Abstract
In the early proximal tubule, Na+-glucose cotransporter 2 (SGLT2) mediates the bulk of renal glucose reabsorption. Gene deletion in mice (Sglt2−/−) was used to determine the role of SGLT2 in acute kidney injury induced by bilateral ischemia-reperfusion (IR). In Sglt2−/− and littermate wild-type mice, plasma creatinine increased similarly on day 1 after IR. This was associated with an equal increase in both genotypes in the urinary kidney injury molecule-1-to-creatinine ratio, a tubular injury marker, and similarly reduced urine osmolality and increased plasma osmolality, indicating impaired urine concentration. In both IR groups, FITC-sinistrin glomerular filtration rate was equally reduced on day 14, and plasma creatinine was similarly and incompletely restored on day 23. In Sglt2−/− mice subjected to IR, fractional urinary glucose excretion was increased on day 1 but reduced and associated with normal renal Na+-glucose cotransporter 1 (Sglt1) mRNA expression on day 23, suggesting temporary SGLT1 suppression. In wild-type mice subjected to IR, renal Sglt1 mRNA was likewise normal on day 23, whereas Sglt2 mRNA was reduced by 57%. In both genotypes, IR equally reduced urine osmolality and renal mRNA expression of the Na+-K+-2Cl− cotransporter and renin on day 23, suggesting thick ascending limb dysfunction, and similarly increased renal mRNA expression of markers of injury, inflammation, oxidative stress, and fibrosis (kidney injury molecule-1, neutrophil gelatinase-associated lipocalin, monocyte chemoattractant protein-1, transforming growth factor-β1, NADPH oxidase-2, and collagen type 1). This was associated with equal increases in kidney histological damage scores and similar degree of capillary loss in both genotypes. The data indicate that genetic deletion of SGLT2 did not protect the kidneys in the initial injury phase or the subsequent recovery phase in a mouse model of IR-induced acute kidney injury.
Keywords: acute kidney injury, Na+-glucose cotransporter 2, sodium-glucose cotransport
INTRODUCTION
Acute kidney injury (AKI) is defined by a rapid decline in renal excretory function (within hours to days) causing an impairment in fluid and electrolyte homeostasis and retention of metabolic waste products such as creatinine and urea (8, 24). Renal ischemia-reperfusion (IR) injury is an important cause of AKI. Renal IR injury is defined by significant renal hypoperfusion causing local or diffuse ischemia followed by restoration of blood flow and reoxygenation, as it can occur in conditions such as cardiovascular shock, decompensation of congestive heart failure, cardiac bypass surgery, or kidney transplantation (10, 26). The pathophysiology of IR-induced kidney injury includes hypoxic injury of the tubular epithelium and endothelial and microcirculatory dysfunction, which cause enhanced oxidative stress, mitochondrial dysfunction, tubulointerstitial inflammation, and tubular necrosis (10, 26, 53). Furthermore, recurring episodes of AKI enhance the risk to develop chronic kidney disease and end-stage renal disease (7, 15). Available treatments include supportive care rather than prevention or restorative therapies, indicating the need to better define and understand the involved pathophysiology and develop new therapeutic approaches (12).
In normoglycemia and with normal glomerular filtration rate (GFR), the two kidneys of an adult individual filter large amounts of glucose (~180 g daily), and almost all filtered glucose is reabsorbed by the tubular system, predominantly by the proximal tubule (PT). The latter is due to the coordinated action of multiple apical and basolateral epithelial glucose transporters (49). At the whole kidney level, the low-affinity, high-capacity Na+-glucose cotransporter [Na+-glucose cotransporter 2 (SGLT2)] reabsorbs ~97% of all filtered glucose (44). SGLT2 is expressed in the apical brush border of the early PT (S1/S2 segments) and takes up glucose from the lumen into the cytoplasm. The facilitative glucose transporter [glucose transporter (GLUT)2] allows intracellular glucose to diffuse across the basolateral membrane and return to the systemic blood circulation, or the peritubular glucose is taken up by more distal tubular cells, which, in contrast to normal PT cells, readily metabolize glucose. The filtered glucose that has not been reabsorbed by SGLT2 reaches the further downstream part of the PT [S2/S3 segments, which eventually enters the outer medulla (OM)], where higher-affinity, lower-capacity Na+-glucose cotransporter 1 (SGLT1) mediates apical glucose uptake (36, 49). SGLT-mediated glucose transport is coupled to Na+ reabsorption, which is driven by the electrochemical gradient for Na+ established by basolateral Na+-K+-ATPase. Thus, SGLT2 and SGLT1 are secondary active transporters that require energy and oxygen to reabsorb the large amounts of filtered glucose (49).
Inhibitors of SGLT2 constitute a new class of antihyperglycemic drugs that have been approved for the treatment of type 2 diabetes mellitus (T2DM) (20, 40). Blockade of SGLT2 decreases glucose reabsorption in the early PT and induces glucosuria and ultimately lowers blood glucose levels. Studies in gene-targeted mice have demonstrated that SGLT1, which is localized downstream of SGLT2 in the late PT, compensates in part for glucose reabsorption, thereby limiting urinary glucose loss when SGLT2 is genetically deleted or pharmacologically blocked (36, 37). Large clinical outcome trials have demonstrated cardioprotective and renoprotective benefits of SGLT2 inhibitors in patients with T2DM with high cardiovascular risk and preserved kidney function (28, 48, 52). More recently, the SGLT2 inhibitor canagliflozin showed cardiovascular and renoprotective effects in patients with T2DM and established kidney disease (33), and the compound has been approved by the United States Food and Drug Administration to slow the progression of diabetic nephropathy in patients with T2DM. The proposed potential mechanisms by which SGLT2 inhibitors protect the kidneys go beyond blood glucose control (30, 31, 46).
Meta-analyses of the large clinical safety trials concluded that SGLT2 inhibition was associated with a reduction in AKI in patients with T2DM (16, 32). Previous studies using murine models of renal IR injury have also shown that treatment with SGLT2 inhibitors improved kidney function impairment and recovery (11, 51). On the other hand, and given that SGLT2 inhibition shifts glucose reabsorption to the downstream S2/S3 segment, SGLT2 blockade could increase SGLT1 activity in the OM and further enhance IR injury or worsen recovery. Based on studies in gene-targeted mice, a deleterious role for SGLT1 transport activity in the hypoxia-vulnerable OM was recently proposed during recovery after IR injury induced by bilateral renal artery clamping (29). In addition, SGLT2-mediated glucose uptake may provide an alternate energy substrate in AKI, as glycolysis has been proposed to increase in PTs under these conditions (22). Moreover, the question whether the renoprotective effects mediated by pharmacological inhibitors of SGLT2 are due to direct or off-target effects needs to be addressed, as studies in the ischemic heart have proposed that SGLT2 inhibitors can act through direct effects on Na+/H+ exchangers (NHEs) (41, 42).
Therefore, in the present study, we aimed to determine whether genetic deletion of SGLT2 is beneficial or detrimental in a murine model of AKI induced by bilateral renal artery clamping, including the initial phase of kidney injury and the early recovery phase over the subsequent 23 days.
MATERIALS AND METHODS
Animals.
All animal experiments were conducted in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD) and were approved by the local Institutional Animal Care and Use Committee. Experiments were performed on 16- to 19-wk-old male Sglt2-deficient (Sglt2−/−) mice and wild-type (WT) littermates (on C57BL/6J background), the generation of which has been previously described (44). All animals were housed in the same room with a 12:12-h light-dark cycle and free access to tap water and standard rodent chow.
Induction of renal IR injury.
Induction of renal IR injury was performed as previously described (29). Briefly, nonfasted mice were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg body wt)-xylazine (8 mg/kg body wt) and placed on a temperature-controlled surgical table with a rectal probe to maintain body temperature between 36 and 37°C. The kidneys were exposed with bilateral flank incisions. The surrounding fat tissue was dissected to expose renal pedicles. Both renal arteries were carefully dissected and clamped for 25 min using nontraumatic microvessel clamps; renal veins remained unoccluded. Cessation of blood flow was documented by visual inspection. After the clamps were released, wounds were sutured using 9-mm wound clips. Sterile saline (0.9% NaCl) was injected subcutaneously (30 µL/g body wt) to replenish fluid loss. Mice recovering from anesthesia were returned to a prewarmed cage (placed on a heating pad) until fully ambulatory (~2 h). Sham-operated (sham) mice underwent the same procedures without artery clamping. The analgesic buprenorphine (50 µg/kg) was injected subcutaneously immediately after completion of surgery and twice a day for the first 3 days postsurgery. The following four groups of mice were studied: WT sham (n = 7), WT IR (n = 10), Sglt2−/− sham (n = 7), and Sglt2−/− IR (n = 15).
Urine and blood collection.
