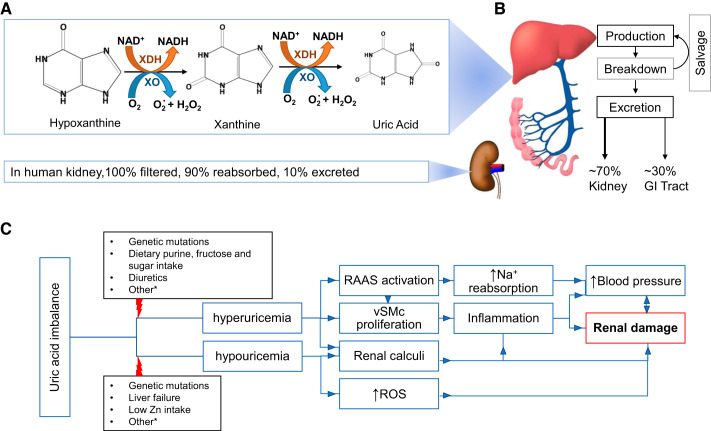
Fig. 1.
Uric acid (UA) metabolism and its effects. A: the xanthine oxidoreductase (XOR) enzyme group includes both xanthine dehydrogenase (XDH) and xanthine oxidase (XO). This enzyme group is responsible for converting hypoxanthine and xanthine to UA, which is the end product of purine catabolism in humans. B: UA homeostasis. Production and breakdown of UA takes place in the liver and excretion occurs in the gastrointestinal (GI) tract and kidney. C: proposed mechanisms by which deviations in UA homeostasis affects renal tissues. *Other factors leading to hyperuricemia include kidney dysfunction causing decreased excretion, alcohol consumption, and endocrine dysfunctions. Several other reasons that give rise to hypouricemia consist of renal reabsorption problems, iron and molybdenum deficiencies, high estrogen levels, and some uricosuric drugs. vSMc, vascular smooth muscle cell; ROS, reactive oxygen species; RAAS, renin-angiotensin-aldosterone system.
UA HOMEOSTASIS
UA homeostasis refers to the relatively steady state of UA levels in the body, determined by the balance between the production and excretion of UA. In humans, one-third of UA excretion occurs in the gastrointestinal tract and two-thirds occurs in the kidney (Fig. 1B), where it is freely filtered. Of the filtered UA load, only ~10% is excreted. Reabsorption and secretion happen in the proximal tubule in a species-specific manner. In humans and rats, UA reabsorption is predominant compared with pigs and rabbits, where secretion of UA plays a major role (1, 21). Two key transporters important for UA reabsorption are urate transporter 1 (URAT1), encoded by the SLC22A12 gene, and glucose transporter 9 (GLUT9), encoded by the SLC2A9 gene. URAT1 and GLUT9 have been identified as the main transporter in urate handling on the apical and basolateral sides of the proximal tubule epithelium, respectively (7, 45). URAT1 has been shown to be regulated by α-protein kinase 1 (16), insulin (42), and glucocorticoid receptors (19). Recent large-scale whole exome sequencing association studies of serum urate and kidney function identified functional variants in both SLC22A12 and SLC2A9 genes as influencing serum urate levels (41).
HYPERURICEMIA
Hyperuricemia results from the overproduction or underexcretion of UA, causing increased circulating levels, which can exceed saturation limits and lead to deposits in the blood, joints, tissues, and urine. Traditionally, hyperuricemia’s major clinical relevance is gout. Gout is an inflammatory disease caused by excess UA deposition and is a common finding in patients with CKD (48), often presented with cardiovascular comorbidities. Clinical treatments for gout include lowering UA levels. As an example, the XOR inhibitor allopurinol (an isomer of hypoxanthine) is a Food and Drug Administration-approved drug commonly used in such cases. UA nephrolithiasis or stone formation can also present with or without gout as part of a metabolic disorder. A recent clinical study concluded that higher acid load to the kidney resulting in net urinary excretion is an important factor of idiopathic UA nephrolithiasis (2).
