Abstract
Changes in mitochondrial function are central to many forms of kidney disease, including acute injury, diabetic nephropathy, hypertension, and chronic kidney diseases. As such, there is an increasing need for reliable and fast methods for assessing mitochondrial respiratory function in renal cells. Despite being indispensable for many mechanistic studies, cultured cells or isolated mitochondria, however, often do not recapitulate in vivo or close to in vivo situations. Cultured and/or immortalized cells often change their bioenergetic profile and phenotype compared with in vivo or ex vivo situations, and isolated mitochondria are simply removed from their cellular milieu. This is especially important for extremely complex organs such as the kidney. Here, we report the development and validation of a new approach for the rapid assessment of mitochondrial oxygen consumption on freshly isolated glomeruli or proximal tubular fragments using Agilent SeaHorse XFe24 and XF96 Extracellular Flux Analyzers. We validated the technique in several healthy and diseased rodent models: the C57BL/6J mouse, the diabetic db/db mouse and matching db/+ control mouse, and the Dahl salt-sensitive rat. We compared the data to respiration from isolated mitochondria. The method can be adapted and used for the rapid assessment of mitochondrial oxygen consumption from any rodent model of the investigator’s choice. The isolation methods presented here ensure viable and functional proximal tubular fragments and glomeruli, with a preserved cellular environment for studying mitochondrial function within the context of their surroundings and interactions.
Keywords: bioenergetics, glomeruli, mitochondria, proximal tubules
INTRODUCTION
Changes in mitochondrial function have been implicated in several forms of kidney disease (28, 29, 32). The list includes, for example, diabetic nephropathy (17, 29, 30), chronic kidney disease (27, 31, 32), acute kidney injury, injury induced by nephrotoxicants (9, 10, 16, 20), or hypertensive kidney disease (8, 15, 18). As such, there is a growing interest to measure renal mitochondrial respiratory parameters adequately and in detail. Each of them, including basal respiration, ATP-linked respiration, maximal respiration, reserve capacity, proton leak, and residual (nonmitochondrial) oxygen consumption, is informative about various functional aspects of the mitochondrion and the cell. The kidney is an extremely heterogeneous organ, consisting of many different cell types (24). Each of these may differ in mitochondrial substrate selection and respiration as well as how they change in health and disease. Comparing mitochondrial function in specific renal cells or nephron segments in renal physiology and pathophysiology, therefore, carries important basic, clinical, and translational value.
With today’s technology, various methods to measure oxygen consumption from isolated mitochondria or cultured renal cells are available to the investigator. Many researchers use isolated mitochondria or cell culture to address a mechanistic question of choice. The disadvantage of using isolated mitochondria is that, as the term says, these mitochondria are indeed isolated. They are taken out of the cellular environment and milieu. They may be even damaged during the lengthy isolation process, and robust control of the isolation quality is required to ensure rigor and reproducibility of these studies. Therefore, isolated mitochondria, although often a useful tool that can be used to a researcher’s advantage, unfortunately lack the natural cell environment. Hence, changes in the respiratory function of the electron transport chain complexes may be difficult to interpret or compare with in vivo situations (1, 2). Isolating mitochondria from kidney cortexes is not a difficult process, and while comparing cortexes can carry information about more robust changes (27), these mitochondria will originate from a mixture of cells.
Cells in culture often change the substrate used for fuel and show a more energetic/glycolytic phenotype (7, 33). This has been shown to be the case for renal cells, for example, in primary renal proximal tubular epithelial cells, where great efforts have been made to optimize culture conditions (21, 22). Many of the nonprimary cell lines are either immortalized or otherwise manipulated. Such alterations may impact mitochondrial substrate selection and metabolism or alter metabolic behavior. Therefore, results from these models may not entirely reflect those changes in mitochondrial respiration and metabolism that occur in kidney physiology or pathophysiology.
Here, we developed and tested an approach for measuring mitochondrial respiratory function in isolated, intact primary mouse proximal tubular (PT) fragments and freshly isolated rat glomeruli. We based our isolation protocols on previously described protocols (34, 35) that ensure as close as possible intactness of the PT and of glomerular structure and preserve mitochondria in their natural environment. We demonstrated that the protocol provides consistent measures of mitochondrial oxygen consumption in three model systems of choice: the C57BL/6J mouse, in the db/+ and db/db mouse PT as a homogenous cell model, and in Dahl salt-sensitive (SS) rat glomeruli (mixed cell model) and is easily adaptable to any disease model of the investigator’s choice.
