Abstract
Ubiquitination of the epithelial Na+ channel (ENaC) in epithelial cells may influence trafficking and hormonal regulation of the channels. We assessed ENaC ubiquitination (ub-ENaC) in mouse and rat kidneys using affinity beads to capture ubiquitinated proteins from tissue homogenates and Western blot analysis with anti-ENaC antibodies. Ub-αENaC was observed primarily as a series of proteins of apparent molecular mass of 40–70 kDa, consistent with the addition of variable numbers of ubiquitin molecules primarily to the NH2-terminal cleaved fragment (~30 kDa) of the subunit. No significant Ub-βENaC was detected, indicating that ubiquitination of this subunit is minimal. For γENaC, the protein eluted from the affinity beads had the same apparent molecular mass as the cleaved COOH-terminal fragment of the subunit (~65 kDa). This suggests that the ubiquitinated NH2 terminus remains attached to the COOH-terminal moiety during isolation through disulfide bonds. Consistent with this, under nonreducing conditions, eluates contained material with increased molecular mass (90–150 kDa). In mice with a Liddle syndrome mutation (β566X) deleting a putative binding site for the ubiquitin ligase neural precursor cell expressed developmentally downregulated 4-2, the amount of ub-γENaC was reduced as expected. To assess aldosterone dependence of ubiquitination, we fed rats either control or low-Na+ diets for 7 days before kidney harvest. Na+ depletion increased the amounts of ub-αENaC and ub-γENaC by three- to fivefold, probably reflecting increased amounts of fully cleaved ENaC. We conclude that ubiquitination occurs after complete proteolytic processing of the subunits, contributing to retrieval and/or disposal of channels expressed at the cell surface. Diminished ubiquitination does not appear to be a major factor in aldosterone-dependent ENaC upregulation.
Keywords: epithelial Na+ channel, Liddle syndrome, mice, proteolysis, rats
INTRODUCTION
Proper trafficking and function of the epithelial Na+ channel (ENaC) are essential for maintaining Na+ balance, extracellular fluid volume, and blood pressure (21, 32–34). Aldosterone, an adrenal corticosteroid that plays a major role in Na+ homeostasis, acts in part by increasing the surface expression of ENaC subunits (13, 14, 22, 23).
As with other proteins, ubiquitination influences the trafficking of ENaC protein within the cell, including the rates of internalization from the cell surface and degradation. Staub et al. (42) first showed that αENaC and γENaC subunits can be ubiquitinated in heterologous expression systems. Subsequently, ubiquitination of βENaC was also reported (46). Mutation of NH2-terminal lysines to arginines in these subunits inhibited ubiquitination and increased channel activity in Xenopus oocytes (42). Neural precursor cell expressed developmentally downregulated 4-2 (Nedd4-2) is a ubiquitin ligase that appears to mediate ENaC ubiquitination (17). Coexpression of Nedd4-2 in human embryonic kidney (HEK)-293 cells increased ubiquitination of ENaC at the cell surface and accelerated its internalization and degradation (46). Subsequent work indicated the involvement of impaired ubiquitination in the hyperactivation of ENaC in Liddle syndrome, a monogenic form of hypertension most commonly caused by mutations in PY motifs in the cytoplasmic COOH terminus of βENaC or γENaC (37, 40). Nedd4-2 binds to these regions of the subunits, and overexpression of the ligase inhibits channel activity in oocytes. Subsequently, a connection was made between these effects and the action of aldosterone. Serum/glucocorticoid-regulated kinase 1 (Sgk1), an aldosterone-induced protein (8, 25), phosphorylates Nedd4-2 and reduces its binding to ENaC (10, 38). This suggested the plausible hypothesis that the hormone might increase ENaC surface expression through diminished ubiquitination and inhibition of channel internalization and degradation.
These phenomena were obtained in heterologous expression systems but have not been thoroughly examined in native tissues. Here, we explored ubiquitination of ENaC in mouse and rat kidneys and examined its role in the regulation of channel activity. We addressed several specific questions. First, which, if any, ENaC subunits are ubiquitinated in vivo? Second, does a mutation mimicking Liddle syndrome reduce ubiquitination? Third, does aldosterone reduce levels of ENaC ubiquitination?
METHODS
Animals.
All procedures using animals were approved by the Institutional Animal Care and Use Committee of Weill Cornell Medical College or by the responsible regulatory authority at the city of Erlangen (I/39/EE006) and the animal welfare officer of the Friedrich-Alexander-Universität Erlangen-Nürnberg (TS-11/2017 ZellPhys).
Sprague-Dawley rats (150–250 g) and C57BL mice (20–30 g, Charles River Laboratories, Kingston, NY) were raised free of viral infections and fed either a Na+-deficient diet containing 0.004% Na+ by weight or a matched diet supplemented with 1% NaCl (MP Biomedicals, Solon, OH). Both male and female animals were used in approximately equal numbers. We observed no systematic differences between the sexes, consistent with previous reports (20, 45), and therefore combined data for presentation and analysis. Animals were euthanized with isoflurane anesthesia, and the kidneys were removed and either processed immediately or frozen for future processing.
An established mouse model for Liddle syndrome (9, 31) was a generous gift of Dr. Edith Hummler (University of Lausanne). This mouse line was maintained at Erlangen as previously described (3, 26). To ensure stability of the genetic background, the mouse line was periodically backcrossed to the C57BL/6J strain (Jax stock no. 000664, Charles River Laboratories, Sulzfeld, Germany). Animals used in the present study were 10–12 wk old (7 female animals weighing 17–21 g and 6 male animals weighing 25–30 g) and were either homozygous for the R566X Liddle syndrome mutation in βENaC (L/L or Liddle mice) or homozygous for wild-type (WT) ENaC. Seven days before euthanasia, six L/L mice (3 male and 3 female mice) and seven WT mice (3 male and 4 female mice) were switched from a standard salt diet (3.2 g Na+/kg of food, tap water) to a low-Na+ diet (0.13 g Na+/kg, deionized water) with food and fluid intake ad libitum. Mice were euthanized by CO2 inhalation followed by terminal interruption of circulation. Kidneys were removed, quickly frozen, and shipped on dry ice to New York for further processing.
Identification of ubiquitinated subunits.