Hyperuricemia as a Disease Marker and a Causal Factor
More recent research shows that hyperuricemia is associated with pathologies other than gout. Hindering the production of UA by blocking XOR should have a protective effect against CVD and CKD. This has been tested yielding positive results (9, 32, 39). For example, inhibition of XOR with a nonpurine inhibitor, febuxostat, in spontaneously hypertensive rats improved HTN and endothelial dysfunction (39). Interestingly, a recent randomized clinical trial in healthy young men showed that the decrease of UA level should be severe and accompanied by nitric oxide (NO) synthase (NOS) inhibition (5) to observe changes in the endothelial function. However, this study was done in healthy individuals. Therefore, the interpretation might be different for older patients with previously diagnosed vascular conditions (5). Furthermore, it was reported that UA-lowering allopurinol, tested in a randomized trial in adolescents with essential HTN, attenuated their blood pressure to normotensive levels (9).
Groups with high CVD risk, including men, obese individuals, postmenopausal women, and patients with HTN, exhibit increased UA levels, resulting from a variety of mechanisms (14). Even though gastrointestinal excretion of UA is significant, decreased GFR can be a mechanism of changes in uricemia (43). Increased net tubular reabsorption and increased production of UA are other major factors affecting UA levels. In addition to being a marker of disease, there is evidence to suggest that UA can lead to CVD and CKD. Thus, in vitro studies revealed that UA stimulates cell proliferation in vascular smooth muscle cells (28). Vascular smooth muscle cell proliferation leads to atherosclerosis, a primary cause of many CVD. UA is also known to be proinflammatory (12); therefore, its increase could lead to HTN and endothelial dysfunction. In addition, UA can act as a potent antioxidant in the extracellular environment (1, 15, 17). It is thought that hyperuricemia in CVD might be a compensatory mechanism to counteract oxidative damage caused by reactive oxygen species (ROS). However, UA can also be considered a prooxidant, because ROS are made as byproducts during intracellular UA synthesis.
Previous research has elucidated a mechanism of CKD caused by hyperuricemia leading to UA crystal deposition in renal tubules (38). The models used for these studies have a severe and rapid increase in serum UA, therefore precluding studies of disease pathogenesis before crystal development. To study the potential pathogenic role of UA without crystal deposition, Mazzali et al. (23) conducted experiments using a rat model of mild hyperuricemia. The previous rat model of hyperuricemia was supplemented with both oxonic acid, a uricase inhibitor and UA itself (38), to produce a seven- to eightfold increase in UA. In contrast, the model used by Mazzali and colleagues was supplemented with only 2% oxonic acid and exhibited only a 1.5-fold increase in serum UA (23). Renal injury and fibrosis occurred in parallel with the development of HTN in mildly hyperuricemic rats but not normouricemic rats (23). These observations were accompanied by increased juxtaglomerular renin levels and decreased expression of NOS in the macula densa (23), which are known to result in afferent and efferent arteriolar vasoconstriction. Treatment of hyperuricemic rats with enalapril, an angiotensin-converting enzyme inhibitor, prevented renal damage and HTN (23). A similar effect was achieved using l-arginine to stimulate NO synthesis. This study suggests a hyperuricemia-renin-vasoconstriction mechanism of UA pathogenesis in the kidney. This mechanism is supported by human studies, which have also indicated renin activation in the presence of hyperuricemia (32). Collectively, these studies demonstrated that increased serum UA is pathogenic and contributes to the development of CKD, potentially through stimulation of the renin-angiotensin system and inhibition of NO synthesis. This process appears to be independent of UA crystal deposition.
The genetic differences in expression of uricase enzyme between rodents and humans pose a challenge in using rodent models in studying related pathophysiology. To avoid this interference from uricase enzyme and develop a spontaneously hyperuricemic model that more effectively recapitulate human disease, a knockout was created in C57BL/6J mice (Uox−/−) (20). Compared with wild-type mice, these hyperuricemic mice had significantly elevated blood urea nitrogen alongside kidney damage. They demonstrated renal lesions followed by dilated Bowman’s capsules and tubules. This model exhibited apparent inflammation and immune cell infiltration as well as elevation in mRNA transcripts for inflammatory cytokines in the kidneys. These results suggest a mechanism of adverse renal outcomes in hyperuricemia through an inflammatory pathway.
Shown on Fig. 1C are a few examples of proposed mechanisms by which deviations in UA homeostasis affects renal tissues. However, more research is needed to validate these mechanisms and to explore additional mechanisms by which hyperuricemia may contribute to CKD progression.