METHODS
Materials
All solutions and materials needed for the experiments were purchased in cell culture grade or the highest grade available. DMEM with 4.5 g/L glucose, with or without phenol red, was purchased from Life Technologies/ThermoFisher Scientific (Waltham, MA) and Agilent Technologies (Billerica, MA). RPMI-1640 media was from ThermoFisher Scientific. Collagenase type I was obtained from Life Technologies. Sieves were purchased from Gilson (Middleton, WI). MatriGel was from Corning (Corning, NY). All other chemicals were from Sigma-Aldrich (St. Louis, MO). The following antibodies were from Abcam (Cambridge, MA): aquaporin (AQP)1 (ab15080, 1:1,000), AQP2 (ab110496, 1:1,000), and β-actin (ab8226, 1:10,000). Secondary antibodies (anti-rabbit or anti-mouse, horseradish peroxidase conjugated, ThermoFisher Scientific) were used at 1:5,000 dilution. General immunoblot techniques were used to assess the purity of PT preparations using a Novex 4–12% gel (ThermoFisher Scientific), 0.45-µm nitrocellulose membrane for transfer in a semidry transfer apparatus, 5% dry milk for blocking, and a chemiluminescent system (ThermoFisher Scientific) and X-ray film for development.
Animal Models Used (As Proof-of-Principle Experiments)
Male and female C57BL/6J mice as well as male db/+ and db/db mice (BKS background, strain code 000697) were used (Jackson Laboratories, Bar Harbor, ME) for harvesting kidneys for PT isolation. Mice were obtained at 8 wk of age (initial weight of 20–22 g), and, after an acclimation period at Pennington Biomedical, they were housed in a room with air conditioning and a 12:12-h light-dark cycle. Mice were fed Purina 5001 chow and had access to water ad libitum. For the experiments, after CO2 euthanasia, the kidneys were excised and immediately placed in ice-cold PBS and used fresh for digestion and PT isolation. We used mice from 12 wk of age up to 12 mo of age to compare the quality of PT isolation. In case of the diabetic mice model, db/+ mice were used at 4 mo of age and were compared with aged diabetic db/db mice at 12 mo of age. All experiments were approved by the Institutional Animal Care and Use Committee of the Pennington Biomedical Research Center and adhered with National Institutes of Health guidelines for the care and handling of experimental animals.
For the isolation of fresh glomeruli, or mitochondria, 11-wk-old male Dahl SS rats were obtained from Charles River Laboratories (strain code 320). The reason for selecting rats for glomerular isolation was based on our previous experience: it is much more straightforward to obtain a pure (~95%) glomerular fraction from rats compared with mice. We do, however, encourage investigators to use a similar sieving method for isolating mouse glomeruli while carefully checking the purity of their preps, which, in our experience (Stadler laboratory, unpublished observations), will contain some PT fragments as well. Rats were fed a normal-salt (NS; 0.4% NaCl) AIN-76A-based diet upon arrival (D113755, Dyets, Bethlehem, PA). Rats were housed at the Medical University of South Carolina Department of Laboratory Animal Resources under a 12:12-h light-dark cycle. Access to food and water and environmental enrichment were provided ad libitum. All animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals following protocol review and approval by the Institutional Animal Care and Use Committee of the Medical University of South Carolina.
Isolation of Fresh PT Fragments and Glomeruli
Isolation of fresh PT fragments.
Murine PT fragments were isolated using collagenase digestion of kidney cortexes and sieving according to the method described by Van Kerkhove et al. (34) with modifications. We detailed the modification and critical steps below. Both kidneys from a mouse were harvested into ice-cold PBS. Kidneys were minced using scissors and razor blades in a petri dish. Minced kidneys were placed into DMEM containing 1 mg/mL collagenase type I (∼10 mL for two mouse kidneys, pH 7.4), and the tube was rotated at 37°C for 30 min. In the meantime, 25 mL DMEM + 5 mL of 30% albumin (BSA) solution was prepared for the sieving process. Thirty milliliters of this solution is sufficient for sieving a pair of mouse kidneys; investigators should increase the amount accordingly. While the cortexes were digested, the wells of a XFe24 cell culture plate were coated with Matrigel (1:1 dilution made with DMEM, 3 µL gel/well). The plate was air dried for 1 h.
critical step:
In our experience, PT fragments adhered better to the plate when MatriGel was used.
For sieving, we used a 250-µm sieve and a 75-µm sieve. Sieves were wetted with the premade DMEM + BSA solution; a small circular spot was made closer to the side of the sieve, as later manipulations are made easier this way. The digested kidney mixture was sieved through the 250-µm mesh first.
critical steps:
We used a flat, wide spatula, applying pressure through the sieve with long continuous motions to help the process. This is only necessary for the first sieve, but it is critical that it is done to help all the digested kidney fragments through the sieve.
The suspension that sieved through was collected into a petri dish. This process was repeated two more times, each time applying more DMEM + BSA onto the spot to help sieving (use small transfer pipettes). Next, the sieve was tilted up and the residue on the other side of the sieve was scraped using a razor blade to collect any remaining material. The suspension was homogenized using a transfer pipette. This mixture was then sieved through the 75-µm mesh. For this step, there was no need to use a spatula anymore, just repeating the process twice adding more DMEM + BSA solution was sufficient. Everything that sieved through the 75-µm mesh was discarded. What remained on top were 80- to 100-µm PT fragments, without much substantial contamination of other nephron segments or glomeruli.
critical step:
The top of the 75-µm sieve was rinsed off (wash the sieve in reverse direction) using the DMEM + BSA solution onto a new petri dish and collected into a 50-mL Falcon tube. The pellet was set for 10 min and then centrifuged for 5 min at 170 g. Excess media were carefully removed, and the pellet was resuspended in 4 mL of fresh serum-free/phenol red-free DMEM for protein measurement using a standard BCA assay.