We followed the procedure proposed by the manufacturer of the ubiquitination detection kit (Signal Seeker, Cytoskeleton, Denver, CO). Normally, about four-fifths of one mouse kidney or about one-half of one rat kidney was quickly homogenized in 1.5 or 4 mL, respectively, of ice-cold BlastR lysis buffer with a glass Dounce homogenizer. Lysis buffer contained deubiquitination inhibitors [10 mM N-ethylmaleimide and 50 μM N,N,N,N’-tetrakis(2-pyridylmethyl)ethylenediamine] and a protease inhibitor cocktail included in the kit. The remaining parts of each kidney were separately homogenized in sucrose lysis buffer and prepared for Western blot analysis as either a whole kidney homogenate or a microsomal pellet as previously described (12, 14). The viscous Blast lysate was transferred into a syringe plunger filter included in the kit to remove DNA and unbroken tissue fragments. One milliliter of the filtered lysate was mixed with 4 mL cold BlastR dilution buffer and centrifuged at 10,000 g for 10 min in a cold room. The supernatant was then collected, aliquoted, and frozen for further processing. Protein concentration of the diluted lysate was measured using the Lowry method. To prepare it for affinity isolation of ubiquitinated proteins, it was further diluted to obtain a protein concentration of ~1 mg/mL with a mixture of BlastR lysis (1 part) and BlastR dilution buffers (4 parts). We used beads containing cross-linked tandem ubiquitin-binding elements that capture both mono- and polyubiquitinated proteins with high affinity and also control beads (identical to the active beads except for two point mutations in the ubiquitin-binding peptides) with markedly reduced ubiquitin binding. Thirty microliters of either bead suspension were incubated with 1.5 mL lysate on an oscillating platform for 2 h in a cold room. Subsequently, the beads were washed three times with 1 mL cold wash buffer for 5 min each. Finally, the captured proteins were eluted from the beads at room temperature for 5 min with 40 μL of 1× elution buffer (loading buffer without reducing agent). After spinning, the supernatant was saved, 5 μL of 500 mM DDT were added, and the mixture was heated at 70°C for 10 min. In some experiments, DTT was omitted to achieve nonreducing conditions.
Western blot analysis.
For Western blot analysis, 45 μL of ubiquitin-affinity eluate or 50 μg of total kidney protein were loaded into one lane of a gel. The eluate represents 1,500 μg of total kidney protein, a 30× amplication factor. Samples were electrophoresed on 4–12% bis-Tris gels (Invitrogen), and proteins were transferred electrophoretically to polyvinylidene difluoride membranes. For nonreducing gels, antioxidants were omitted from the running buffer. After being blocked, membranes were incubated overnight at 4°C with primary antibodies. Anti-rabbit IgG conjugated with alkaline phosphatase was used as a secondary antibody. Bound antibody was visualized on autoradiography film (HyBlot CL, Denville Scientific) or with a Syngene Pxi imager using a chemiluminescence substrate (Western Breeze, Invitrogen). Semiquantitative densitometry of protein bands was performed with background subtraction using AdobePhotoshop CS5. After detection of the ENaC subunits, images in blots of ubiquitinated proteins, and the membranes were reprobed without stripping with anti-ubiquitin-horseradish peroxidase antibody to confirm that ubiquitinated proteins had been selectively enriched using the affinity beads. Blots of homogenates and microsomal fractions were stained with Coomassie blue to assess protein loading in individual lanes, which varied by <10%.
Antibodies.
Polyclonal antibodies against the β- and γ-subunits of rat ENaC were based on short peptide sequences in the COOH termini as previously described (12, 24). Antisera were purified using peptide-linked agarose bead affinity columns (Sulfolink Kit, Pierce Biotechnology) and used at 1:500 dilution. The antibody against the NH2 terminus of mouse αENaC (39) was a gift of Prof. Johannes Loffing (Univeristy of Zürich), and it was used at 1:1,000 dilution. The antibody against ubiquitin-horseradish peroxidase was provided in the ubiquitin detection kit (Cytoskeleton).
Statistics.
For analysis of quantitative data, values from different groups were compared using an unpaired two-tailed Student’s t test. P < 0.05 was considered significant.
RESULTS
Mouse kidney extracts were prepared for detection of ubiquitinated proteins as described in methods. Samples from individual animals were loaded onto separate lanes of the gel for Western blot analysis. Figure 1A shows staining with anti-ubiquitin-horseradish peroxidase. This procedure detects all ubiquitinated proteins, and individual peptides cannot be resolved. Compared with starting material, the eluate was highly enriched for ubiquitinated proteins. In contrast, very little ubiquitinated protein was detected in the eluate from control beads containing a peptide with reduced affinity for ubiquitin.
Fig. 1.
Pulldown of ubiquitinated proteins and the α-subunit of the epithelial Na+ channel (αENaC) from the mouse kidney. Lanes of the Western blot were loaded with 50 μg of whole kidney extract (input to the affinity beads), 45 μL of eluates from the ubiquitin-affinity beads, or 45 μL eluates from the control-affinity beads. A: blots were stained using anti-ubiquitin horseradish peroxidase. B: a similar blot stained with anti-αENaC antibody. Mice were fed either a control diet or a low-Na+ diet for 1 wk. Results are representative of at least 3 experiments with mouse and rat kidneys.
Figure 1B shows a similar blot stained for αENaC. The whole kidney extract showed two bands representing the subunit as previously described (12–14). The ~85-kDa species corresponds to the full-length subunit, while the smaller ~30-kDa band represents a NH2-terminal cleavage product; both of these forms increase in abundance in animals on a Na+-deficient diet for 1 wk (compare input lanes in Fig. 1B) as expected (12). The band just below 80 kDa may represent nonspecific staining as it is not seen in the rat kidney (Fig. 7). In the eluate from the ubiquitin-affinity beads, anti-αENaC antibody detected peptides with apparent molecular masses of 40–70 kDa. Since each ubiquitin molecule adds 8.6 kDa to the mass of the protein, this is consistent with mono- and polyubiquitination of the NH2-terminal cleavage product of αENaC, since the bands migrate faster than the full-length peptide. Some of the eluted material that reacted with anti-αENaC antibody had an apparent molecular mass of 110–150 kDa. This could represent ubiquitination of either the cleaved or full-length peptide. Effects of dietary Na+ restriction are discussed quantitatively below. The eluate from the control beads contained no material that interacted with the antibody. In contrast, we detected no βENaC protein in either the ubiquitin or control eluates (Fig. 2). This indicates that in lysis buffer (containing 0.3% SDS) the ENaC subunits dissociate, since if they remained together βENaC would be pulled down with the ubiquitinated αENaC and γENaC subunits.