HYPOURICEMIA
Hypouricemia, a decrease in serum UA (below 2 mg/dL in humans), leads to xanthinuria and, ultimately, to xanthine and hypoxanthine crystal deposition in the joints, muscles, and/or kidney. It can cause nephrolithiasis/urolithiasis with secondary renal damage and pain if deposited in muscles (30). Hypouricemia can result from inherited or transient causes (Fig. 1C). Mutations in genes encoding urate transporters (2, 20) or liver enzymes [XDH (Ref. 18), molybdenum cofactor sulfurase (MOCOS; Ref. 10), purine nucleoside phosphorylase (PNP; Ref. 21), and phosphoribosyl pyrophosphate (PRPP)] have been identified in patients presenting hereditary hypouricemia. Hypouricemia and renal tubule dysfunctions associated with mutations in these genes have been reported in many studies (2, 4, 8). Liver failure hindering the production of UA, high levels of estrogen (estrogen is a uricosuric agent; Ref. 43), and lack of micronutrients used as cofactors with XOR complex are among other causes of hypouricemia.
Associations Between Hypouricemia and Kidney Disease
Although research exploring hypouricemia is sparse, a mutation in the Xdh gene has been revealed to cause renal failure and early death in a knockout mouse model (Xdh−/− mouse) (27). The homozygous animals in this model were smaller in body size compared with their wild-type littermates (27). Studies in Xdh−/− mice have shown that the renal damage is due to increased ROS production and the resulting interstitial fibrosis (26). The renal damage was accompanied by crystal and lipid depositions (26). These mice also exhibited an increased excretion of hypoxanthine and xanthine in their urine (26). These results, in combination with increased blood urea nitrogen (27), showed that Xdh−/− mice most likely die due to renal failure as the underlying cause. A significant increase in markers for fibrosis, inflammation, ischemia, and oxidative stress was also seen in protein and mRNA levels in hypouricemic mice (26). One of the markers tested was transforming growth factor-β (TGF-β). A recent study done on hepatocellular carcinoma cells revealed that the downregulation of XDH can promote the TGF-β signaling pathway (5). TGF-β can induce cyclooxygenase-2 expression (9), which can ultimately produce prostaglandins promoting inflammation. Even though in humans hypouricemia is mostly a rare and asymptomatic condition, animal and cellular study evidence points to a potential mechanism of hypouricemia leading to kidney diseases through inflammatory signaling pathways.
Hereditary Diseases of Hypouricemia
Hereditary xanthinuria (HX) is an autosomal recessive disease characterized by low UA and elevated xanthine in the urine caused by mutations relating to xanthine production. There are two mutation-specific forms of HX. Type I is caused by a mutation in the XDH gene, and type II is due to a mutation in the MOCOS gene. There is a nonclassical clinically distinct third type that usually associated with severe neurological diseases (11) in addition to the symptoms of hypouricemia mentioned above.
In contrast with hypouricemic mice (27), both types of HX in humans are usually clinically mild or asymptomatic (31). The difference is likely due to the evolutionary pathway variance between mice and humans. Also, patients with HX have enhanced salvage pathway activity, recycling hypoxanthine into purines efficiently (22). This suggests that there is a compensatory mechanism to account for xanthine accumulation in patients with HX. Finally, during fetal development, humans can derive XDH from their mother, which is not possible in mice (26).
Genetic diseases of UA overexcretion (hyperuricosuria) are called renal hypouricemia. Like HX, they have been categorized into two types. The mutation causing types 1 and 2 are in genes SLC22A12 and SLC2A9, respectively (29). Renal hypouricemia can be asymptomatic until the patients are subjected to strenuous exercise, which can lead to acute renal injury (10, 34). Therefore, identifying these mutations is critical to avoid such episodes. Research indicates that this occurs due to oxidative damage caused by increased ROS production during exercise leading to renal vasoconstriction and ischemia (28).
CONCLUSIONS
Recently, many studies have elucidated the relationship between UA, CVD, and CKD, among other chronic conditions. Because of its common occurrence with and without gout, hyperuricemia was studied more thoroughly in research. This review focused on describing facets of hyperuricemia and hypouricemia linked with an increased risk for cardiovascular events as pathological states mediated by the disturbance in UA homeostasis and renal dysfunction. Given the sheer amount of evidence, it is apparent that serum UA levels should be maintained to protect the kidneys and prevent cardiovascular risks. Comprehensive studies are necessary to identify how UA homeostasis in the kidney is a pivotal mechanistic player in cardiovascular and renal health.