Glomeruli isolation from rat kidneys.
For renal tissue collection, rats were anesthetized with 2.5% isoflurane, the abdominal aorta was catheterized, and kidneys were then flushed with PBS (3 mL/min per kidney until blanched) as previously described (11, 12). The surgical procedures have been previously described in detail (13, 14). Tissues were then used for immediate glomeruli isolation. The renal cortex was excised and minced using a new singled edge razor blade; isolation was then performed with differential sieving as previously described (13, 14). Briefly, the minced tissue was sequentially pushed through a steel 150-μm mesh sieve and then pipetted through a 106-μm sieve using the culture medium solution (RPMI-1640) supplemented with 5% BSA. The tissue homogenate was then pipetted onto a 75-μm mesh sieve (Sigma-Aldrich), and glomerulus-containing fraction was rinsed from the sieve surface and stored on ice. Glomeruli were used within 3 h postisolation. Generally, ~1.5–2 g of cortical tissue, preferably collected from both kidneys, were used for isolations. We used a final volume of 500 μL to resuspend glomeruli and plate them on 96-well dishes.
Isolation of Renal Cortical Mitochondria for Comparison
To isolate mitochondria from kidney cortexes, we used the method previously described in Ref. 27. Briefly, kidney cortexes were homogenized using a “dounce” homogenizer (Wheaton 358034, 5 strokes of a PTFE pestle) and mitochondrial isolation buffer (10 mM Tris-MOPS, 1 mM EGTA/Tris, and 0.2 M sucrose, pH 7.4) on ice. The homogenate was centrifuged in 2-mL microtubes at 1,000 rpm for 10 min at 4°C. The resulting supernatant was centrifuged at 7,000 rpm at 4°C. The supernatant was then discarded, and the pellet was resuspended in 100 µL of isolation buffer. The resuspended pellet was centrifuged again at 7,000 rpm for 10 min at 4°C, and the pellet was resuspended in 100 µL of isolation buffer. The final protein concentration was 20 mg/mL.
Mitochondrial Respiratory Measurements
Mitochondrial oxygen consumption [and, simultaneously, extracellular acidification rates (ECARs)] of freshly isolated PTs were measured using a SeaHorse XFe24 or XF96 extracellular flux analyzer (Agilent Technologies, Billerica, MA). For each measurement, the cartridges were hydrated overnight according to the manufacturer’s instructions in a non-CO2 incubator. On the day of the measurements, the ports of the XFe24 (or XF96) cartridges were loaded for injections of choice. For a standard mitochondrial oxygen consumption assessment, we used sequential injections of oligomycin (final concentration: 2 µM, port A), FCCP (final concentration: 3 µM, port B), and antimycin A (1.5 µg/mL, port C). For a model system to test Na+-K+-ATPase-linked oxygen consumption, the Na+-K+-ATPase inhibitor ouabain (final concentration: 500 µM, port A) was used. The cartridge then was loaded into the XF analyzer for calibration and equilibration. After calibration, the utility plate was replaced with the cell culture plate containing the freshly isolated PTs or glomeruli.
For glomerular mitochondria respiration measurements, the general preparation procedures were similar to those of the PT, according to the manufacturer’s recommendations; however, we used 1 μM oligomycin, 1 μM FCCP, 2 μM rotenone, and 1 μM antimycin A. In case of isolated mitochondria, the optimal concentrations were as follows: 1 mM ADP, 4 µM oligomycin, 4 µM FCCP, and 4 µM antimycin A + rotenone. We recommend that investigators always optimize the concentration of these compounds in their model system. The reason to do this is to obtain maximal responses on each injection. Such responses may require a change in the concentration of oligomycin, FCCP, or antimycin A + rotenone slightly depending on the model (PT or glomeruli) or whether laboratories use isolated mitochondria.
critical step:
We recommend careful optimization of seeding of the PT fragments or glomeruli for each model of choice. The goal is to obtain a baseline of around 100–200 pmol/min oxygen consumption rate (OCR), so maximal responses on the sequential injections to modulate mitochondrial respiration can be achieved. In our case, for mouse PTs, we found 30–50 µg protein/well to be the optimal. The optimal concentration to obtain a baseline will also depend on the choice of substrate used for energizing the mitochondria. Gentle vortexing of the suspension between seeding into each well was also critical to achieve consistent well-to-well results. If vortexing is omitted, PT fragments tend to sediment rapidly, leading to inconsistent seeding and variation of OCR measurement between wells.