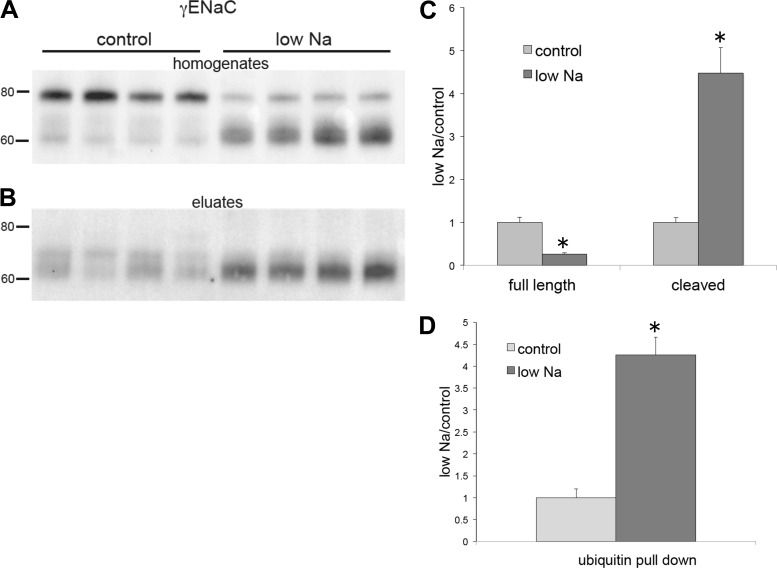
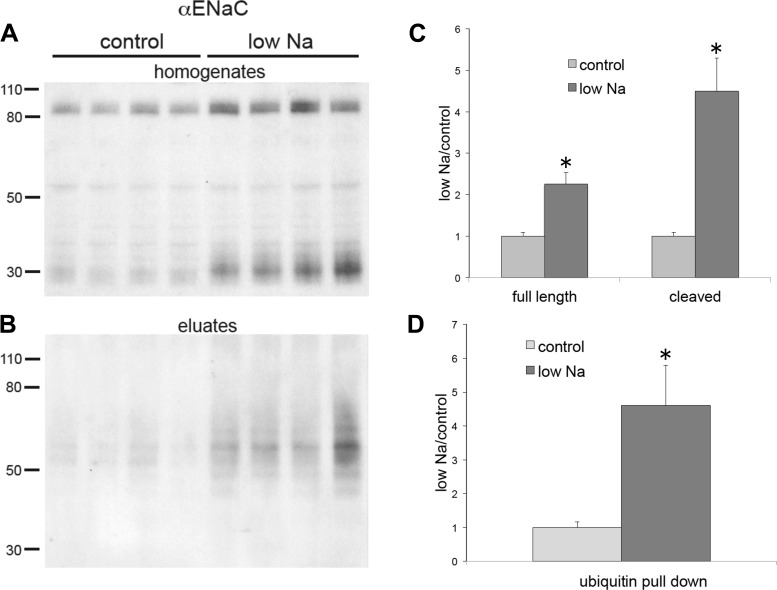
Fig. 7.
Expression of total and ubiquitinated γ-subunit of the epithelial Na+ channel (γENaC) from kidneys of rats on the control diet or low-Na+ diet. A: whole-kidney homogenates of animals fed either control-Na or low-Na food for 1 wk. Lanes of the Western blots were loaded with 30 μg protein. B: eluates from ubiquitin-affinity beads. Lanes were loaded with 45 μL of eluates. Blots were stained using anti-γENaC antibody. C: quantitation of expression in kidney homogenates. D: quantitation of the ubiquitin pulldown. Data were normalized to values for the control diet and are shown as means ± SE for 4 rats in each condition. Full-length γENaC: P = 0.0008; cleaved γENaC: P = 0.001; ubiquitinated γENaC: P = 0.0003 vs. control.
Fig. 2.
Pulldown of the ubiquitinated β-subunit of the epithelial Na+ channel (βENaC) from the mouse kidney. Lanes of the Western blot were loaded with 50 μg of whole kidney extract (input to the affinity beads), 45 μL of eluates from the ubiquitin-affinity beads, or 45 μL eluates from the control-affinity beads. Blots were stained using anti-βENaC antibody. Animals were fed either a control diet or a low-Na+ diet for 1 wk. The bottom bands in the lanes for the whole cell extracts represent nonspecific staining, as they are also present in the absence of the COOH-terminal epitope (see Fig. 5). Results are representative of at least 3 experiments with mouse and rat kidneys.
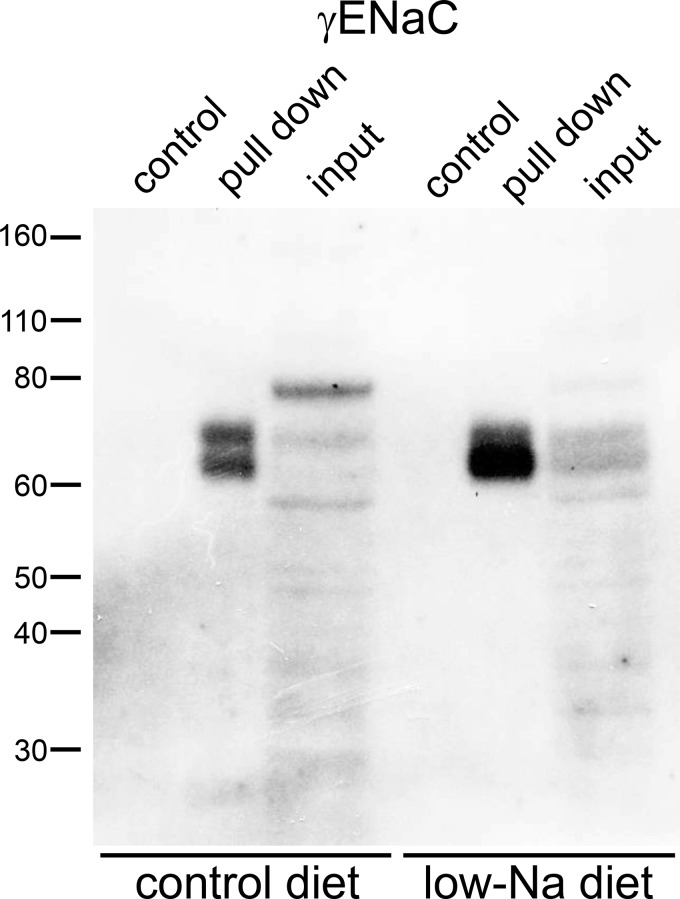
Figure 3 shows the analysis of ubiquitination of the γENaC subunit. Here, we could distinguish three separate bands in the whole kidney extract: the full-length subunit at ~80 kDa, a partially cleaved species at ~70 kDa, and a fully cleaved product at ~65 kDa. Unlike the case for αENaC, the cleaved forms represent COOH-terminal fragments, since the antibody is raised against a COOH-terminal peptide. Dietary Na+ depletion greatly increased the amount of cleaved subunits and decreased the amount of the full-length form, as previously reported (12–14). The eluate from the ubiquitin-affinity beads contained material that ran at the same apparent molecular mass as the cleaved bands from the input material. With Na+ depletion, much more of the fully cleaved subunit was eluted. Again, we detected no γENaC protein in the control eluates.
Fig. 3.
Pulldown of the ubiquitinated γ-subunit of the epithelial Na+ channel (γENaC) from the mouse kidney. Lanes of the Western blot were loaded with 50 μg of whole kidney extract (input to the affinity beads), 45 μL of eluates from the ubiquitin-affinity beads, or 45 μL eluates from the control-affinity beads. Blots were stained using anti-γENaC antibody. Animals were fed either a control diet or a low-Na+ diet for 1 wk. Results are representative of at least 3 experiments with mouse and rat kidneys.