Urine collection was performed in awake mice 2 days before surgery to obtain basal values as well as 1, 3, 7, and 23 days after surgery and in general paired with body weight and blood glucose measurements. To minimize stress-induced blood glucose elevation, blood glucose was measured first (by tail snip, Contour glucometer, Bayer, Mishawaka, IN). Urine was then obtained by grabbing the mice and provoking spontaneous urination. Samples were stored at −80°C for later analyses. Blood samples were collected 2 days before surgery to obtain basal values as well as on days 1, 7, and 23 after surgery. Briefly, under short isoflurane anesthesia (3 min, 3% isoflurane, 2.5 L/min O2), the retroorbital plexus was punctured using a 10-µL Na+-heparin microcapillary (Hirschmann Laborgeräte, Eberstadt, Germany), and blood was collected into a 70-µL heparinized microhematocrit capillary tube (Fisherbrand, Hampton, NH). Capillaries were sealed (Châ-Seal tube sealing compound, Kimble Chase, Vineland, NJ) and centrifuged for 3 min at 12,000 rpm. Plasma was stored at −80°C for later analyses.
Determination of GFR in conscious mice.
Fourteen days after surgery, GFR was determined using plasma elimination kinetics of FITC-sinistrin, as previously described (29, 34, 35, 43). Briefly, 2 µL/g body wt of FITC-sinistrin (Fresenius-Kabi, Linz, Austria, 2% in 0.85% NaCl, which also served to establish the standard curve) was injected into the retroorbital plexus during brief isoflurane anesthesia (3 min, 3% isoflurane, 2.5 L/min O2). Blood was then collected into Na+-heparinized 10-µL microcapillaries from a small tail nick at 3, 5, 7, 10, 15, 35, 56, and 75 min after injection. After centrifugation (3 min at 12,000 rpm), 2 µL plasma was diluted 1:10 in 0.5 mol/L HEPES (pH 7.4), and the fluorescence signal was measured at 470 nm in 2-µL samples using a NanoDrop ND-3300 Fluorospectrometer (Nanodrop Technologies, Wilmington, DE). GFR was calculated using a two-compartment model of two-phase exponential decay (GraphPad Prism, San Diego, CA).
Kidney harvest.
Twenty-three days after surgery and under terminal isoflurane anesthesia, blood was collected by retroorbital plexus puncture (see above), kidneys were subsequently harvested, and the renal capsule was gently removed. Kidneys were weighed, cut in half, frozen in liquid nitrogen, and stored at −80°C for later quantitative RT-PCR analyses. One kidney half was kept in 3.5 mL of 10% formalin fixation solution for 48 h at 4°C. Fixed samples were transferred in tissue cassettes, immersed into 70% ethanol, and prepared for histological analyses (see Histological analyses below).
Plasma and urine analyses.
Urine glucose concentration was determined by the hexokinase/glucose-6-phosphate dehydrogenase method (Infinity, Thermo Scientific, Louisville, CO) and plasma creatinine concentration by the isotope dilution liquid chromatography-tandem mass spectrometry method (LC-MS/MS) at the O’Brien Center for Acute Kidney Injury Research at the University of Alabama at Birmingham (Birmingham, AL). Urine creatinine was measured by the kinetics of the alkaline picrate Jaffe reaction (Infinity, Thermo Scientific). Plasma and urine phosphate concentration was measured using the QuantiChrom Phosphate Assay Kit (BioAssay Systems). Fractional excretions of glucose (FE-glucose) and phosphate (FE-phosphate) were calculated as the urine-to-plasma concentration ratio of glucose and phosphate, respectively, divided by the urine-to-plasma concentration ratio of creatinine. Plasma electrolytes (Na+, K+, and Cl−) were measured using an electrolyte analyzer (EasyElectrolytes, Medica, Bedford, MA). Plasma and urine osmolality were measured using a Vapro vapor pressure osmometer 5520 (Wescor, Logan, UT). On day 1 after the surgery, urinary kidney injury molecule (KIM)-1 concentrations were determined in samples diluted 1:10,000 using the mouse TIM-1/KIM-1/HAVCR Quantikine ELISA kit (Bio-Techne, Minneapolis, MN) following the manufacturer’s instructions and normalized to creatinine. On day 23 after surgery, urinary albumin concentration was determined using a mouse albumin ELISA kit (Bethyl Laboratories) and normalized to creatinine.
Reverse transcription and real-time PCR.
Total kidney RNA was isolated using the RNeasy Plus Mini Kit (Qiagen, Germantown, MD), and cDNA was prepared using the SuperScript IV First-Strand Synthesis System (Thermo Scientific). For quantification, Taqman Universal PCR Master Mix (Applied Biosystems) and specific primers were used in a 7500 Real-Time PCR System (2 min at 50°C and 10 min at 95°C, with 40 cycles of 15 s at 95°C and 1 min at 60°C). Each experiment was performed in duplicate to determine the relative expression of the following genes: KIM-1 [hepatitis A virus cellular receptor 1 (Havcr1)], neutrophil gelatinase-associated lipocalin [NGAL; lipocalin 2 (Lcn2)], collagen type I-α1 (Col1a1), monocyte chemoattractant protein-1 [chemokine (C-C motif) ligand 2 (Ccl2)], transforming growth factor-β1 (TGF-β1; Tgfb1), peroxisome proliferator-activated receptor-γ coactivator-1α (Ppargc1a), hexokinase-2 (Hk2), aquaporin-2 (Aqp2), solute carrier family 5 member 1 (Slc5a1, SGLT1), solute carrier family 5 member 2 (Slc5a2, SGLT2), solute carrier family 12 member 1 [Slc12a1, Na+-K+-2Cl− cotransporter (NKCC2)], NADPH oxidase-2 [NOX2; cytochrome b(558) subunit-β (Cybb)], vascular endothelial growth factor A (VEGF-A; Vegfa), and renin (Ren). Primer details are shown in Table 1.
Table 1.
Real-time PCR primers used
| Target Gene | Assay ID |
|---|---|
| Aqp2 | Mm00437575_m1 |
| MCP1/Ccl2 | Mm00441242_m1 |
| Col1a1 | Mm00801666_g1 |
| KIM-1/Havcr1 | Mm00506686_m1 |
| Hk2 | Mm00443385_m1 |
| Hprt | Mm00446968_m1 |
| Ppargc1a | Mm01208835_m1 |
| Ren | Mm02342889_g1 |
| SGLT1/Slc5a1 | Mm00451203_m1 |
| SGLT2/Slc5a2 | Mm00453831_m1 |
| NKCC2/Slc12a1 | Mm01275821_m1 |
| NOX2/Cybb | Mm01287743_m1 |
| Tgfb1 | Mm01178820_m1 |
| Vegfa | Mm00437306_m1 |
| NGAL/Lcn2 | Mm01324470_m1 |
Aqp2, aquaporin 2; MCP1, monocyte chemoattractant protein-1; Ccl2, chemokine (C-C motif) ligand 2; Col1a1, collagen type I-α1; KIM-1, kidney injury molecule 1; Havcr1, hepatitis A virus cellular receptor 1; Hk2, hexokinase-2; Hprt, hypoxanthine-guanine phosphoribosyltransferase; Ppargc1a, peroxisome proliferator-activated receptor-γ coactivator-1α; Ren, renin; SGLT, Na+-glucose cotransporter; NKCC2, Na+-K+-2Cl− cotransporter; NOX2, NADPH oxidase-2; Cybb, cytochrome b(558)β; Tgfb1, transforming growth factor-β1; Vegfa, vascular endothelial growth factor-A; NGAL, neutrophil gelatinase-associated lipocalin; Lcn2, lipocalin 2.
Histological analyses.
Formalin-fixed kidney samples were embedded in paraffin blocks, and 5-µm sections were prepared for histological analyses by the University of California-San Diego Tissue Technology Shared Resource (supported by National Cancer Institute Cancer Center Support Grant P30-CA-23100). For tubular injury semiquantification, periodic acid-Schiff staining was performed, and slides were analyzed by a renal pathologist who was blinded to experimental groups. For each animal, tubular injury was defined as tubular dilation, tubular atrophy, tubular cast formation, sloughing of tubular epithelial cells, or loss of the brush border and thickening of the tubular basement membrane, using the following scoring system: 0, no tubular injury; 1, <25% of tubules injured; 2, 25–50% of tubules injured; and 3, >50% of tubules injured.
Immunostaining.