We set up the following simple protocol to measure PT respiration, using the supplied Wave software (Agilent Technologies):
Calibration
Equilibration
Baseline measurement: 2 cycles with 30 s of mixing, 2 min of wait/equilibration time, and 3 min of measurement per each cycle
Injection 1 (port A): oligomycin, with 2 cycles with 30 s of mixing, 2 min of wait/equilibration time, and 3 min of measurement per each cycle*
Injection 2 (port B): FCCP, with 2 cycles with 30 s of mixing, 2 min of wait/equilibration time, and 3 min of measurement per each cycle
-
Injection 3 (port C): antimycin A, with 2 cycles with 30 s of mixing, 2 min of wait/equilibration time, and 3 min of measurement per each cycle
Of note, in case of testing the Na+-K+-ATPase-linked respiration, ouabain was injected from port A and the other ports were left empty. Measurements continued for 3 cycles, with 30 s of mixing, 2 min of waiting, and 3 min of measurement per each cycle.
For isolated rat glomeruli, XF96 Seahorse analyzer and Agilent Seahorse XF96 microplates were used; we found 2−10 µg/protein per well to be optimal for obtaining consistent results. The day before analysis, the sensor cartridge was placed in the calibration buffer provided by the manufacturer. The following day, the media were replaced with low-phosphate DMEM and warmed up in a 37°C non-CO2 incubator. The injection ports of the sensor plate were filled with 25 μL of compounds or vehicle diluted in buffer. The cartridge with the utility plate was placed into the XF96 analyzer for calibration. After calibration, the utility plate was removed and the cell plate was loaded for analysis. The measurement protocol was 2-min mix and 3-min measurement. There were three rate measurements postinjection (basal levels, oligomycin, FCCP, and antimycin A + rotenone), and each injection had three measurement cycles. OCR was normalized to protein concentrations (a standard BCA assay was performed on tested wells and OCR results were normalized to protein content). For isolated mitochondria used for comparison, measurements were done as previously described in Ref. 6.
After the XFe24 measurement, each well was photographed using a Zeiss Axiovert 200 microscope to verify seeding, viability, and consistency between plates. Results were analyzed using the Wave software export option and/or GraphPad Prism or OriginPro. The following parameters were monitored and analyzed: basal respiration, ATP-linked respiration (= last rate before injection of oligomycin – minimum rate after injection of oligomycin), maximal respiration (= maximum rate after FCCP injection), spare or reserve respiratory capacity (= maximal respiration − basal respiration), proton leak (= minimum rate after oligomycin injection − nonmitochondrial respiration), nonmitochondrial OCR (= minimum rate after injection of antimycin A), and coupling efficiency [= (ATP-linked respiration/basal respiration) × 100].
RESULTS
Primary Mouse PT Fragments and Rat Glomeruli
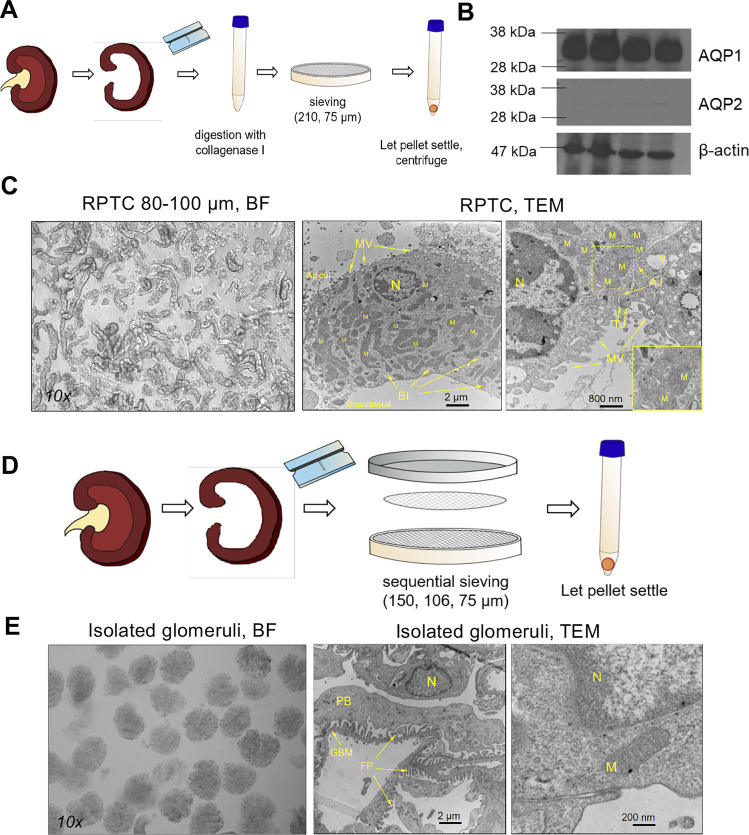
The isolation procedure we used was based on that of van Kerkhove et al. (34), which combines dissection of the kidney, enzymatic digestion, and sieving, yielded a very clean fraction of PT fragments, generally 80–100 µm long (Fig. 1, B and C). In a given viewing area, only a few glomeruli were seen, which supports the purity of isolation. Western blot analyses for the water channel AQP1 showed positive bands at the expected size, while AQP2 (a generally used marker for collecting ducts) showed only very faint bands (Fig. 1B). Representative transmission electron microphotographs of our preparation showed, in detail, microvilli, tight junctions, basolateral infold, and abundant mitochondria (Fig. 1C). In general, PT preparations from older mice contained more glomerular contamination, due to the bigger size of some glomeruli that were retained on the 75-µm sieve. Based on this observation, we recommend increasing the mesh size to 80 µm if aged mice (>12 mo) are used in the experiments. Please see Fig. 1E for a representative image of a pure isolated glomerular fraction and a representative transmission electron microphotograph showing the glomerular basement membrane, foot processes of podocytes, and some mitochondria. The glomerular isolation technique has been used by others and us in multiple previously published papers (13, 14, 23) and is significantly more powerful in rats (95% purity) than in mice. We have also previously shown that the glomeruli isolated from rats remain intact and functional.