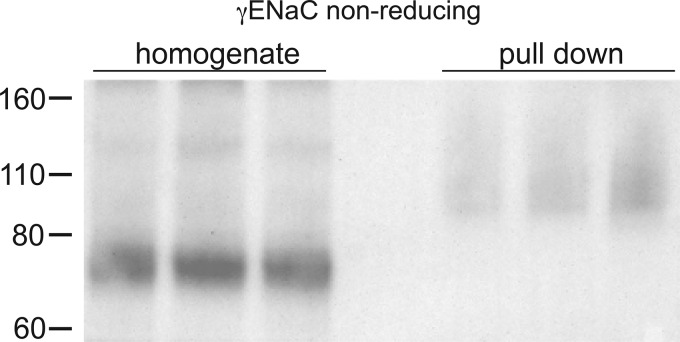
Based on the in vitro study (42), we expected ubiquitination of γENaC near the NH2 terminus, but this modification should increase the apparent molecular mass of the subunit. However if, like the αENaC subunit, ubiquitination is specific for the cleaved form of γENaC, the ubiquitinated NH2-terminal fragment would not be detected using our antibody. We therefore suspected that the COOH-terminal fragment remains connected to the ubiquitinated NH2-terminal fragment in the starting extract and is pulled down with the NH2 terminus by the affinity beads. Consistent with this idea, structural as well as functional studies have indicated that the two parts of the subunit are joined together by an extracellular disulfide bond, specifically between (mouse) γENaC C200 and C289 (27, 36). To test this further, we performed Western blot analysis under nonreducing conditions (Fig. 4). Here, total protein from the kidneys of Na+-depleted mice showed that most of the γENaC protein had an apparent molecular mass of 70–80 kDa; the fully cleaved 60- to 65-kDa fragment was not observed, presumably because the NH2- and COOH-terminal parts of the subunit were still attached. Under these conditions, anti-γENaC antibody detected material in the ubiquitin-affinity eluates with molecular masses between 90 and 150 kDa, consistent with ubiquitination of the S-S coupled subunit.
Fig. 4.
Pulldown of the ubiquitinated γ-subunit of the epithelial Na+ channel (γENaC) from mouse kidneys analyzed under nonreducing conditions. Lanes of the Western blot were loaded with 15 μg of whole kidney (homogenate) or 40 μL of eluates from the ubiquitin-affinity beads (pulldown) from three different animals fed a low-Na+ diet for 1 wk. Blots were stained using anti-γENaC antibody. Results are representative of 3 experiments from rats and mice.
In Liddle syndrome, mutations or truncations in the cytoplasmic COOH terminus of βENaC or γENaC are thought to abrogate binding of the ubiquitin ligase Nedd4-2 and therefore to reduce ENaC ubiquitination (17, 19, 46). To test this in vivo, we isolated ubiquitinated proteins from kidneys of mice with a truncation at βENaC R566, mimicking one of the human mutations (31). We have previously shown that in Na+-depleted mice the enhancement of ENaC function measured by electrophysiology in both the cortical collecting duct (9), late distal convoluted tubule/connecting segment (26), and colon (3) was exaggerated in animals with the mutation. Under these conditions, plasma aldosterone was increased in both groups, although to a larger extent in WT animals (3). In contrast, L/L mice had larger amiloride-sensitive short-circuit currents in the colon (3) and whole cell ENaC currents in distal nephron segments (26).
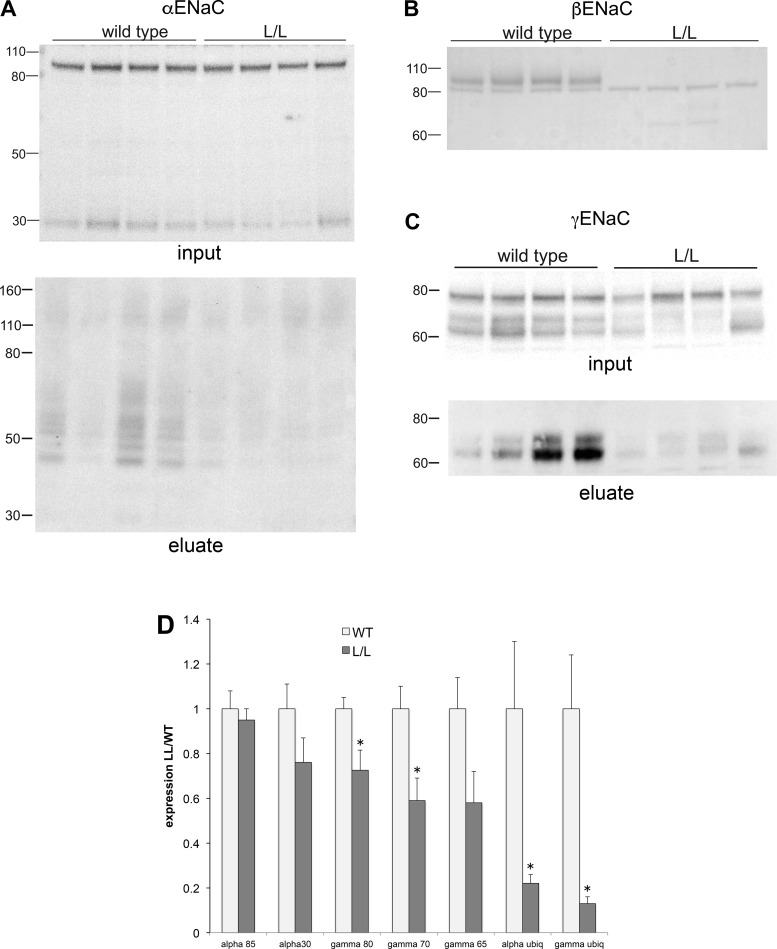
Figure 5 shows Western blots for ENaC in total kidney homogenates and for γENaC ubiquitin-affinity eluates from WT and L/L mice fed a low- Na+ diet for 7 days. Expression of full-length and cleaved αENaC was similar for WT and L/L animals (Fig. 5A). βENaC could not be detected in L/L kidneys since the antibody is directed against a COOH-terminal epitope that is lost in the truncated subunit (Fig. 5B). The sharp band at 80 kDa seen with both groups of mice presumably represents nonspecific antibody binding to another protein. Overall, γENaC expression was diminished in L/L animals compared with control animals (Fig. 5C). This reduction of 30–40% was similar for all forms of the subunit (full length, partially cleaved, and fully cleaved), although in the last case the change was not quite statistically significant. The effect of the mutation on the amount of ubiquitinated γENaC was more dramatic, with an average reduction of ~90%. This is in agreement with the idea that the COOH terminus of βENaC is a key site for the interaction of ubiquitinating enzymes with the channel.