Paraffin-embedded kidney tissue sections were rehydrated, and heat-induced antigen retrieval was performed in 10 mM citrate buffer (pH 6.0). For immunohistochemistry that uses horseradish peroxidase (HRP), tissue sections were incubated in 3% H2O2 for 5 min. Tissues were blocked in 2.5% normal donkey or goat serum in Tris-buffered saline plus 0.1% Tween 20 for 30 min at room temperature and then incubated with primary antibodies overnight at 4°C. After a wash with Tris-buffered saline plus 0.1% Tween 20, sections were incubated with fluorescent dye-conjugated secondary antibodies or ImmPRESS HRP anti-rabbit IgG polymers (MP-7451, Vector Laboratories) for 1 h at room temperature. For immunofluorescent staining, autofluorescence background signals were quenched by a TrueBlack Lipofuscin Autofluorescence Quencher reagent (no. 23007, Biotium) according to the manufacturer’s instructions. A DAB substrate kit (SK-4100, Vector Laboratories) was used for HRP detection. Slides were scanned via an Axio Scan.Z1 (Zeiss) slide scanner.
The primary antibodies used in this study were anti-KIM-1 (1:200, AF1817, R&D Systems), anti-uromodulin (UMOD; 1:1,000, ab207170, Abcam), and anti-CD31 (1:100, no. 77699, Cell Signaling Technology). The secondary antibodies used in this study were anti-goat IgG antibodies conjugated with Alexa Fluor 555 (1:500, A21432, Invitrogen) and anti-rabbit IgG conjugated with Alexa Fluor 488 (1:200, ab150081, Abcam). To localize proximal tubules, fluorescein-labeled Lotus tetragonolobus lectin (LTL; 1:200, FL-1321, Vector Laboratories) was coincubated with secondary antibodies.
Statistical analyses.
Data are presented as means ± SE. Data were analyzed by two-way ANOVA to test for an effect of IR or SGLT2 knockout and for a significant interaction between the two factors. If the interaction was statistically significant, then a pairwise multiple comparison procedure (Holm-Sidak method) was used to identify the significant effects (38). An unpaired t test was used when only two groups were compared for basal data. Survival was analyzed with the log-rank test. An overall significance level of P < 0.05 was considered as statistically significant. SigmaPlot (version 11.0, Systat Software, San Jose, CA) was used for all statistical analyses.
RESULTS
Absence of SGLT2 caused glucosuria under basal conditions.
Quantitative RT-PCR analyses confirmed the effective elimination of renal Sglt2 mRNA expression in Sglt2−/− sham and Sglt2−/− IR mice (Fig. 1A). Renal Sglt1 mRNA expressions were similar in Sglt2−/− sham versus WT sham mice (Fig. 1A). Previous studies had reported unchanged renal mRNA expression of Glut1 and Glut2 in Sglt2−/− versus WT mice (44, 45).
Fig. 1.
Absence of Na+-glucose cotransporter 2 (SGLT2) did not affect the early glomerular and tubular impairment in response to ischemia-reperfusion (IR) or subsequent recovery. A: renal mRNA expression of Slc5a2 (Sglt2) and Slc5a1 [Na+-glucose cotransporter (Sglt1)] were measured on day 23 after IR or sham surgery [normalized to the housekeeping gene hypoxanthine-guanine phosphoribosyltransferase (Hprt)]. B: plasma creatinine rose to similar levels in Sglt2−/− and wild-type (WT) mice on day 1 after IR, indicating similar initial glomerular filtration rate (GFR) reduction. Plasma creatinine recovery was similar in Sglt2−/− versus WT mice and remained incomplete by day 23. C: direct measurement on day 14 after IR confirmed similar GFR values in Sglt2−/− versus WT mice. D: urinary levels of the proximal tubular injury marker kidney injury molecule-1 (KIM-1) were similarly increased in Sglt2−/− versus WT mice on day 1 after IR. E and F: transient increase in fractional renal excretion of glucose (FE-glucose) in both genotypes on day 1 after IR, with higher levels observed in Sglt2−/− versus WT mice. G: blood glucose was significantly reduced in Sglt2−/− and WT mice on day 1 after IR versus sham. H: a similar decrease in body weight was observed in Sglt2−/− and WT mice following IR. Results are means ± SE. Two-way ANOVA was performed to probe for a significant effect of Sglt2−/− (PSglt2), IR (PIR), or the interaction between the two factors (Pinter). If the interaction was statistically significant, a pairwise multiple-comparison procedure (Holm-Sidak method) identified the significant effects. #P < 0.05 vs. sham mice; *P < 0.05 vs. WT mice. A: n = 7–11 mice/group; B: n = 6–11 mice/group and per time point; C: n = 7–10 mice/group; D: sham n = 4 mice/group and IR n = 8–9 mice/group; E–H: n = 4–11 mice/group and per time point. ND, not detected.
At baseline, plasma creatinine concentrations did not differ between genotypes, indicating similar GFRs (Table 2). This finding was confirmed by additional plasma creatinine measurements on days 1, 7, and 23 in the sham groups (Fig. 1B) and by actual GFR measurements on day 14 (Fig. 1C). Mean urinary KIM-1-to-creatinine ratios, a marker of proximal tubular injury, were likewise not different between Sglt2−/− and WT sham groups (Fig. 1D).
Table 2.
Baseline values in WT and Sglt2−/− mice
| Genotype |
Blood glucose, mg/dL |
Urinary glucose, µmol/mg creatinine |
FE-glucose, % |
Body weight, g |
Hematocrit, % |
Plasma creatinine, mg/dL |
Urine osmolality, mosm/kg |
Plasma osmolality, mosm/kg |
|---|---|---|---|---|---|---|---|---|
| WT | 151 ± 5 | 8.0 ± 0.6 | 0.10 ± 0.01 | 27.3 ± 0.7 | 43.1 ± 0.4 | 0.105 ± 0.002 | 1,736 ± 91 | 284 ± 3.3 |
| Sglt2−/− | 122 ± 5* | 1,581 ± 124* | 26.2 ± 2.3* | 25.1 ± 0.4* | 44.7 ± 0.4* | 0.115 ± 0.004 (P = 0.058) | 1,455 ± 42* | 293 ± 3.5 (P = 0.079) |
Results are means ± SE; n = 14–18 mice/group. Baseline data were not significantly different between sham-operated and ischemia-reperfused groups for a given genotype and thus were pooled. WT, wild type; Sglt2−/−, Na+-glucose transporter 2 knockout; FE-glucose, fractional renal excretion of glucose.
P < 0.05 vs. WT mice using an unpaired t test.
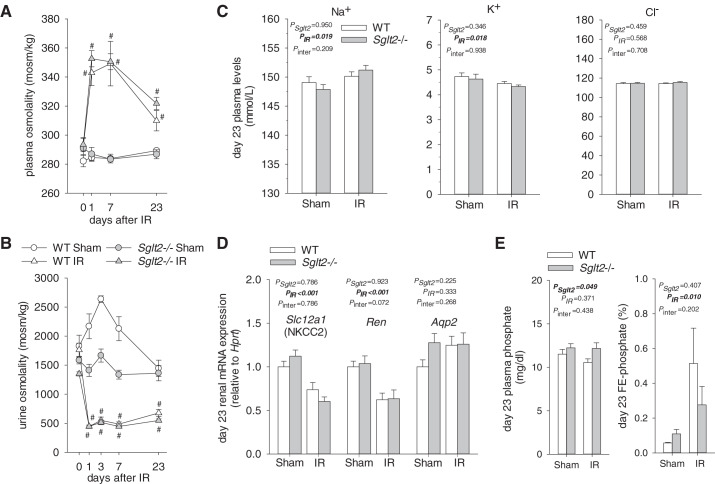
Consistent with a major contribution of SGLT2 to basal renal glucose reabsorption in the normal kidney under euglycemia (44), urinary glucose-to-creatinine ratios and FE-glucose were greater (Fig. 1, E and F) and blood glucose levels were lower (Table 2) in Sglt2−/− versus WT sham mice. Absence of SGLT2 lowered urine osmolality in sham mice, consistent with an osmotic diuretic effect of the excreted glucose (Table 2). This was associated with a slightly greater hematocrit in Sglt2−/− compared with WT sham mice (Table 2).
Absence of SGLT2 did not affect the early glomerular and tubular impairment in response to IR.
Five animals died during the followup period after IR surgery and were removed from the analyses: of 15 Sglt2−/− mice subjected to IR, 2 mice died on day 1 after IR and 2 mice died on day 3. Of 10 WT mice subjected to IR, 1 mouse died on day 7 after IR. The cause of death was not investigated. No mortality was observed in the sham groups. A log-rank test did not reveal a statistically significant difference between the survival curves of all four groups [P = 0.185, not significant (NS)].
On day 1 after IR, plasma creatinine rose to similar levels in Sglt2−/− and WT mice (Fig. 1B), indicating a similar initial decrease in GFR. IR increased urinary KIM-1 excretion as measured on day 1 of reperfusion. Absence of SGLT2 did not significantly affect the increase in urinary KIM-1, suggesting similar initial PT injury in both IR groups (Fig. 1D).