Fig. 1.
Isolation and morphologic characteristics of murine proximal tubular (PT) fragments and rat glomeruli. A: isolation procedure for obtaining PT fragments from the mouse kidney, using mincing, collagenase type I digestion, sieving, and centrifugation. B: confirmation of the purity of isolation. Shown is Western blot analysis of PT lysates for the water channel aquaporin-1 (AQP1) and collecting duct marker aquaporin-2 (AQP2). n = 4 replicates. β-Actin was used as the loading control. C: representative microphotographs [bright field (BF): ×10 and transmission electron microscopy (TEM): ×8,000 and ×20,000] of freshly isolated PT fragments. RPTC, renal PT cells; MV, microvilli; TJ, tight junction; AJ, adherens junction; BI, basolateral infold; M, mitochondria; N, nucleus. D: isolation procedure for obtaining a pure glomerular fraction from rat kidneys using mincing and sequential sieving. E: representative microphotographs [BF: ×10 and TEM: ×8,000 and ×80,000X) of freshly isolated glomeruli. FP, foot processes; PB, podocyte body; GBM, glomerular basement membrane.
Mitochondrial Oxygen Consumption of Mouse Primary PTs on Pyruvate-Supported Respiration
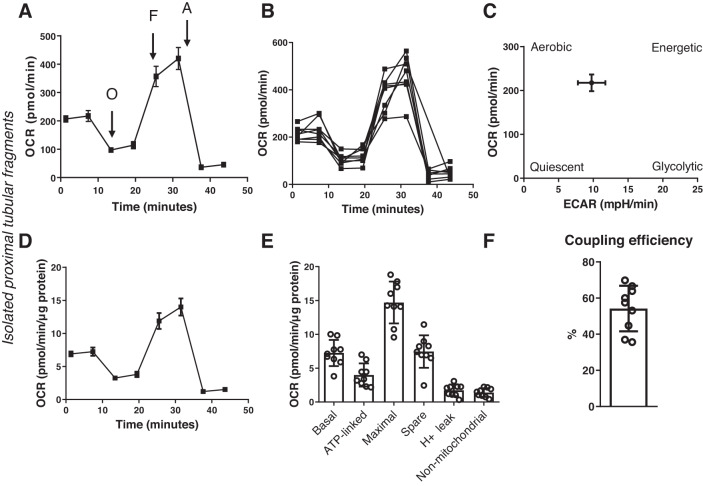
Primary PT fragments plated at 30 µg/well protein amount gave a steady baseline respiration of around 200 pmol/min OCR using 5 mM pyruvate as the energizing substrate (Fig. 2A). Oligomycin was added (2 µM) to block the F1F0 subunit of ATPase, to assess ATP-linked respiration. The resulting slope in OCR was used to calculate ATP turnover (Fig. 2E). Next, the uncoupler FCCP was added at 3 µM to measure maximal respiratory capacity. The addition of FCCP resulted in an approximately twofold increase in OCR in freshly isolated PT fragments (Fig. 2, A, D, and E). Finally, antimycin A was added (1.5 µg/mL) to block all mitochondrial respiration and to estimate oxygen consumption linked to nonmitochondrial sources. All respiratory parameters were normalized to micrograms of protein for a straightforward and easy comparison between the PT and glomerular preparations. From the energy map shown in Fig. 2C, it can be appreciated that the fresh PT largely retained its energetic/aerobic phenotype rather than switching to glycolysis. Coupling efficiency of PT fragments was calculated from the following parameters: ATP-linked respiration divided by basal respiration multiplied by 100 (Fig. 2F).
Fig. 2.
Mitochondrial oxygen consumption analysis in freshly isolated proximal tubular (PT) fragments. PT fragments were seeded on XFe24 cell culture plates at 30 µg/well protein concentration. Mitochondria were energized using 5 mM pyruvate and analyzed using an Agilent SeaHorse XFe24 Extracellular Flux Analyzer. A: representative oxygen consumption rate (OCR) graph showing a typical respiratory graph of a PT. Values are means ± SE; n = 5 biological replicates through 5 days and n = 7–10 technical replicates for each day. O, oligomycin; F, FCCP; A, antimycin A. B: well-to-well comparison of mitochondrial OCR in PTs showing the average error, variation in seeding, and reproducibility of the method throughout one assay. C: energy map of freshly isolated PT fragments obtained by graphing baseline OCR versus baseline extracellular acidification rate (ECAR) values. D: same as in A but oxygen consumption was normalized to micrograms of protein. E: analysis of the various mitochondrial respiratory parameters obtained from the normalized XFe24 graph, including basal respiration, ATP-linked respiration, maximal respiration and reserve (spare) capacity, proton leak, nonmitochondrial respiration. F: coupling efficiency. n = 5 biological replicates through 5 days and n = 8–10 technical replicates for each day.