Fig. 5.
Expression and pulldown of the ubiquitinated γ-subunit of the epithelial Na+ channel (γENaC) from kidneys of wild-type (WT) mice or those with the Liddle syndrome mutation β566X (L/L). Animals were fed a low-Na+ diet for 1 wk. A–C: kidney lysates. Each lane of the Western blots was loaded with 50 μg protein from a different WT or L/L mouse and stained using antibodies against the α-subunit of the epithelial Na+ channel (αENaC; A), β-subunit of the epithelial Na+ channel (βENaC; B), and γENaC (C). A and C, bottom, show ubiquitin-affinity bead eluates. Lanes were loaded with 45 μL of eluates and stained using antibodies against αENaC (A) or γENaC (C). D: quantitation of the blots in A–C. Data were normalized to values in WT mice and are shown as means ± SE for 7 WT mice and 6 L/L mice. 80-kDa γENaC: P = 0.02; 70-kDa γENaC: P = 0.01; 65-kDa γENaC: P = 0.06; ubiquitinated αENaC: P = 0.04; and ubiquitinated γENaC: P = 0.007 vs. WT mice.
We next tested whether increases in circulating aldosterone elicited by a Na+-deficient diet altered ENaC ubiquitination. For these measurements, we used rat kidneys although similar effects are observed in mice (see Figs. 1–3). Animals were fed either low-Na+ chow or a matched control diet for 7 days. Previous experiments revealed large increases in plasma aldosterone from 20 to 1,200 ng/dL with the same protocol of dietary Na+ restriction (28). Figure 6 shows effects on αENaC in whole-kidney extracts (Fig. 6A) and in ubiquitin-affinity eluates (Fig. 6B). Figure 6, C and D, shows the quanitification of the blots. Na+ depletion produced a 2.3-fold increase in the full-length subunit and a 4.5-fold increase in the cleaved form of the subunit, similar to previous results (12). A third band appeared at 50 kDa. We have not identified this species but suspect that it is nonspecific as it is not present in the mouse kidney (see Fig. 1) and did not change with Na+ depletion. To quantify ubiquitination, we measured the total signal over the region of the blot ranging from 50 to 80 kDa. In Na+-depleted animals, this increased by 4.6-fold.
Fig. 6.
Expression of total and ubiquitinated α-subunit of the epithelial Na+ channel (αENaC) from kidneys of rats on the control diet or low-Na+ diet. A: whole kidney homogenates of animals fed either control diet or low-Na+ food for 1 wk. Lanes of the Western blots were loaded with 30 μg protein. B: eluates from ubiquitin-affinity beads. Lanes were loaded with 45 μL of eluates. Blots were stained using anti-αENaC antibody. C: quantitation of expression in kidney homogenates. D: quantitation of the ubiquitin pulldown. Data were normalized to values for the control diet and are shown as means ± SE for 4 rats in each condition. Full-length αENaC: P = 0.005; cleaved αENaC: P = 0.0007; ubiquitinated αENaC: P = 0.04 vs. control.
Figure 7 shows a similar analysis of γENaC expression. In whole kidney extracts, the abundance of the full-length subunit decreased by 74%, while that of the fully cleaved form increased 4.5-fold, consistent with previous results (12–14). The amount of cleaved γENaC in the ubiquitin-affinity eluate increased by 4.3-fold. Thus, for both subunits, Na+ depletion increased ubiquitination in proportion to the amount of their fully processed (i.e., cleaved) forms. ENaC in mouse kidneys showed similar effects (Figs. 1–3), but these were not assessed systematically.
DISCUSSION
Comparison with in vitro results.
Our basic results on the identification of ubiquitinated ENaC subunits are a striking confirmation of previous studies of ENaC modification in heterologous expression systems. Staub et al. (42) first demonstrated ubiquitination of ENaC subunits by isolating ubiquitinated proteins in HEK-293 cells cotransfected with both ENaC and ubiquitin. They further showed that the ubiquitination occurred on αENaC and γENaC, but not βENaC, and identified NH2-terminal lysines on these subunits as the major sites that were modified. Our results recapitulate all of these findings in the kidneys in vivo. In extracts of mouse and rat kidneys, ubiquitin-affinity-purified eluates contained αENaC and γENaC but not βENaC. This differs from subsequent studies showing ubiquitination of the β subunit in other cell lines (44, 46). Our results were also consistent with specific modification of the NH2 termini. We showed ubiquitination of the NH2-terminal fragment of αENaC directly as peptides of molecular mass higher than that of the cleaved but lower than that of the full-length subunit (Fig. 1). Results with γENaC also indicated exclusive ubiquitination of its NH2 terminus. Ubiquitin-affinity purification of this subunit showed modification of the conjoined NH2- and COOH-terminal cleavage fragments but not of the separated COOH-terminal fragment (Figs. 3 and 4). Together, these results provide strong evidence for the ubiquitination of ENaC subunits. Definitive proof will require proteomic analysis of ENaC protein.
Selectivity of ubiquitination.
The α- and γ-subunits of ENaC undergo proteolytic cleavage during the maturation process (18, 35). These steps are thought to occur in both the Golgi apparatus and, in the case of γENaC, at the plasma membrane. Most ENaC at the apical surface of the kidney and in the early endosome compartment, as well as all ENaC in urinary exosomes, is in the cleaved form (13, 14). Here, we showed selective ubiquitination of the cleaved subunits. We cannot exclude some modification of full-length αENaC, since we did see some material in the pulldown at molecular masses greater than that of the uncleaved subunit, but for γENaC the specificity was quite strong. This suggests that this modification affects channels either at the plasma membrane or after retrieval within endosomes. This could reflect either restriction of the ubiquitinating enzymes to these compartments, enhanced susceptibility of the mature channels to these enzymes, or activation of ubiquitin ligase bound to channels with cleaved subunits. Further studies will be required to distinguish these possibilities. To our knowledge, this is the first direct demonstration of selective ubiquitination of the cleaved forms of ENaC. However, Kabra et al. (16) reported that trypsin cleavage decreases the surface lifetime of mature (cleaved) but not immature ENaC in HEK-293 cells. This effect likely involves increased ubiquitination of the cleaved species. Furthermore, our finding agrees with previous studies showing that overexpression of a deubiquitinating enzyme (ubiquitin-specific peptidase 8) specifically increased the surface lifetime of mature subunits (47). Knight et al. (19) showed a negative correlation between ubiquitination and cleavage of ENaC. As discussed by those authors, one interpretation of this result is that cleavage increases the susceptibility of the channels to ubiquitination, leading to selective removal of the cleaved species from the membrane. This interpretation is consistent with our results.