GRANTS
This work was partially supported by National Heart, Lung, and Blood Institute Grants R35-HL-135749 and P01-HL-116264 and Department of Veteran Affairs Grant I01-BX-004024.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.V.D. prepared figure; L.V.D. and A.S. drafted manuscript; L.V.D., D.R.S., O.P., and A.S. edited and revised manuscript; L.V.D., D.R.S., O.P., and A.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We apologize if additional relevant publications were not directly and/or fully discussed due to space limitation of this review. We also would like to highlight the review article by Jung and colleagues (Am J Physiol Renal Physiol; doi: 10.1152/ajprenal.00272.2019) focused on uric acid and inflammation in kidney disease, which was accepted at the same time as this review.
REFERENCES
- 1.Bobulescu IA, Moe OW. Renal transport of uric acid: evolving concepts and uncertainties. Adv Chronic Kidney Dis 19: 358–371, 2012. doi: 10.1053/j.ackd.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bobulescu IA, Park SK, Xu LHR, Blanco F, Poindexter J, Adams-Huet B, Davidson TL, Sakhaee K, Maalouf NM, Moe OW. Net acid excretion and urinary organic anions in idiopathic uric acid nephrolithiasis. Clin J Am Soc Nephrol 14: 411–420, 2019. doi: 10.2215/CJN.10420818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cappola TP, Kass DA, Nelson GS, Berger RD, Rosas GO, Kobeissi ZA, Marbán E, Hare JM. Allopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathy. Circulation 104: 2407–2411, 2001. doi: 10.1161/hc4501.098928. [DOI] [PubMed] [Google Scholar]
- 4.Chen C, Lü JM, Yao Q. Hyperuricemia-related diseases and xanthine oxidoreductase (XOR) inhibitors: an overview. Med Sci Monit 22: 2501–2512, 2016. doi: 10.12659/MSM.899852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen GL, Ye T, Chen HL, Zhao ZY, Tang WQ, Wang LS, Xia JL. Xanthine dehydrogenase downregulation promotes TGFβ signaling and cancer stem cell-related gene expression in hepatocellular carcinoma. Oncogenesis 6: e382, 2017. doi: 10.1038/oncsis.2017.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ekelund UE, Harrison RW, Shokek O, Thakkar RN, Tunin RS, Senzaki H, Kass DA, Marbán E, Hare JM. Intravenous allopurinol decreases myocardial oxygen consumption and increases mechanical efficiency in dogs with pacing-induced heart failure. Circ Res 85: 437–445, 1999. doi: 10.1161/01.RES.85.5.437. [DOI] [PubMed] [Google Scholar]
- 7.Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, Hosoyamada M, Takeda M, Sekine T, Igarashi T, Matsuo H, Kikuchi Y, Oda T, Ichida K, Hosoya T, Shimokata K, Niwa T, Kanai Y, Endou H. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 417: 447–452, 2002. doi: 10.1038/nature742. [DOI] [PubMed] [Google Scholar]
- 8.Fabbrini E, Serafini M, Colic Baric I, Hazen SL, Klein S. Effect of plasma uric acid on antioxidant capacity, oxidative stress, and insulin sensitivity in obese subjects. Diabetes 63: 976–981, 2014. doi: 10.2337/db13-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang L, Chang HM, Cheng JC, Leung PC, Sun YP. TGF-β1 induces COX-2 expression and PGE2 production in human granulosa cells through Smad signaling pathways. J Clin Endocrinol Metab 99: E1217–E1226, 2014. doi: 10.1210/jc.2013-4100. [DOI] [PubMed] [Google Scholar]