IR induced a transient increase in the urinary glucose-to-creatinine ratio on day 1 in WT mice; in contrast, the ratio was decreased in Sglt2−/− mice and remained at similar lower levels until day 23 (Fig. 1E). IR induced a transient increase in FE-glucose on day 1 in both WT and Sglt2−/− mice (Fig. 1F). Unexpectedly, FE-glucose was also slightly increased in Sglt2−/− sham mice on days 1 and 3 and returned to baseline on day 23. The mechanism remained unclear. It could theoretically have resulted from downregulation of SGLT1 activity, possibly due to prolonged anesthesia or the sham surgical procedure, but further studies are needed to support this hypothesis. A proposed effect of SGLT1 downregulation would not be detectable as an increase in FE-glucose in WT sham mice due to the large glucose reabsorption capacity of intact SGLT2.
Blood glucose levels decreased in both Sglt2−/− and WT mice on day 1 after IR (Fig. 1G). This was associated with a more sustained and similar body weight reduction in both genotypes in response to IR (Fig. 1H), possibly as a consequence of enhanced glucosuria and the resulting osmotic diuresis and urinary caloric loss, but potentially also due to plasma accumulation of uremic toxins, which may have suppressed appetite.
IR increased plasma osmolality and reduced urine osmolality to a similar extent in both genotypes on day 1 after IR (Fig. 2, A and B), indicating a similar initial impairment in urine concentration. Sham surgery transiently increased urine osmolality in WT mice, possibly due to surgical stress-induced release of vasopressin, which enhanced water reabsorption in the collecting ducts. Such an effect may have been partly offset in Sglt2−/− mice by the osmotic effect of glucose.
Fig. 2.
Absence of Na+-glucose cotransporter 2 (SGLT2) did not affect alterations in plasma and urine osmolality after ischemia-reperfusion (IR). A: on day 1 after IR, plasma osmolality was increased to a similar extent in Sglt2−/− and wild-type (WT) mice. The subsequent recovery was equal in both genotypes and remained incomplete on day 23. B: on day 1 after IR, urine osmolality was reduced to a similar extent in Sglt2−/− and WT mice. Subsequent recovery was incomplete and not different between Sglt2−/− and WT mice. C: effect of IR on plasma concentrations of Na+, K+, and Cl− was not affected by genotype. D: on day 23 after IR, renal mRNA expression of Na+-K+-2Cl− cotransporter (Nkcc2) and renin was similarly decreased in Sglt2−/− and WT mice versus their respective sham groups, whereas aquaporin-2 (Aqp2) mRNA was unchanged. E: effect of IR on plasma phosphate concentrations and fractional renal excretion of phosphate (FE-phosphate) was not affected by genotype. Lack of SGLT2 was associated with slightly higher plasma phosphate concentrations, independent of IR. Results are means ± SE. Two-way ANOVA was performed to probe for a significant effect of Sglt2−/− (PSglt2), IR (PIR), or the interaction between the two factors (Pinter). If the interaction was statistically significant, a pairwise multiple-comparison procedure (Holm-Sidak method) identified the significant effects. #P < 0.05 vs. sham mice; *P < 0.05 vs.WT mice. A and B: n = 4–11 mice/group and per time point; C: n = 6–11 mice/group; D: n = 6–11 mice/group; E: n = 5–9 mice/group.
Absence of SGLT2 did not affect the recovery of GFR and of markers of tubular function and kidney injury following IR.
On day 14 after IR, GFR was reduced to similar levels in Sglt2−/− IR and WT IR mice (Fig. 1C). In accordance, on days 7 and 23 after IR, the recovery profiles of plasma creatinine levels were similar in Sglt2−/− IR and WT IR mice (Fig. 1B). Notably, plasma creatinine on day 23 after IR surgery remained significantly greater than in sham animals, indicating incomplete recovery of glomerular filtration. Urinary albumin-to-creatinine ratios were not different on day 23 after IR versus sham mice and tended to be lower in Sglt2−/− versus WT mice independent of IR (WT sham: 15.7 ± 1.2 µg/mg, Sglt2−/− sham: 12.3 ± 1.6 µg/mg, WT IR: 16.6 ± 1.9 µg/mg, and Sglt2−/− IR: 12.1 ± 2.3 µg/mg, n = 5–8 mice/group, PSglt2 = 0.057, PIR = 0.841, Pinter = 0.774).
In WT mice, FE-glucose progressively normalized from day 7 to day 23 after IR but remained slightly higher in IR versus sham groups on day 23 (0.28 ± 0.02 vs. 0.15 ± 0.02%, P = 0.039; Fig. 1F). This was associated with an ~60% downregulation in Sglt2 mRNA in WT IR versus WT sham mice (Fig. 1A). In comparison, renal Sglt1 mRNA levels were not significantly affected in WT by IR (Fig. 1A). Overall, this indicated better preservation or quicker recovery of Sglt1 versus Sglt2 mRNA expression at day 23 after IR in WT mice.
In Sglt2−/− IR mice, FE-glucose returned to baseline on day 7 after IR and further decreased on day 23, resulting in lower FE-glucose in Sglt2−/− IR compared with Sglt2−/− sham mice (12 ± 2% vs. 26 ± 3%, P = 0.002; Fig. 1F). This likely reflected a sustained reduction in GFR and thus less filtered glucose in the Sglt2−/− IR group, such that SGLT1 could more fully compensate. This would be consistent with the observed similar Sglt1 mRNA expression in Sglt2−/− IR versus Sglt2−/− sham mice on day 23, as well as with previous data in Sglt2−/− mice showing a positive correlation between filtered glucose and FE-glucose (44).
Plasma osmolality recovery from day 3 to day 23 after IR was similar between Sglt2−/− and WT mice and remained incomplete on day 23 (Fig. 2A). Similarly, lack of SGLT2 did not affect urine osmolality recovery, which hardly increased from day 3 to day 23 after IR (Fig. 2B). In both genotypes, plasma concentrations of Na+ were slightly higher and plasma K+ slightly lower in IR versus sham mice on day 23, whereas plasma Cl− concentrations were not affected (Fig. 2C). Overall, lack of SGLT2 did not significantly affect plasma electrolyte concentrations in IR or sham groups (Fig. 2C).
Whole kidney quantitative RT-PCR analyses on day 23 after IR revealed that renal mRNA expressions of Nkcc2 and renin were similarly reduced in Sglt2−/− and WT mice (Fig. 2D). This may indicate a sustained impairment of NaCl reabsorption in the thick ascending limb, which is expected to impair urine concentration and, through effects on NaCl delivery to the juxtaglomerular apparatus, lower renin expression and GFR. In comparison, mRNA expression of Aqp2, a marker of the connecting tubule and collecting duct, was similar in IR versus sham mice of both genotypes (Fig. 2D). Plasma phosphate concentrations were not significantly changed on day 23 after IR versus sham in Sglt2−/− or WT mice (Fig. 2E), whereas FE-phosphate was increased in both genotypes (Fig. 2E). SGLT2 inhibition can cause a small increase in plasma phosphate levels in humans (9, 39). In accordance, absence of SGLT2 was associated with slightly higher plasma phosphate concentrations; this was independent of IR (Fig. 2E).