The reproducibility of the assay was assessed using multiple preparations: from different mice at different days, measuring protein concentration from PT preparations, and seeding 30–50 µg protein/well. PT preparations consistently showed a baseline of around ~200–300 pmol/min OCR. Well-to-well results showed the daily variability of the technical replicates (Fig. 2B).
Testing the Functionality of Isolated PT Fragments
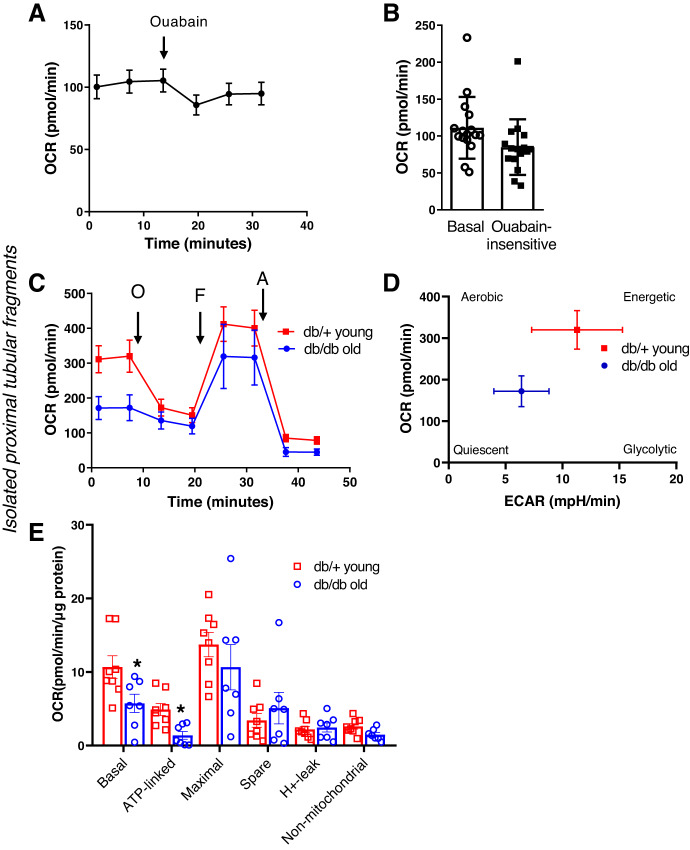
Next, we further tested the functionality of PT fragments in two different experiments. First, we assessed Na+-K+-ATPase-linked oxygen consumption using ouabain as an inhibitor of the Na+-K+-ATPase pump. In a second set of experiments, we isolated fresh PT fragments from young db/+ and old db/db mice. We asked the question of whether deficiencies in mitochondrial respiration, which can occur in aging or diabetic mice, would be detectable using our method. The results are shown in Fig. 3. Similarly to the general mitochondrial oxygen consumption experiments, PT fragments equal to 30−50 µg/well protein produced a steady baseline OCR of around ~100 pmol/min (Fig. 3A). Ouabain is an inhibitor of Na+-K+-ATPase in the membrane. Adding ouabain to a functional PT should therefore lead to the influx of Na+ (and water) into the cell and indirectly to a decrease in OCR coupled to a decrease in ATP usage linked to Na+-K+-ATPase. When ouabain was added (final concentration of 500 µM in the well), a significant drop in OCR was observed, which represents the amount of oxygen used linked to Na+-K+-ATPase (Fig. 3B). Experiments using the db/+ and db/db models revealed that the method is suitable to detect impairments in mitochondrial respiration in an established diabetic nephropathy model. Aged db/db mice (12 mo) had significantly lower basal respiration and almost completely lost their ATP-linked capacity in PTs compared with young, healthy controls (db/+; Fig. 3C–E). Energy map analysis revealed that PTs from db/db mice were significantly less energetic and switched to a more quiescent phenotype (Fig. 3D).
Fig. 3.
Functional applications of the method in isolated proximal tubular (PT) fragments. A: mitochondrial oxygen consumption analysis and functionality of Na+-K+-ATPase. PT fragments were seeded on XFe24 cell culture plates at 30–50 µg/well protein concentration, and mitochondria were energized using 5 mM pyruvate/4 mM lactate. The representative oxygen consumption rate (OCR) graph shows basal respiration and the response of PTs to the Na+-K+-ATPase blocker ouabain (500 µM). Values are means ± SE; n = 3 biological replicates through 3 days and n = 10–15 technical replicates for each day. B: analysis of basal and ouabain-insensitive respiration, obtained from the points of a representative OCR graph before and after ouabain injection. C: comparison of PT mitochondrial function in young, healthy (db/+, 4 mo) and old, type 2 diabetic (db/db, 12 mo) mice. PT segments isolated with the method were seeded as in A. Representative OCR graphs show impaired mitochondrial basal respiration and an almost complete loss of ATP-linked respiration, together with a significantly less energetic phenotype from old db/db mice, as shown in the energy map (D). E: analysis of mitochondrial respiratory parameters obtained from normalized XFe24 graphs. n = 8 technical replicates. *P < 0.05 by Student’s t test.