In vitro studies have indicated a role for ubiquitination in the process of endoplasmic reticulum-associated degradation (5). This suggests that subunits that have not undergone proteolytic cleavage in the Golgi or at the surface would be ubiquitinated. We cannot rule out the presence of such a population in native kidney tissue. It is possible, for example, that ubiquitinated, unprocessed subunits are degraded so quickly that they do not accumulate to detectable levels. It is also possible, however, that this degradation pathway is more important in heterologous overexpression systems than in native tissue.
Liddle syndrome.
Liddle syndrome is a monogenic form of salt-sensitive hypertension that is most commonly caused by mutations or truncations in the COOH termini of βENaC or γENaC subunits (7). The PY motifs that are most often affected by the mutations were shown to be sites of interaction with the WW domains of Nedd4-2 (17, 41, 46). This led to the predictions that the syndrome involves decreased ubiquitination of the subunits, leading to decreased rates of channel retrieval from the cell surface and of subsequent proteasomal degradation. Higher expression levels at the apical surface then produce overreabsorption of Na+ from urine, expanded extracellular volume, and elevated blood pressure.
In the present study, the first of these predictions was verified in mice harboring a Liddle syndrome truncation (βENaC R566X); ubiquitination of γENaC in these mice was strongly reduced (Fig. 5). Although L/L mice had 20–30% lower total amounts of the γ-subunit compared with WT mice, this cannot account for the entire reduction in γENaC ubiquitination. Thus, the COOH terminus of βENaC, which is lacking in L/L mice, appears to be crucial for the ubiquitination of γENaC, presumably by binding Nedd4-2.
Given that ubiquitin presumably facilitates internalization and degradation of the channels (17, 42, 46), we were somewhat surprised that the total amount of γENaC protein, particularly that of the cleaved species, was not higher in L/L animals than in WT animals, especially under conditions of Na+ depletion in which ENaC-mediated currents are larger (9, 26). However, we measured the total cellular content, and it is possible that channel abundance increases specifically at the surface. In addition, in a previous study (3), L/L mice on a low-Na+ diet had lower levels of plasma aldosterone than did WT mice. This could contribute to the decreased expression of γENaC in L/L mice relative to WT mice.
Mechanism of action of aldosterone.
One well-documented effect of aldosterone on ENaC in the kidney is to increase surface expression of subunit proteins (13, 14, 22, 23, 26). One mechanism proposed to account for this effect involves the induction of the kinase SGK1, which phosphorylates Nedd4-2, reducing binding of its WW domain to PY motifs on βENaC and γENaC and the subsequent ubiquitination of the channel (10, 38). All of these steps have been verified in vitro, but their relevance in vivo is unclear. In A6 cells, the only aldosterone-responsive epithelium in which surface lifetime has been measured, aldosterone had no measurable effect on the lifetime of ENaC protein at the cell surface (1). Furthermore, in the Xenopus laevis oocyte expression system, the stimulatory effect of coexpressed SGK1 on ENaC persisted even when the COOH termini of all three subunits were truncated (2). If inhibition of ubiquitination were the main mechanism underlying stimulation of ENaC in the kidney by aldosterone or Na+ depletion, then the amounts of ubiquitinated subunits should be lower than, or at most equal to, those under control conditions. Instead, we found an increase in ubiquitination that correlates with the overall levels of mature subunits. In contrast, this result is expected from a model in which the main effect of the hormone is to promote the forward trafficking of ENaC to the apical membrane, with the amounts of ubiquitinated subunits reflecting the size of the pool of mature channels that are susceptible to the modification. We previously invoked this model to explain changes in ENaC expression in various subcellular compartments (14).
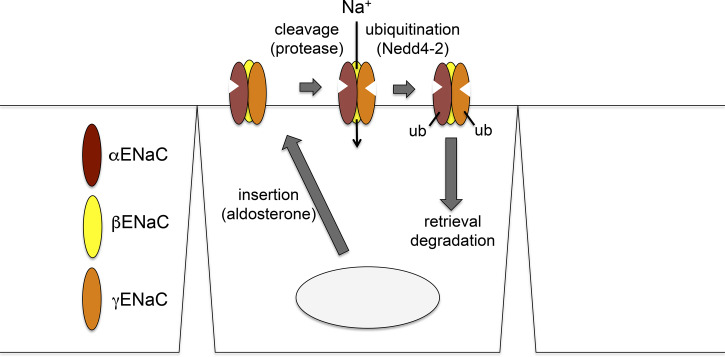
Our working model for ENaC processing and trafficking is shown in Fig. 8. The major steps are as follows. Step 1 is movement through the endoplasmic reticulum and Golgi to the plasma membrane. We propose that this is the major step regulated by aldosterone, based on the results in shown Figs. 6 and 7 and those presented previously (14). The α-subunit is fully cleaved during step 2: proteolytic cleavage of γENaC at the cell surface. Here, we invoke the hypothesis of Kleyman and colleagues (4) that this subunit is cleaved once in the intracellular compartment (probably the Golgi apparatus) by furin or furin-like convertases and again by an extracellular or membrane-bound proteases, releasing an inhibitory peptide. The enzyme or enzymes responsible for this step in vivo have not yet been identified, but candidates include prostasin (4, 43) and kallikrein (29, 30). This second cleavage step converts silent or near-silent channels to active forms (6, 11, 15), stimulating Na+ influx. Step 3 is ubiquitination of cleaved subunits. Once the channels have been moved to the cell surface and proteolytically activated, they are ubiquitinated on α- and γ-subunits, presumably through Nedd4-2 ubiquitin ligase. Step 4 is internalization/degradation of ENaC. The ubiquitinated channels are rapidly internalized and degraded. In this scheme, the rates of ubiquitination, internalization, and degradation are not strongly regulated by aldosterone but depend on the number of channels at the surface. This permits the tight regulation of active channels at the surface by limiting their lifetime. The process is analogous to the inactivation of voltage-gated ion channels that shuts off ion flows after the channels open.
Fig. 8.
Model for epithelial Na+ channel (ENaC) trafficking in the kidney. The major effect of aldosterone is on the rate of trafficking to the apical membrane. Ubiquitination accelerates the rate of internalization and degradation of the channels. These rates depend on the ubiquitin ligase neural precursor cell expressed developmentally downregulated 4-2 (Nedd4-2) and on the number of channels at the surface but are not strongly influenced by aldosterone.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-111380 from the National Institutes of Health and Deutsche Forschungsgemeinschaft (German Research Foundation), Project 387509280, SFB 1350.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.F., M.B., C.K., and L.G.P. conceived and designed research; G.F., M.B., and L.G.P. performed experiments; G.F. and L.G.P. analyzed data; G.F., C.K., and L.G.P. interpreted results of experiments; L.G.P. prepared figures; L.G.P. drafted manuscript; G.F., M.B., C.K., and L.G.P. edited and revised manuscript; G.F., M.B., C.K., and L.G.P. approved final version of manuscript.