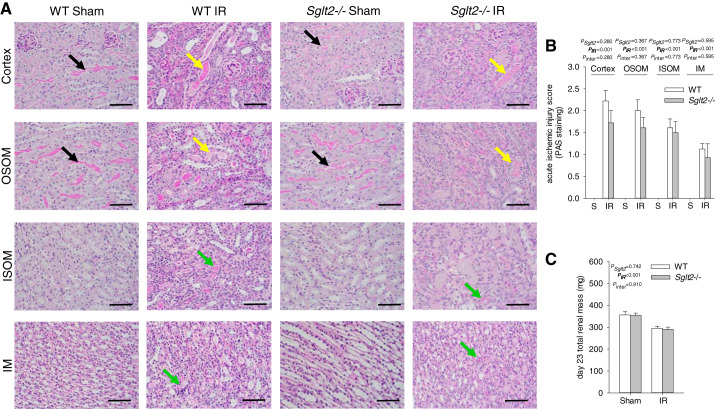
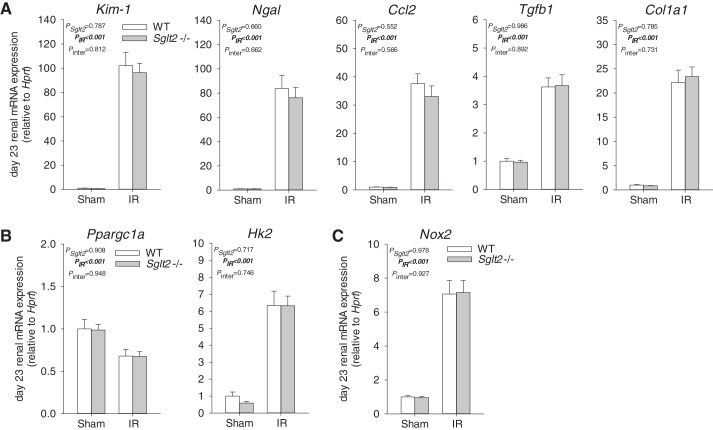
Tubular injury was quantified in kidneys harvested on day 23 after IR by a kidney pathologist blinded to study groups. In sham animals, no significant differences were observed between genotypes (Fig. 3, A and B). Moreover, total renal mass was similar between Sglt2−/− sham and WT sham mice (Fig. 3C). IR induced cortical and medullary tubular damage and cast formation, brush border loss, tubular simplification, and immune cell infiltration, the severity of which appeared to be similar between Sglt2−/− and WT mice (Fig. 3, A and B). This was associated with an equal reduction in total renal mass (Fig. 3C), indicative of similar development of progressive fibrotic renal lesions (2, 21, 23). These histological changes were associated with similar upregulation of renal mRNA expression of the tubular injury markers Kim-1 and Ngal as well as renal inflammation and fibrosis markers Ccl2, Tgfb1, and Col1a1 in Sglt2−/− and WT mice (Fig. 4A). Immunostaining indicated that Sglt2−/− and WT sham kidneys did not display positive KIM-1 staining and that IR enhanced renal KIM-1 expression in the OM and cortex in both genotypes (Fig. 5). Absence of SGLT2 did not affect the increase and localization of KIM-1 immunostaining in response to IR (Fig. 5). Proximal tubule-specific LTL staining and distal tubule-specific UMOD immunodetection were used to assess in what tubule segment KIM-1 was expressed. LTL staining revealed decreased PT brush-border abundance and height in IR groups, consistent with sustained renal dysfunction on day 23. KIM-1 expression was detected primarily in LTL-negative and UMOD-negative tubules, which appeared to be atrophic tubules (Fig. 5). Some KIM-1-positive cells colocalized with LTL-positive tubules. In addition, mRNA expression of Hk2, a key glycolytic gene, and Ppargc1a, a master regulator of mitochondrial biogenesis and function, were similarly upregulated and downregulated, respectively, in both genotypes in response to IR (Fig. 4B), indicating similar renal metabolic disturbance in Sglt2−/− and WT IR mice. Furthermore, renal mRNA expression of Nox2, a major contributor to IR-induced oxidative stress (19), was similarly upregulated in both genotypes in response to IR (Fig. 4C).
Fig. 3.
Absence of Na+-glucose cotransporter 2 (SGLT2) did not affect cortical and medullary injury during recovery from ischemia-reperfusion (IR). On day 23 after IR, kidneys were harvested for histological analysis and semiquantification of IR injury by a renal pathologist blinded with regard to study groups. A: representative pictures of cortical and medullary tubular injury (periodic acid-Schiff staining). Black arrows indicate proximal tubules with an intact brush border. Yellow arrows indicate ectatic proximal tubules with flattened epithelial cells, loss of the brush border, and containing necrotic debris/casts. Green arrows indicate ectatic tubules containing casts. B: semiquantitative analysis of cortical and medullary ischemic injury. Sglt2−/− sham animals had normal histology features similar to that of the wild-type (WT) sham animals. IR-induced tubular injury was equal in Sglt2−/− and WT mice, with greater damage score in the cortex and outer medulla (OM) compared with the inner medulla (IM). C: total renal mass measured on day 23 of reperfusion. Absence of SGLT2 did not affect renal mass in sham animals. IR reduced renal mass to similar levels in Sglt2−/− and WT mice. S, sham; OSOM, outer stripe of OM; ISOM, inner stripe of the OM. Results are means ± SE. Two-way ANOVA was performed to probe for a significant effect of Sglt2−/− (PSglt2), IR (PIR), or the interaction between the two factors (Pinter). If the interaction was statistically significant, a pairwise multiple-comparison procedure (Holm-Sidak method) identified the significant effects. #P < 0.05 vs. sham mice; *P < 0.05 vs. WT mice. Sham: n = 6 mice/group and per region; IR: n = 7–9 mice/group and per region. Scale bars = 100 µm.
Fig. 4.
Absence of Na+-glucose cotransporter 2 (SGLT2) did not affect renal mRNA expression of markers of tubular injury, inflammation, and fibrosis during recovery from ischemia-reperfusion (IR). Kidneys were harvested on day 23 after IR for quantitative RT-PCR analyses of mRNA expression. mRNA expression was normalized to the housekeeping gene hypoxanthine-guanine phosphoribosyltransferase (Hprt). A: absence of SGLT2 had no effect on the IR-induced increase in renal expression of markers of tubular injury [kidney injury molecule-1 (Kim-1) and neutrophil gelatinase-associated lipocalin (Ngal)], inflammation [chemokine (C-C motif) ligand 2 (Ccl2) and transforming growth factor-β1 (Tgfb1)], and fibrosis [collagen type I-α1 (Col1a1)]. B: absence of SGLT2 did not affect the upregulation of hexokinase-2 (Hk2), a key glycolytic enzyme, and did not affect the downregulation of peroxisome proliferator-activated receptor-γ coactivator-1α (Ppargc1a), a master regulator of mitochondrial biogenesis and function. C: upregulation of NAPDH oxidase (Nox2; a mediator of IR-induced oxidative stress) was not affected by Sglt2 knockout. Results are means ± SE. Two-way ANOVA was performed to probe for a significant effect of Sglt2−/− (PSglt2), IR (PIR), or the interaction between the two factors (Pinter). If the interaction was statistically significant, a pairwise multiple-comparison procedure (Holm-Sidak method) identified the significant effects. #P < 0.05 vs. sham mice; *P < 0.05 vs. wild-type (WT) mice. n = 6–11 mice/group.
Fig. 5.
Enhanced kidney injury molecule 1 (KIM-1) immunostaining in the renal outer medulla and cortex during recovery from ischemia-reperfusion (IR). On day 23 after IR, kidneys were harvested to localize KIM-1 protein expression by immunostaining. Representative pictures are shown. IR enhanced renal KIM-1 immunostaining in the outer medulla and cortex in both genotypes. KIM-1-positive cells were mostly Lotus tetragonolobus lectin (LTL)-negative and uromodulin (UMOD)-negative cells that localized in atrophic tubules. Absence of Na+-glucose cotransporter 2 (SGLT2) did not affect the increase in KIM-1 immunostaining in either region. Both Sglt2−/− and wild-type (WT) IR kidneys displayed similar UMOD-positive dilated tubules. Double-stained images are magnified images of the white boxed area (left) from consecutive tissue sections. Insets in the left bottom corners show tissues stained without primary antibody. n = 6 mice/group.
Absence of SGLT2 did not protect the kidneys from IR-induced capillary rarefaction.
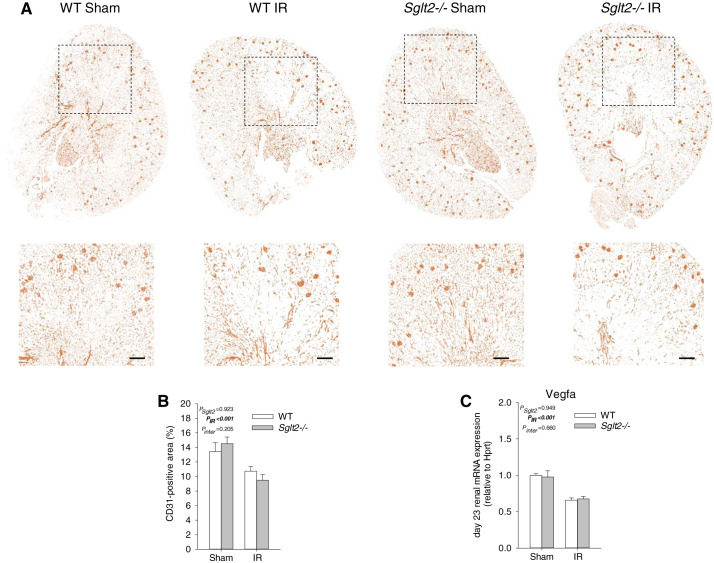
A reduction in vascular density occurs following acute IR injury. Immunodetection of CD31, a marker of endothelial cells, was performed on kidney sections to assess capillary loss on day 23 after surgery. There was no difference in the CD31-positive area between Sglt2−/− sham and WT sham mice (Fig. 6, A and B). IR induced a similar decrease in CD31-positive area in both genotypes, indicative of comparable capillary rarefaction (Fig. 6, A and B). Endothelial loss was associated with a similar downregulation of renal Vegfa mRNA expression in Sglt2−/− IR and WT IR (Fig. 6C).
Fig. 6.