Mitochondrial OCRs in Freshly Isolated Rat Glomeruli and Comparison With Isolated Mitochondria
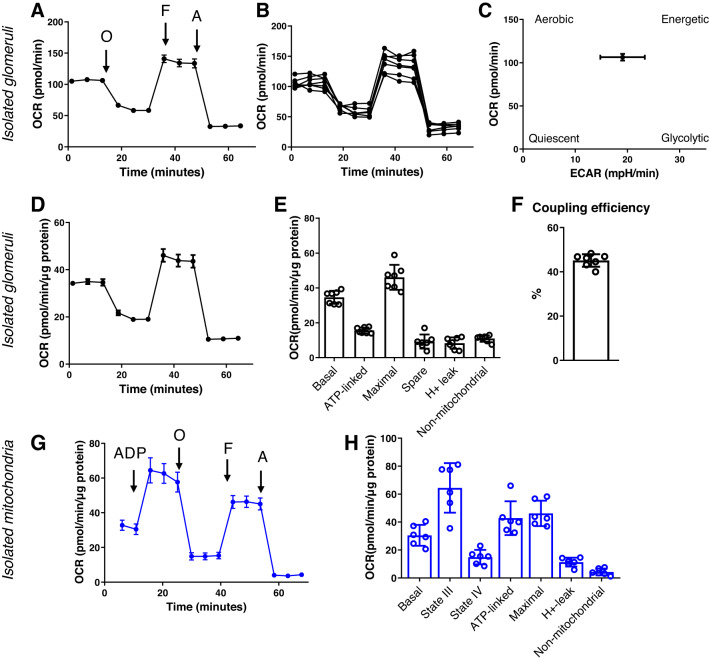
In this assay, we added oligomycin [which blocks the proton channel of ATP synthase (V), so that phosphorylation of ADP is no longer possible], FCCP (uncoupler of oxidative phosphorylation, which allows the electron transport chain to function at the maximal capacity), as well as antimycin and rotenone (complex III complex I inhibitors, which shut down the electron transport chain entirely) to freshly isolated glomerular preparations. As shown from the summarized OCR transients in Fig. 4, A and D, the basal OCR obtained from rat glomeruli (3 μg protein/well in a 96-well plate) was established to be ~100 −150 pmol/min. The data shown in Fig. 4A are representative from one animal and seven experimental replicates, with low variation among replicates (~15%). Experiments have been repeated through multiple days from multiple rats. Similar to the measurements from the PT, all respiratory parameters were normalized to micrograms of protein (Fig. 4, D and E) for comparison. We have recently published an article featuring a comparison of mitochondrial respiration in Dahl SS rat glomeruli isolated from animals fed different experimental diets (6) and were able to successfully use this technique to show a reduction in mitochondrial OCR in glomeruli of rats challenged with a high-salt diet to induced hypertensive glomerulosclerosis. Finally, we asked the question of whether results from the new method were comparable with those obtained from isolated kidney mitochondria using previously established methods. Figure 4, G and H, shows that when normalized to protein content, OCR values were in a similar range using either method.
Fig. 4.
Mitochondrial oxygen consumption analysis in freshly isolated rat glomeruli. Glomeruli from Dahl salt-sensitive rats were seeded on XF96 cell culture plates at 3 µg/well protein concentration. Mitochondria were energized using 5 mM pyruvate and analyzed using an Agilent SeaHorse XF96 Extracellular Flux Analyzer. A: representative oxygen consumption rate (OCR) graph showing a typical respiratory graph of freshly isolated glomeruli. Values are means ± SE; n = 7 technical replicates for an isolation from one animal. O, oligomycin; F, FCCP; A, antimycin A. B: well-to-well comparison of mitochondrial OCRs in fresh glomerular preparations showing the average error, variation in seeding, and reproducibility of the method throughout one isolation. C: energy map of freshly isolated rat glomeruli, obtained by plotting the baseline OCR versus baseline extracellular acidification rate (ECAR) values. D: same as in A but oxygen consumption was normalized to micrograms of protein. E: analysis of the various mitochondrial respiratory parameters obtained from the normalized XF96 graph, including basal respiration, ATP-linked respiration, maximal respiration and reserve (spare) capacity, proton leak, and nonmitochondrial respiration. F: coupling efficiency. n = 7 technical replicates from one animal. G: comparison of the method and analysis in isolated kidney cortex mitochondria. The representative OCR graph obtained from mitochondria isolated from the kidneys of Dahl salt-sensitive rats shows similar normalized OCR values compared with measurements from nephron segments. H: analysis of mitochondrial respiratory parameters obtained from isolated mitochondria. Values are means ± SE; n = 8 technical replicates.