- 10.Furuto Y, Kawamura M, Namikawa A, Takahashi H, Shibuya Y, Mori T, Sohara E. Non-urate transporter 1, non-glucose transporter member 9-related renal hypouricemia and acute renal failure accompanied by hyperbilirubinemia after anaerobic exercise: a case report. BMC Nephrol 20: 433, 2019. doi: 10.1186/s12882-019-1618-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ichida K, Amaya Y, Okamoto K, Nishino T. Mutations associated with functional disorder of xanthine oxidoreductase and hereditary xanthinuria in humans. Int J Mol Sci 13: 15475–15495, 2012. doi: 10.3390/ijms131115475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson RJ. Why focus on uric acid? Curr Med Res Opin 31, Suppl 2: 3–7, 2015. doi: 10.1185/03007995.2015.1087979. [DOI] [PubMed] [Google Scholar]
- 13.Johnson RJ, Gaucher EA, Sautin YY, Henderson GN, Angerhofer AJ, Benner SA. The planetary biology of ascorbate and uric acid and their relationship with the epidemic of obesity and cardiovascular disease. Med Hypotheses 71: 22–31, 2008. doi: 10.1016/j.mehy.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson RJ, Kang DH, Feig D, Kivlighn S, Kanellis J, Watanabe S, Tuttle KR, Rodriguez-Iturbe B, Herrera-Acosta J, Mazzali M. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension 41: 1183–1190, 2003. doi: 10.1161/01.HYP.0000069700.62727.C5. [DOI] [PubMed] [Google Scholar]
- 15.Kanda E, Muneyuki T, Kanno Y, Suwa K, Nakajima K. Uric acid level has a U-shaped association with loss of kidney function in healthy people: a prospective cohort study. PLoS One 10: e0118031, 2015. doi: 10.1371/journal.pone.0118031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuo TM, Huang CM, Tu HP, Min-Shan Ko A, Wang SJ, Lee CP, Ko YC. URAT1 inhibition by ALPK1 is associated with uric acid homeostasis. Rheumatology (Oxford) 56: 654–659, 2017. doi: 10.1093/rheumatology/kew463. [DOI] [PubMed] [Google Scholar]
- 17.Lee SY, Park W, Suh YJ, Lim MJ, Kwon SR, Lee JH, Joo YB, Oh YK, Jung KH. Association of serum uric acid with cardiovascular disease risk scores in Koreans. Int J Environ Res Public Health 16: 4632, 2019. doi: 10.3390/ijerph16234632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehto S, Niskanen L, Rönnemaa T, Laakso M. Serum uric acid is a strong predictor of stroke in patients with non-insulin-dependent diabetes mellitus. Stroke 29: 635–639, 1998. doi: 10.1161/01.STR.29.3.635. [DOI] [PubMed] [Google Scholar]
- 19.Li G, Han L, Ma R, Saeed K, Xiong H, Klaassen CD, Lu Y, Zhang Y. Glucocorticoids increase renal excretion of urate in mice by downregulating urate transporter 1. Drug Metab Dispos 47: 1343–1351, 2019. doi: 10.1124/dmd.119.087700. [DOI] [PubMed] [Google Scholar]
- 20.Lu J, Hou X, Yuan X, Cui L, Liu Z, Li X, Ma L, Cheng X, Xin Y, Wang C, Zhang K, Wang X, Ren W, Sun R, Jia Z, Tian Z, Mi QS, Li C. Knockout of the urate oxidase gene provides a stable mouse model of hyperuricemia associated with metabolic disorders. Kidney Int 93: 69–80, 2018. doi: 10.1016/j.kint.2017.04.031. [DOI] [PubMed] [Google Scholar]
- 21.Martinez F, Manganel M, Montrose-Rafizadeh C, Werner D, Roch-Ramel F. Transport of urate and p-aminohippurate in rabbit renal brush-border membranes. Am J Physiol Renal Physiol 258: F1145–F1153, 1990. doi: 10.1152/ajprenal.1990.258.5.F1145. [DOI] [PubMed] [Google Scholar]
- 22.Mateos FA, Puig JG, Jiménez ML, Fox IH. Hereditary xanthinuria. Evidence for enhanced hypoxanthine salvage. J Clin Invest 79: 847–852, 1987. doi: 10.1172/JCI112893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, Lan HY, Kivlighn S, Johnson RJ. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 38: 1101–1106, 2001. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 24.Nakagawa T, Kang DH, Feig D, Sanchez-Lozada LG, Srinivas TR, Sautin Y, Ejaz AA, Segal M, Johnson RJ. Unearthing uric acid: an ancient factor with recently found significance in renal and cardiovascular disease. Kidney Int 69: 1722–1725, 2006. doi: 10.1038/sj.ki.5000391. [DOI] [PubMed] [Google Scholar]