REFERENCES
- 1.Alvarez de la Rosa D, Li H, Canessa CM. Effects of aldosterone on biosynthesis, traffic, and functional expression of epithelial sodium channels in A6 cells. J Gen Physiol 119: 427–442, 2002. doi: 10.1085/jgp.20028559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez de la Rosa D, Zhang P, Náray-Fejes-Tóth A, Fejes-Tóth G, Canessa CM. The serum and glucocorticoid kinase sgk increases the abundance of epithelial sodium channels in the plasma membrane of Xenopus oocytes. J Biol Chem 274: 37834–37839, 1999. doi: 10.1074/jbc.274.53.37834. [DOI] [PubMed] [Google Scholar]
- 3.Bertog M, Cuffe JE, Pradervand S, Hummler E, Hartner A, Porst M, Hilgers KF, Rossier BC, Korbmacher C. Aldosterone responsiveness of the epithelial sodium channel (ENaC) in colon is increased in a mouse model for Liddle’s syndrome. J Physiol 586: 459–475, 2008. doi: 10.1113/jphysiol.2007.140459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruns JB, Carattino MD, Sheng S, Maarouf AB, Weisz OA, Pilewski JM, Hughey RP, Kleyman TR. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the gamma-subunit. J Biol Chem 282: 6153–6160, 2007. doi: 10.1074/jbc.M610636200. [DOI] [PubMed] [Google Scholar]
- 5.Buck TM, Brodsky JL. Epithelial sodium channel biogenesis and quality control in the early secretory pathway. Curr Opin Nephrol Hypertens 27: 364–372, 2018. doi: 10.1097/MNH.0000000000000438. [DOI] [PubMed] [Google Scholar]
- 6.Caldwell RA, Boucher RC, Stutts MJ. Serine protease activation of near-silent epithelial Na+ channels. Am J Physiol Cell Physiol 286: C190–C194, 2004. doi: 10.1152/ajpcell.00342.2003. [DOI] [PubMed] [Google Scholar]
- 7.Chang SS, Grunder S, Hanukoglu A, Rösler A, Mathew PM, Hanukoglu I, Schild L, Lu Y, Shimkets RA, Nelson-Williams C, Rossier BC, Lifton RP. Mutations in subunits of the epithelial sodium channel cause salt wasting with hyperkalaemic acidosis, pseudohypoaldosteronism type 1. Nat Genet 12: 248–253, 1996. doi: 10.1038/ng0396-248. [DOI] [PubMed] [Google Scholar]
- 8.Chen SY, Bhargava A, Mastroberardino L, Meijer OC, Wang J, Buse P, Firestone GL, Verrey F, Pearce D. Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proc Natl Acad Sci USA 96: 2514–2519, 1999. doi: 10.1073/pnas.96.5.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahlmann A, Pradervand S, Hummler E, Rossier BC, Frindt G, Palmer LG. Mineralocorticoid regulation of epithelial Na+ channels is maintained in a mouse model of Liddle’s syndrome. Am J Physiol Renal Physiol 285: F310–F318, 2003. doi: 10.1152/ajprenal.00016.2003. [DOI] [PubMed] [Google Scholar]
- 10.Debonneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, Münster C, Chraïbi A, Pratt JH, Horisberger JD, Pearce D, Loffing J, Staub O. Phosphorylation of Nedd4-2 by Sgk1 regulates epithelial Na(+) channel cell surface expression. EMBO J 20: 7052–7059, 2001. doi: 10.1093/emboj/20.24.7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diakov A, Bera K, Mokrushina M, Krueger B, Korbmacher C. Cleavage in the gamma-subunit of the epithelial sodium channel (ENaC) plays an important role in the proteolytic activation of near-silent channels. J Physiol 586: 4587–4608, 2008. doi: 10.1113/jphysiol.2008.154435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ergonul Z, Frindt G, Palmer LG. Regulation of maturation and processing of ENaC subunits in the rat kidney. Am J Physiol Renal Physiol 291: F683–F693, 2006. doi: 10.1152/ajprenal.00422.2005. [DOI] [PubMed] [Google Scholar]
- 13.Frindt G, Ergonul Z, Palmer LG. Surface expression of epithelial Na channel protein in rat kidney. J Gen Physiol 131: 617–627, 2008. doi: 10.1085/jgp.200809989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frindt G, Gravotta D, Palmer LG. Regulation of ENaC trafficking in rat kidney. J Gen Physiol 147: 217–227, 2016. doi: 10.1085/jgp.201511533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haerteis S, Krappitz M, Diakov A, Krappitz A, Rauh R, Korbmacher C. Plasmin and chymotrypsin have distinct preferences for channel activating cleavage sites in the γ subunit of the human epithelial sodium channel. J Gen Physiol 140: 375–389, 2012. doi: 10.1085/jgp.201110763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kabra R, Knight KK, Zhou R, Snyder PM. Nedd4-2 induces endocytosis and degradation of proteolytically cleaved epithelial Na+ channels. J Biol Chem 283: 6033–6039, 2008. doi: 10.1074/jbc.M708555200. [DOI] [PubMed] [Google Scholar]
- 17.Kamynina E, Debonneville C, Bens M, Vandewalle A, Staub O. A novel mouse Nedd4 protein suppresses the activity of the epithelial Na+ channel. FASEB J 15: 204–214, 2001. doi: 10.1096/fj.00-0191com. [DOI] [PubMed] [Google Scholar]
- 18.Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: regulation of epithelial sodium channels by proteases. J Biol Chem 284: 20447–20451, 2009. doi: 10.1074/jbc.R800083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knight KK, Olson DR, Zhou R, Snyder PM. Liddle’s syndrome mutations increase Na+ transport through dual effects on epithelial Na+ channel surface expression and proteolytic cleavage. Proc Natl Acad Sci USA 103: 2805–2808, 2006. doi: 10.1073/pnas.0511184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Xu S, Yang L, Yang J, Wang CJ, Weinstein AM, Palmer LG, Wang T. Sex difference in kidney electrolyte transport II: impact of K+ intake on thiazide-sensitive cation excretion in male and female mice. Am J Physiol Renal Physiol 317: F967–F977, 2019. doi: 10.1152/ajprenal.00125.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loffing J, Korbmacher C. Regulated sodium transport in the renal connecting tubule (CNT) via the epithelial sodium channel (ENaC). Pflügers Arch 458: 111–135, 2009. doi: 10.1007/s00424-009-0656-0. [DOI] [PubMed] [Google Scholar]
- 22.Loffing J, Pietri L, Aregger F, Bloch-Faure M, Ziegler U, Meneton P, Rossier BC, Kaissling B. Differential subcellular localization of ENaC subunits in mouse kidney in response to high- and low-Na diets. Am J Physiol Renal Physiol 279: F252–F258, 2000. doi: 10.1152/ajprenal.2000.279.2.F252. [DOI] [PubMed] [Google Scholar]