Absence of Na+-glucose cotransporter 2 (SGLT2) did not affect capillary rarefaction during recovery from ischemia-reperfusion (IR). On day 23 after IR, kidneys were harvested to localize CD31 protein expression by immunostaining and determine renal vascular endothelial growth factor-A (Vegfa) mRNA levels. A: representative images of CD31 immunostaining of whole kidney sections (top) and magnified images of boxed areas (bottom). B: CD31-positive area quantification. IR decreased renal CD31-positive area to a similar extent in both genotypes. C: renal Vegfa mRNA expression was similarly downregulated in Sglt2−/− and wild-type (WT) mice after IR. Two-way ANOVA was performed to probe for a significant effect of Sglt2−/− (PSglt2), IR (PIR), or the interaction between the two factors (Pinter). If the interaction was statistically significant, a pairwise multiple-comparison procedure (Holm-Sidak method) identified the significant effects. A and B: n = 5–6 mice/group; C: n = 6–11 mice/group. Scale bars = 200 μm.
DISCUSSION
The present study reports that genetic deletion of SGLT2 did not affect the acute impairment in glomerular and tubular function or the subsequent extent of their recovery in a mouse model of IR-induced AKI using bilateral artery clamping. This was indicated by a comparable increase in both genotypes in plasma creatinine, accompanied by similar increases in urinary KIM-1 excretion and impairment in urine concentration ability on day 1 after IR. This was followed by a similarly incomplete recovery of glomerular and tubular function in both genotypes on day 23 of reperfusion, associated with similar upregulation of renal markers of tubular injury (Kim-1 and Ngal), inflammation (Ccl2 and Tgfb1), and fibrosis (Col1a1) and a comparable degree of renal parenchymal damage and capillary rarefaction.
Little is known about the consequences of IR injury on PT glucose handling and its implications. Previous studies in rats found that SGLT2 transport activity was transiently reduced after IR, associated with decreased SGLT2 expression at the apical membrane (18, 27). In accordance, preliminary RNA sequencing data indicated that renal Sglt2 and Sglt1 mRNA expressions were downregulated on day 1 of reperfusion after 15 or 25 min of bilateral renal artery clamping in C57BL/6J mice (14). Twenty-four hours after glycerol-induced AKI in rats, glucosuria was observed associated with a striking depression in tubular glucose reabsorption (47). The present study revealed in WT mice that IR induced a transient rise in FE-glucose but also increased urinary glucose-to-creatinine ratios on day 1 after IR, indicating an increase in absolute glucose excretion, despite a potential strong reduction in GFR and thus in filtered glucose, consistent with a markedly reduced renal glucose reabsorption capacity. Subsequently, FE-glucose and urinary glucose-to-creatinine ratios largely normalized in WT IR mice, indicating sufficient renal glucose reabsorption capacity despite subnormal renal Sglt2 mRNA expression, possibly facilitated by still reduced amounts of filtered glucose and relatively normal renal Sglt1 mRNA expression.
In mice lacking SGLT2, absolute and fractional urinary glucose excretion was greater at baseline, confirming the prominent contribution of SGLT2 to renal glucose reabsorption (44). IR provoked a robust decrease in urinary glucose-to-creatinine ratios on day 1 after IR, which can be attributed to the initial reduction in GFR. This was associated with increased FE-glucose, suggesting decreased SGLT1-mediated transport activity. The initial absolute increase in FE-glucose appeared slightly larger in Sglt2−/− mice (~30 to ~60%) versus WT mice (0 to ~20%), possibly reflecting the greater dependence of renal glucose reabsorption in the former on SGLT1, the expression of which was likely suppressed on day 1 (see above). During the recovery phase, FE-glucose in Sglt2−/− IR mice fell and on day 23 ended up below sham values, potentially resulting from recovery of SGLT1-mediated transport capacity, consistent with its near-normal renal mRNA expression, while GFR and thus the tubular glucose load remained suppressed. A lesser suppression or quicker recovery in renal mRNA in Sglt1 versus Sglt2 following IR was previously reported in WT mice (29). Moreover, by using Sglt1 gene knockout mice, these studies indicated a robust contribution of SGLT1 to glucose reabsorption from day 1 after IR to the end of the study on day 16, potentially as a compensatory mechanism for SGLT2 downregulation and increased glucose load to the downstream late PT (29). Furthermore, the presence of SGLT1-mediated transport activity worsened kidney recovery after IR (29). The present study found evidence for similar initial renal injury and subsequent recovery and damage in Sglt2−/− and WT kidneys following IR, which may be explained, in part, by comparable SGLT1-mediated transport work in the hypoxia-sensitive OM. The net contribution of SGLT2 in this scenario, and thus the detectable difference between Sglt2−/− and WT kidneys, may have been minimized by the robust and sustained downregulation of SGLT2 in WT mice (Fig. 7).
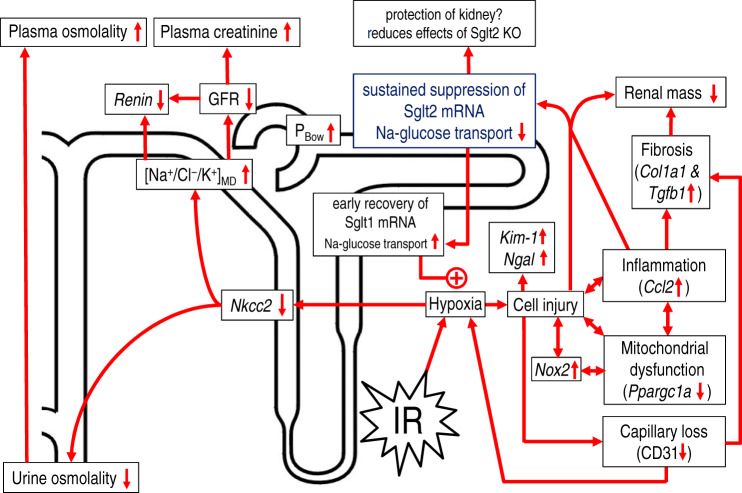
Fig. 7.
Proposed consequences of ischemia-reperfusion (IR)-induced downregulation of Na+-glucose cotransporter 2 (SGLT2). IR induces hypoxia and tubular cell injury. IR initially suppresses SGLT2- and Na+-glucose cotransporter 1 (SGLT1)-mediated Na+-glucose reabsorption, which is associated with glucosuria. The hypoxic environment inhibits Na+-K+-2Cl− cotransporter (NKCC2)-mediated NaCl reabsorption in the thick ascending limb, which impairs urine concentration and enhances Na+-Cl−-K+ delivery to the macula densa ([Na/Cl/K]MD). This reduces renin expression and lowers glomerular filtration rate (GFR) via tubuloglomerular feedback. Reduced Na+ reabsorption and luminal accumulation of cellular debris may also increase tubular back pressure, which lowers GFR by increasing hydrostatic pressure in Bowman’s space (PBow). The reduction in GFR enhances plasma creatinine. While SGLT2 remains partly suppressed, recovery of SGLT1-mediated Na+ reabsorption in the late proximal tubule/outer medulla lowers glucosuria but sustains IR-induced hypoxia. This may further enhance inflammation in the outer medulla, which may spread and further promote suppression of cortical tubular function. Mitochondrial dysfunction, endothelial injury, and associated loss of capillaries further enhances hypoxia and contributes to tissue fibrosis. In the long term, inflammation and fibrosis contribute to loss of renal mass. Thus, sustained suppression of SGLT2 after IR may protect the early proximal tubule but can enhance the glucose load to SGLT1, which may have detrimental consequences during renal recovery after IR. IR-induced sustained SGLT2 suppression may have masked any potential beneficial effects of SGLT2 knockout. Kim-1, kidney injury molecule 1; Ngal, neutrophil gelatinase-associated lipocalin; Col1a1, collagen type I-α1; Tgfb1, transforming growth factor-β1; Ccl2, chemokine (C-C motif) ligand 2; Ppargc1a, peroxisome proliferator-activated receptor-γ coactivator 1α; CD31, cluster of differentiation 31; Nox2, NADPH oxidase 2.
Endothelial dysfunction and capillary rarefaction are hallmarks of renal IR injury and are recognized as major contributors to the transition from AKI to chronic kidney disease, as microvascular injury further enhances renal hypoxia, which, in turn, compromises tubular repair and promotes fibrosis (1, 5, 6). Zhang et al. (51) recently reported in mice that 7-day treatment with the SGLT2 inhibitor luseogliflozin, initiated 6 h after unilateral clamping of renal artery and vein, ameliorated the loss of renal endothelial cells, as shown by a better-preserved CD31-positive area at 4 wk postischemia. The beneficial effect of SGLT2 treatment after IR was thought to be mediated by decreased PT glucose uptake leading to increased production of the proangiogenic factor VEGF-A, which, in turn, helps maintaining peritubular microvasculature integrity. In the present study, IR-induced endothelial rarefaction was likewise indicated in WT mice by a decreased CD31-positive area, associated with a significant downregulation of Vegfa mRNA. Absence of SGLT2, however, did not prevent these changes. The cellular origin of VEGF-A production was not investigated; however, a previous study using tubular-specific Vegfa knockdown in rats demonstrated that VEGF-A of tubular origin contributes to peritubular capillary maintenance in physiological conditions (13). In the present study, Kim-1 and Ngal mRNA (tubular injury markers) as well as renal KIM-1-positive staining were strongly upregulated on day 23 after IR in both genotypes, indicating sustained tubular dysfunction independent of SGLT2.