- 25.Oh TR, Choi HS, Kim CS, Bae EH, Ma SK, Sung S-A, Kim Y-S, Oh KH, Ahn C, Kim SW. Hyperuricemia has increased the risk of progression of chronic kidney disease: propensity score matching analysis from the KNOW-CKD study. Sci Rep 9: 6681, 2019. doi: 10.1038/s41598-019-43241-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohtsubo T, Matsumura K, Sakagami K, Fujii K, Tsuruya K, Noguchi H, Rovira II, Finkel T, Iida M. Xanthine oxidoreductase depletion induces renal interstitial fibrosis through aberrant lipid and purine accumulation in renal tubules. Hypertension 54: 868–876, 2009. doi: 10.1161/HYPERTENSIONAHA.109.135152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohtsubo T, Rovira II, Starost MF, Liu C, Finkel T. Xanthine oxidoreductase is an endogenous regulator of cyclooxygenase-2. Circ Res 95: 1118–1124, 2004. doi: 10.1161/01.RES.0000149571.96304.36. [DOI] [PubMed] [Google Scholar]
- 28.Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest 74: 1156–1164, 1984. doi: 10.1172/JCI111524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pineda C, Soto-Fajardo C, Mendoza J, Gutierrez J, Sandoval H. Hypouricemia: what the practicing rheumatologist should know about this condition. Clin Rheumatol 39: 135–147, 2020. doi: 10.1007/s10067-019-04788-8. [DOI] [PubMed] [Google Scholar]
- 30.Prasad Sah OS, Qing YX. Associations between hyperuricemia and chronic kidney disease: a review. Nephrourol Mon 7: e27233, 2015. doi: 10.5812/numonthly.7(3)2015.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raivio KO, Saksela M, Lapatto R. Xanthine oxidoreductase−role in human pathophysiology and in hereditary xanthinuria. In: OMMBID, edited by Beaudet AL, Vogelstein B, Kinzler KW, Antonarakis SE, Ballabio A, Gibson KM, Mitchell G. New York: McGraw-Hill, 2014. [Google Scholar]
- 32.Saito I, Saruta T, Kondo K, Nakamura R, Oguro T, Yamagami K, Ozawa Y, Kato E. Serum uric acid and the renin-angiotensin system in hypertension. J Am Geriatr Soc 26: 241–247, 1978. doi: 10.1111/j.1532-5415.1978.tb02396.x. [DOI] [PubMed] [Google Scholar]
- 33.Sato Y, Feig DI, Stack AG, Kang D-H, Lanaspa MA, Ejaz AA, Sánchez-Lozada LG, Kuwabara M, Borghi C, Johnson RJ. The case for uric acid-lowering treatment in patients with hyperuricaemia and CKD. Nat Rev Nephrol 15: 767–775, 2019. doi: 10.1038/s41581-019-0174-z. [DOI] [PubMed] [Google Scholar]
- 34.Shimizu Y, Wakabayashi K, Totsuka A, Hayashi Y, Nitta S, Hara K, Akira M, Tomino Y, Suzuki Y. Exercise-induced acute kidney injury in a police officer with hereditary renal hypouricemia. Case Rep Nephrol Dial 9: 92–101, 2019. doi: 10.1159/000501877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shirakura T, Nomura J, Matsui C, Kobayashi T, Tamura M, Masuzaki H. Febuxostat, a novel xanthine oxidoreductase inhibitor, improves hypertension and endothelial dysfunction in spontaneously hypertensive rats. Naunyn Schmiedebergs Arch Pharmacol 389: 831–838, 2016. doi: 10.1007/s00210-016-1239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis 47: 51–59, 2006. doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Son CN, Kim JM, Kim SH, Cho SK, Choi CB, Sung YK, Kim TH, Bae SC, Yoo DH, Jun JB. Prevalence and possible causes of hypouricemia at a tertiary care hospital. Korean J Intern Med (Korean Assoc Intern Med) 31: 971–976, 2016. doi: 10.3904/kjim.2015.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stavric B, Johnson WJ, Grice HC. Uric acid nephropathy: an experimental model. Proc Soc Exp Biol Med 130: 512–516, 1969. doi: 10.3181/00379727-130-33593. [DOI] [PubMed] [Google Scholar]