- 23.Loffing J, Zecevic M, Féraille E, Kaissling B, Asher C, Rossier BC, Firestone GL, Pearce D, Verrey F. Aldosterone induces rapid apical translocation of ENaC in early portion of renal collecting system: possible role of SGK. Am J Physiol Renal Physiol 280: F675–F682, 2001. doi: 10.1152/ajprenal.2001.280.4.F675. [DOI] [PubMed] [Google Scholar]
- 24.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest 104: R19–R23, 1999. doi: 10.1172/JCI7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Náray-Fejes-Tóth A, Canessa C, Cleaveland ES, Aldrich G, Fejes-Tóth G. SGK is an aldosterone-induced kinase in the renal collecting duct. Effects on epithelial Na+ channels. J Biol Chem 274: 16973–16978, 1999. doi: 10.1074/jbc.274.24.16973. [DOI] [PubMed] [Google Scholar]
- 26.Nesterov V, Krueger B, Bertog M, Dahlmann A, Palmisano R, Korbmacher C. In Liddle syndrome, epithelial sodium channel is hyperactive mainly in the early part of the aldosterone-sensitive distal nephron. Hypertension 67: 1256–1262, 2016. doi: 10.1161/HYPERTENSIONAHA.115.07061. [DOI] [PubMed] [Google Scholar]
- 27.Noreng S, Bharadwaj A, Posert R, Yoshioka C, Baconguis I. Structure of the human epithelial sodium channel by cryo-electron microscopy. eLife 7: e39340, 2018. doi: 10.7554/eLife.39340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pácha J, Frindt G, Antonian L, Silver RB, Palmer LG. Regulation of Na channels of the rat cortical collecting tubule by aldosterone. J Gen Physiol 102: 25–42, 1993. doi: 10.1085/jgp.102.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel AB, Chao J, Palmer LG. Tissue kallikrein activation of the epithelial Na channel. Am J Physiol Renal Physiol 303: F540–F550, 2012. doi: 10.1152/ajprenal.00133.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Picard N, Eladari D, El Moghrabi S, Planès C, Bourgeois S, Houillier P, Wang Q, Burnier M, Deschenes G, Knepper MA, Meneton P, Chambrey R. Defective ENaC processing and function in tissue kallikrein-deficient mice. J Biol Chem 283: 4602–4611, 2008. doi: 10.1074/jbc.M705664200. [DOI] [PubMed] [Google Scholar]
- 31.Pradervand S, Wang Q, Burnier M, Beermann F, Horisberger J-D, Hummler E, Rossier BC. A mouse model for Liddle’s syndrome. J Am Soc Nephrol 10: 2527–2533, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Rossier BC. Epithelial sodium channel (ENaC) and the control of blood pressure. Curr Opin Pharmacol 15: 33–46, 2014. doi: 10.1016/j.coph.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Rossier BC, Palmer LG. Mechanisms of aldosterone action on sodium and potassium transport. In: The Kidney: Physiology and Pathophysiology, edited by Seldin DW, Giebisch G. New York: Raven, 1992, p. 1373–1409. [Google Scholar]
- 34.Rossier BC, Pradervand S, Schild L, Hummler E. Epithelial sodium channel and the control of sodium balance: interaction between genetic and environmental factors. Annu Rev Physiol 64: 877–897, 2002. doi: 10.1146/annurev.physiol.64.082101.143243. [DOI] [PubMed] [Google Scholar]
- 35.Rossier BC, Stutts MJ. Activation of the epithelial sodium channel (ENaC) by serine proteases. Annu Rev Physiol 71: 361–379, 2009. doi: 10.1146/annurev.physiol.010908.163108. [DOI] [PubMed] [Google Scholar]
- 36.Sheng S, Maarouf AB, Bruns JB, Hughey RP, Kleyman TR. Functional role of extracellular loop cysteine residues of the epithelial Na+ channel in Na+ self-inhibition. J Biol Chem 282: 20180–20190, 2007. doi: 10.1074/jbc.M611761200. [DOI] [PubMed] [Google Scholar]
- 37.Snyder PM. Minireview: regulation of epithelial Na+ channel trafficking. Endocrinology 146: 5079–5085, 2005. doi: 10.1210/en.2005-0894. [DOI] [PubMed] [Google Scholar]
- 38.Snyder PM, Olson DR, Thomas BC. Serum and glucocorticoid-regulated kinase modulates Nedd4-2-mediated inhibition of the epithelial Na+ channel. J Biol Chem 277: 5–8, 2002. doi: 10.1074/jbc.C100623200. [DOI] [PubMed] [Google Scholar]
- 39.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J. Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 83: 811–824, 2013. doi: 10.1038/ki.2013.14. [DOI] [PubMed] [Google Scholar]
- 40.Staub O, Abriel H, Plant P, Ishikawa T, Kanelis V, Saleki R, Horisberger JD, Schild L, Rotin D. Regulation of the epithelial Na+ channel by Nedd4 and ubiquitination. Kidney Int 57: 809–815, 2000. doi: 10.1046/j.1523-1755.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- 41.Staub O, Dho S, Henry P, Correa J, Ishikawa T, McGlade J, Rotin D. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle’s syndrome. EMBO J 15: 2371–2380, 1996. doi: 10.1002/j.1460-2075.1996.tb00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Staub O, Gautschi I, Ishikawa T, Breitschopf K, Ciechanover A, Schild L, Rotin D. Regulation of stability and function of the epithelial Na+ channel (ENaC) by ubiquitination. EMBO J 16: 6325–6336, 1997. doi: 10.1093/emboj/16.21.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature 389: 607–610, 1997. doi: 10.1038/39329. [DOI] [PubMed] [Google Scholar]
- 44.Wiemuth D, Ke Y, Rohlfs M, McDonald FJ. Epithelial sodium channel (ENaC) is multi-ubiquitinated at the cell surface. Biochem J 405: 147–155, 2007. doi: 10.1042/BJ20060747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang L, Xu S, Guo X, Uchida S, Weinstein AM, Wang T, Palmer LG. Regulation of renal Na transporters in response to dietary K. Am J Physiol Renal Physiol 315: F1032–F1041, 2018. doi: 10.1152/ajprenal.00117.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou R, Patel SV, Snyder PM. Nedd4-2 catalyzes ubiquitination and degradation of cell surface ENaC. J Biol Chem 282: 20207–20212, 2007. doi: 10.1074/jbc.M611329200. [DOI] [PubMed] [Google Scholar]
- 47.Zhou R, Tomkovicz VR, Butler PL, Ochoa LA, Peterson ZJ, Snyder PM. Ubiquitin-specific peptidase 8 (USP8) regulates endosomal trafficking of the epithelial Na+ channel. J Biol Chem 288: 5389–5397, 2013. doi: 10.1074/jbc.M112.425272. [DOI] [PMC free article] [PubMed] [Google Scholar]