Another major mediator of IR injury is the enhanced oxidative burst, which occurs upon organ reoxygenation and includes enhanced reactive oxygen species production. NOX2 has been implicated as a mediator of renal IR injury by enhancing ROS production and eventually contributes to fibrosis development (19). Recent animal studies have indicated that SGLT2 inhibitors may exert cardiovascular and renal protection, in part, through antioxidant mechanisms, including downregulation of NADPH enzyme expression and activity via direct or indirect effects (50). In the present study, genetic deletion of Sglt2 did not prevent the renal upregulation of Nox2 mRNA expression after IR, consistent with the observed similar histological damage in Sglt2−/− and WT mice.
Renal IR injury is associated with mitochondrial dysfunction and a metabolic shift of the recovering PTs toward anaerobic glycolysis (3, 22). This shift is characterized by increased activity of hexokinase in the kidney cortex and outer stripe of the OM at 14 days after IR injury (22). At least in the diabetic setting, SGLT2 inhibitors may improve mitochondrial function and kidney metabolism (for a review, see Ref. 31). In the present study, whole kidney mRNA expression of Hk2, a key glycolytic enzyme, was similarly upregulated on day 23 after IR in both genotypes. Moreover, renal mRNA expression of the transcription factor Ppargc1a, a master regulator of mitochondrial biogenesis and function (25), was similarly downregulated in both genotypes during recovery from IR. These results suggested that the renal transition to anaerobic glycolysis might not have been largely affected by the absence of SGLT2.
The observed SGLT2 downregulation in IR may protect the kidney from further damage but would also make it more difficult to detect beneficial effects of its inhibition. In other words, more benefits may be observed in models and conditions with stronger preservation of SGLT2 function. Moreover, previous studies conducted in isolated murine cardiomyocytes have suggested that SGLT2 inhibitors might reduce myocardial NHE flux, potentially via direct inhibition at the Na+-binding site (4, 41). In mouse isolated perfused heart, the SGLT2 inhibitor empagliflozin delayed ischemic contracture, which was proposed to relate to direct inhibition of cardiac NHE1 (42). A preliminary study using tubular-specific NHE3 knockdown mice has suggested that natriuretic effects of empagliflozin depend in part on tubular NHE3 (17). Further studies are needed to better understand the beneficial effects of SGLT2 inhibitors in AKI and the subsequent recovery, which may involve SGLT2-dependent and independent pathways.
In summary, genetic deletion of SGLT2 did not affect the early tubular injury and impairment of kidney function after IR or the subsequent glomerular and tubular function recovery and induction of proinflammatory and profibrotic markers and of capillary rarefaction. Further studies are needed to better define the protective molecular mechanisms of SGLT2 inhibition and their role in AKI.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01-DK-106102, R01-DK-112042, R01-HL-142814, RF1-AG-061296 (to V. Vallon), University of Alabama at Birmingham/University of California-San Diego O’Brien Center of Acute Kidney Injury NIH Grant P30 DK-079337 (to V. Vallon and P. W. Sanders), and the Department of Veterans Affairs (to V. Vallon).
DISCLOSURES
Over the past 36 months, V. Vallon has served as a consultant and received honoraria from Bayer, Boehringer Ingelheim, Eli Lilly, Janssen Pharmaceutical, Merck, and Retrophin and received grant support for investigator-initiated research from Astra-Zeneca, Bayer, Boehringer Ingelheim, Fresenius, and Janssen Pharmaceutical. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
J.N. and V.V. conceived and designed research; J.N., R.P., H.Z., W.H., B.F., and Y.C.K. performed experiments; J.N., R.P., H.Z., W.H., Y.C.K., and V.V. analyzed data; J.N., H.Z., P.S., Y.C.K., and V.V. interpreted results of experiments; J.N., H.Z., Y.C.K., and V.V. prepared figures; J.N. and V.V. drafted manuscript; J.N., R.P., H.Z., W.H., B.F., P.S., Y.C.K., and V.V. edited and revised manuscript; J.N., R.P., H.Z., W.H., B.F., P.S., Y.C.K., and V.V. approved final version of manuscript.
REFERENCES
- 1.Afsar B, Afsar RE, Dagel T, Kaya E, Erus S, Ortiz A, Covic A, Kanbay M. Capillary rarefaction from the kidney point of view. Clin Kidney J 11: 295–301, 2018. doi: 10.1093/ckj/sfx133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ascon M, Ascon DB, Liu M, Cheadle C, Sarkar C, Racusen L, Hassoun HT, Rabb H. Renal ischemia-reperfusion leads to long term infiltration of activated and effector-memory T lymphocytes. Kidney Int 75: 526–535, 2009. doi: 10.1038/ki.2008.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ash SR, Cuppage FE. Shift toward anaerobic glycolysis in the regenerating rat kidney. Am J Pathol 60: 385–402, 1970. [PMC free article] [PubMed] [Google Scholar]
- 4.Baartscheer A, Schumacher CA, Wüst RCI, Fiolet JWT, Stienen GJM, Coronel R, Zuurbier CJ. Empagliflozin decreases myocardial cytoplasmic Na+ through inhibition of the cardiac Na+/H+ exchanger in rats and rabbits. Diabetologia 60: 568–573, 2017. doi: 10.1007/s00125-016-4134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basile DP. Rarefaction of peritubular capillaries following ischemic acute renal failure: a potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens 13: 1–7, 2004. doi: 10.1097/00041552-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Basile DP. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int 72: 151–156, 2007. doi: 10.1038/sj.ki.5002312. [DOI] [PubMed] [Google Scholar]
- 7.Belayev LY, Palevsky PM. The link between acute kidney injury and chronic kidney disease. Curr Opin Nephrol Hypertens 23: 149–154, 2014. doi: 10.1097/01.mnh.0000441051.36783.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet 380: 756–766, 2012. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 9.Blau JE, Bauman V, Conway EM, Piaggi P, Walter MF, Wright EC, Bernstein S, Courville AB, Collins MT, Rother KI, Taylor SI. Canagliflozin triggers the FGF23/1,25-dihydroxyvitamin D/PTH axis in healthy volunteers in a randomized crossover study. JCI Insight 3: e99123, 2018. doi: 10.1172/jci.insight.99123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang Y-K, Choi H, Jeong JY, Na K-R, Lee KW, Lim BJ, Choi DE. Dapagliflozin, SGLT2 inhibitor, attenuates renal ischemia-reperfusion injury. PLoS One 11: e0158810, 2016. doi: 10.1371/journal.pone.0158810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H, Busse LW. Novel therapies for acute kidney injury. Kidney Int Rep 2: 785–799, 2017. doi: 10.1016/j.ekir.2017.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimke H, Sparks MA, Thomson BR, Frische S, Coffman TM, Quaggin SE. Tubulovascular cross-talk by vascular endothelial growth factor a maintains peritubular microvasculature in kidney. J Am Soc Nephrol 26: 1027–1038, 2015. doi: 10.1681/ASN.2014010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fattah H, Shigeoka A, Huang W, Patel R, Kasimsetty S, Singh P, McKay DB, Vallon V. Diverse gene expression patterns of renal transporters in AKI (Abstract). J Am Soc Nephrol 29: 1045, 2018. [Google Scholar]
- 15.Fiorentino M, Grandaliano G, Gesualdo L, Castellano G. Acute kidney injury to chronic kidney disease transition. Contrib Nephrol 193: 45–54, 2018. doi: 10.1159/000484962. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert RE, Thorpe KE. Acute kidney injury with sodium-glucose co-transporter-2 inhibitors: a meta-analysis of cardiovascular outcome trials. Diabetes Obes Metab 21: 1996–2000, 2019. doi: 10.1111/dom.13754. [DOI] [PubMed] [Google Scholar]
- 17.Huang W, Patel R, Onishi A, Crespo Masip M, Soleimani M, Freeman B, Vallon V. Tubular NHE3 is a determinant of the acute natriuretic and chronic blood pressure lowering effect of the SGLT2 inhibitor empagliflozin (Abstract). FASEB J 32, suppl 1: 620.17, 2018. [Google Scholar]
- 18.Johnston PA, Rennke H, Levinsky NG. Recovery of proximal tubular function from ischemic injury. Am J Physiol 246: F159–F166, 1984. doi: 10.1152/ajprenal.1984.246.2.F159. [DOI] [PubMed] [Google Scholar]