- 39.Stull LB, Leppo MK, Szweda L, Gao WD, Marbán E. Chronic treatment with allopurinol boosts survival and cardiac contractility in murine postischemic cardiomyopathy. Circ Res 95: 1005–1011, 2004. doi: 10.1161/01.RES.0000148635.73331.c5. [DOI] [PubMed] [Google Scholar]
- 40.Suliman ME, Johnson RJ, García-López E, Qureshi AR, Molinaei H, Carrero JJ, Heimbürger O, Bárány P, Axelsson J, Lindholm B, Stenvinkel P. J-shaped mortality relationship for uric acid in CKD. Am J Kidney Dis 48: 761–771, 2006. doi: 10.1053/j.ajkd.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 41.Tin A, Li Y, Brody JA, Nutile T, Chu AY, Huffman JE, Yang Q, Chen M-H, Robinson-Cohen C, Macé A, Liu J, Demirkan A, Sorice R, Sedaghat S, Swen M, Yu B, Ghasemi S, Teumer A, Vollenweider P, Ciullo M, Li M, Uitterlinden AG, Kraaij R, Amin N, van Rooij J, Kutalik Z, Dehghan A, McKnight B, van Duijn CM, Morrison A, Psaty BM, Boerwinkle E, Fox CS, Woodward OM, Köttgen A. Large-scale whole-exome sequencing association studies identify rare functional variants influencing serum urate levels. Nat Commun 9: 4228, 2018. doi: 10.1038/s41467-018-06620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toyoki D, Shibata S, Kuribayashi-Okuma E, Xu N, Ishizawa K, Hosoyamada M, Uchida S. Insulin stimulates uric acid reabsorption via regulating urate transporter 1 and ATP-binding cassette subfamily G member 2. Am J Physiol Renal Physiol 313: F826–F834, 2017. doi: 10.1152/ajprenal.00012.2017. [DOI] [PubMed] [Google Scholar]
- 43.Vaziri ND, Freel RW, Hatch M. Effect of chronic experimental renal insufficiency on urate metabolism. J Am Soc Nephrol 6: 1313–1317, 1995. [DOI] [PubMed] [Google Scholar]
- 44.Verdecchia P, Schillaci G, Reboldi G, Santeusanio F, Porcellati C, Brunetti P. Relation between serum uric acid and risk of cardiovascular disease in essential hypertension. The PIUMA study. Hypertension 36: 1072–1078, 2000. doi: 10.1161/01.HYP.36.6.1072. [DOI] [PubMed] [Google Scholar]
- 45.Vitart V, Rudan I, Hayward C, Gray NK, Floyd J, Palmer CNA, Knott SA, Kolcic I, Polasek O, Graessler J, Wilson JF, Marinaki A, Riches PL, Shu X, Janicijevic B, Smolej-Narancic N, Gorgoni B, Morgan J, Campbell S, Biloglav Z, Barac-Lauc L, Pericic M, Klaric IM, Zgaga L, Skaric-Juric T, Wild SH, Richardson WA, Hohenstein P, Kimber CH, Tenesa A, Donnelly LA, Fairbanks LD, Aringer M, McKeigue PM, Ralston SH, Morris AD, Rudan P, Hastie ND, Campbell H, Wright AF. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet 40: 437–442, 2008. doi: 10.1038/ng.106. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe S, Kang DH, Feng L, Nakagawa T, Kanellis J, Lan H, Mazzali M, Johnson RJ. Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension 40: 355–360, 2002. doi: 10.1161/01.HYP.0000028589.66335.AA. [DOI] [PubMed] [Google Scholar]
- 47.Yeldandi AV, Chu R, Pan J, Zhu Y, Usuda N. Peroxisomal purine metabolism. Ann N Y Acad Sci 804: 165–175, 1996. doi: 10.1111/j.1749-6632.1996.tb18615.x. [DOI] [PubMed] [Google Scholar]
- 48.Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007−2008. Am J Med 125: 679–687.e1, 2012. doi: 10.1016/j.amjmed.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 49.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007−2008. Arthritis Rheum 63: 3136–3141, 2011. doi: 10.1002/art.30520. [DOI] [PubMed] [Google Scholar]