- 19.Karim AS, Reese SR, Wilson NA, Jacobson LM, Zhong W, Djamali A. Nox2 is a mediator of ischemia reperfusion injury. Am J Transplant 15: 2888–2899, 2015. doi: 10.1111/ajt.13368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kramer CK, Zinman B. Sodium-glucose cotransporter-2 (SGLT-2) inhibitors and the treatment of type 2 diabetes. Annu Rev Med 70: 323–334, 2019. doi: 10.1146/annurev-med-042017-094221. [DOI] [PubMed] [Google Scholar]
- 21.Kwak W, Jang H-S, Belay T, Kim J, Ha YS, Lee SW, Ahn B-C, Lee J, Park KM, Yoo J. Evaluation of kidney repair capacity using 99mTc-DMSA in ischemia/reperfusion injury models. Biochem Biophys Res Commun 406: 7–12, 2011. doi: 10.1016/j.bbrc.2011.01.085. [DOI] [PubMed] [Google Scholar]
- 22.Lan R, Geng H, Singha PK, Saikumar P, Bottinger EP, Weinberg JM, Venkatachalam MA. Mitochondrial pathology and glycolytic shift during proximal tubule atrophy after ischemic AKI. J Am Soc Nephrol 27: 3356–3367, 2016. doi: 10.1681/ASN.2015020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Clef N, Verhulst A, D’Haese PC, Vervaet BA. Unilateral renal ischemia-reperfusion as a robust model for acute to chronic kidney injury in mice. PLoS One 11: e0152153, 2016. doi: 10.1371/journal.pone.0152153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey AS, James MT. Acute kidney Iinjury. Ann Intern Med 167: ITC66–ITC80, 2017. doi: 10.7326/AITC201711070. [DOI] [PubMed] [Google Scholar]
- 25.Lynch MR, Tran MT, Parikh SM. PGC1α in the kidney. Am J Physiol Renal Physiol 314: F1–F8, 2018. doi: 10.1152/ajprenal.00263.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malek M, Nematbakhsh M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J Renal Inj Prev 4: 20–27, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molitoris BA, Kinne R. Ischemia induces surface membrane dysfunction. Mechanism of altered Na+-dependent glucose transport. J Clin Invest 80: 647–654, 1987. doi: 10.1172/JCI113117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377: 644–657, 2017. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 29.Nespoux J, Patel R, Hudkins KL, Huang W, Freeman B, Kim YC, Koepsell H, Alpers CE, Vallon V. Gene deletion of the Na+-glucose cotransporter SGLT1 ameliorates kidney recovery in a murine model of acute kidney injury induced by ischemia-reperfusion. Am J Physiol Renal Physiol 316: F1201–F1210, 2019. doi: 10.1152/ajprenal.00111.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nespoux J, Vallon V. SGLT2 inhibition and kidney protection. Clin Sci (Lond) 132: 1329–1339, 2018. doi: 10.1042/CS20171298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nespoux J, Vallon V. Renal effects of SGLT2 inhibitors: an update. Curr Opin Nephrol Hypertens 29: 190–198, 2020. doi: 10.1097/MNH.0000000000000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neuen BL, Young T, Heerspink HJL, Neal B, Perkovic V, Billot L, Mahaffey KW, Charytan DM, Wheeler DC, Arnott C, Bompoint S, Levin A, Jardine MJ. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 7: 845–854, 2019. doi: 10.1016/S2213-8587(19)30256-6. [DOI] [PubMed] [Google Scholar]
- 33.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu P-L, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380: 2295–2306, 2019. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 34.Pill J, Kraenzlin B, Jander J, Sattelkau T, Sadick M, Kloetzer H-M, Deus C, Kraemer U, Gretz N. Fluorescein-labeled sinistrin as marker of glomerular filtration rate. Eur J Med Chem 40: 1056–1061, 2005. doi: 10.1016/j.ejmech.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 35.Qi Z, Whitt I, Mehta A, Jin J, Zhao M, Harris RC, Fogo AB, Breyer MD. Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol 286: F590–F596, 2004. doi: 10.1152/ajprenal.00324.2003. [DOI] [PubMed] [Google Scholar]
- 36.Rieg T, Masuda T, Gerasimova M, Mayoux E, Platt K, Powell DR, Thomson SC, Koepsell H, Vallon V. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am J Physiol Renal Physiol 306: F188–F193, 2014. doi: 10.1152/ajprenal.00518.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song P, Huang W, Onishi A, Patel R, Kim YC, van Ginkel C, Fu Y, Freeman B, Koepsell H, Thomson S, Liu R, Vallon V. Knockout of Na+-glucose cotransporter SGLT1 mitigates diabetes-induced upregulation of nitric oxide synthase NOS1 in the macula densa and glomerular hyperfiltration. Am J Physiol Renal Physiol 317: F207–F217, 2019. doi: 10.1152/ajprenal.00120.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steel RGD, Torrie JH, Dicky DA. Principles and Procedures of Statistics, A Biometrical Approach (3rd ed). New York: McGraw Hill, Inc., 1997. [Google Scholar]
- 39.Taylor SI, Blau JE, Rother KI. Possible adverse effects of SGLT2 inhibitors on bone. Lancet Diabetes Endocrinol 3: 8–10, 2015. doi: 10.1016/S2213-8587(14)70227-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomson SC, Vallon V. Renal effects of sodium-glucose co-transporter inhibitors. Am J Cardiol 124, Suppl 1: S28–S35, 2019. doi: 10.1016/j.amjcard.2019.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uthman L, Baartscheer A, Bleijlevens B, Schumacher CA, Fiolet JWT, Koeman A, Jancev M, Hollmann MW, Weber NC, Coronel R, Zuurbier CJ. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na+/H+ exchanger, lowering of cytosolic Na+ and vasodilation. Diabetologia 61: 722–726, 2018. doi: 10.1007/s00125-017-4509-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uthman L, Nederlof R, Eerbeek O, Baartscheer A, Schumacher C, Buchholtz N, Hollmann MW, Coronel R, Weber NC, Zuurbier CJ. Delayed ischaemic contracture onset by empagliflozin associates with NHE1 inhibition and is dependent on insulin in isolated mouse hearts. Cardiovasc Res 115: 1533–1545, 2019. doi: 10.1093/cvr/cvz004. [DOI] [PubMed] [Google Scholar]
- 43.Vallon V, Gerasimova M, Rose MA, Masuda T, Satriano J, Mayoux E, Koepsell H, Thomson SC, Rieg T. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Renal Physiol 306: F194–F204, 2014. doi: 10.1152/ajprenal.00520.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vallon V, Platt KA, Cunard R, Schroth J, Whaley J, Thomson SC, Koepsell H, Rieg T. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol 22: 104–112, 2011. doi: 10.1681/ASN.2010030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vallon V, Rose M, Gerasimova M, Satriano J, Platt KA, Koepsell H, Cunard R, Sharma K, Thomson SC, Rieg T. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol 304: F156–F167, 2013. doi: 10.1152/ajprenal.00409.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia 60: 215–225, 2017. doi: 10.1007/s00125-016-4157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Westenfelder C, Arevalo GJ, Crawford PW, Zerwer P, Baranowski RL, Birch FM, Earnest WR, Hamburger RK, Coleman RD, Kurtzman NA. Renal tubular function in glycerol-induced acute renal failure. Kidney Int 18: 432–444, 1980. doi: 10.1038/ki.1980.156. [DOI] [PubMed] [Google Scholar]
- 48.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde A-M, Sabatine MS; DECLARE–TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 380: 347–357, 2019. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 49.Wright EM, Loo DDF, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 91: 733–794, 2011. doi: 10.1152/physrev.00055.2009. [DOI] [PubMed] [Google Scholar]
- 50.Yaribeygi H, Atkin SL, Butler AE, Sahebkar A. Sodium-glucose cotransporter inhibitors and oxidative stress: an update. J Cell Physiol 234: 3231–3237, 2019. doi: 10.1002/jcp.26760. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Nakano D, Guan Y, Hitomi H, Uemura A, Masaki T, Kobara H, Sugaya T, Nishiyama A. A sodium-glucose cotransporter 2 inhibitor attenuates renal capillary injury and fibrosis by a vascular endothelial growth factor-dependent pathway after renal injury in mice. Kidney Int 94: 524–535, 2018. doi: 10.1016/j.kint.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 52.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373: 2117–2128, 2015. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 53.Zuk A, Bonventre JV. Acute kidney injury. Annu Rev Med 67: 293–307, 2016. doi: 10.1146/annurev-med-050214-013407. [DOI] [PMC free article] [PubMed] [Google Scholar]