DISCUSSION
Here, we developed and tested a method for rapid assessment of mitochondrial respiratory function in isolated primary PTs and glomeruli. The primary goal was to mimic an in vivo situation where renal mitochondria are present in their native environment. Two “proof of concept” models were tested: PTs from mice and glomeruli from Dahl SS rats. The reason for selecting the PT as a model system was that the renal tubular epithelium is unique in the sense of being able to regenerate and repair after injury. The process often involves mitochondria and their biogenesis (25, 26), making the assessment of mitochondrial function in this nephron segment specifically important. To our knowledge, there are not many studies using fresh glomeruli for mitochondrial function measurements (5). However, there are now numerous novel reports in isolated podocytes demonstrating the different metabolic characteristics of these cells compared with PTs (3, 19). As such, there is a vast interest in studying the mitochondria in the context of glomerular function.
Since the sieving and isolation method required little to no adjustments dependent on the disease model of choice or species, we believe our approach provides the basis for a universal application for other investigators. Due to the small amount of protein required for successful data obtainment on the XF24 or XF96 plates, further use of the remaining PTs or glomeruli for functional tests, imaging, or other experiments is possible. This can be important for interpretation of data where the same day, same mouse, or same batch minimizes variability. The method avoids time-consuming mitochondrial isolation and keeps potential damage to mitochondria minimal, which we illustrated with transmission electron microphotographs. This is important for two reasons. First, the majority of mechanistic information [with some exceptions (4, 9, 16)] has largely been obtained from mitochondrial electron transport chain component activity assays, where mitochondria are isolated from their native surroundings from tissue or cells. These assays are performed under nonphysiological basal conditions, with substrate concentrations being different compared with those in vivo. Importantly, assays of activities outside of the intact mitochondrial environment cannot identify defects in mitochondrial function. Generally, substrates are added to isolated mitochondria, and either oxygen consumption or ATP generation is measured (2). While widely recognized as the standard assay, a significant limitation is the requirement for viable functioning mitochondria. This puts a limitation on the assay, requiring fast preparation/isolation of mitochondria immediately after the tissue is obtained. Second, the process of isolating mitochondria places the organelle outside of a truly physiological condition. This may be of crucial importance in the case of the kidney with its complex and heterogeneous cell environment.
In our experience, plating of PT fragments or glomeruli was repeatable and reproducible, with low variation of baseline respiration between batches. Notably, in our hands, a permeabilization step (which would also perturb cellular environment) was not necessary. The method offers and combines a fast and clean isolation approach with a high-throughput functional measurement and analysis of multiple parameters at the same time. Importantly, both PT segments and glomeruli seemed to retain their more energetic phenotype, as confirmed by plotting the OCR versus ECAR values on an “energy map” of the cells. These freshly isolated segments, therefore, could represent a much closer to in vivo bioenergetic situation for mitochondrial functional studies. Interestingly, both PTs and isolated glomeruli displayed similar ratios of respiratory parameters (such as maximal and spare capacity) and an ~50% coupling efficiency when normalized to protein content. When comparing our method with data obtained from isolated mitochondria, we found that OCR values were in range and comparable using either our method or previously well-established mitochondrial respiration measurement methods (Fig. 4). However, the two approaches differ somewhat in the information they can offer. To isolated mitochondria, ADP can be given to measure state II (“basal”) and state III respiration followed by oligomycin to assess state IV respiration. Direct effects of nephrotoxicants or other compounds or genetic manipulations on these respiratory states are somewhat easier to pinpoint in a “traditional” isolated mitochondria approach. Information about cell context-dependent processes and their effects on mitochondrial function is, however, lost. It is not possible to define “ECAR” and no opportunity to test energetic/glycolytic changes and quiescence. Cell context-dependent measurements, such as, for example, testing how much of ATP-linked respiration is coupled to the Na+-K+-ATPase pump of the PT, are not possible using isolated mitochondria.
The functional validation assays we used also revealed that the model system is suitable to answer questions related to disease models or pathology. In a well-accepted model of diabetic nephropathy (the db/db mouse), our results showed that impaired renal mitochondrial function related to aging and diabetes can be detected using freshly isolated nephron segments (Fig. 3, C–E).
The following points below summarize the main advantages of the model system:
Freshly isolated PT fragments that were 80- to 100-µm-long tubes had an intact brush border, functionality (Fig. 1) (34), and preserved mitochondrial milieu.
The mixed cell model (freshly isolated glomeruli) demonstrates a preserved glomerular basement membrane, podocytes with their foot processes attached to the capillaries, and an intact filtration barrier and microvasculature, with mitochondria remaining in their cell environment (Fig. 1). This is an enormous advantage for studies of glomerular function.
There is no need for long-term culturing cells or outgrowths. The approach provides same-day data, with multiple respiratory parameters, a short isolation process in the morning, and measurement and immediate analysis in the afternoon.
The process is easily applicable to the model of choice (mouse or rat model of kidney disease) studied by the investigator.
There can be further optimization to the investigator’s model of choice, and changing to various respiratory substrates is straightforward.
Respiratory parameters and potential changes detected in a disease model are closer to an in vivo situation where mitochondria and other organelles are present together and interact.
Potential caveats of the model system include the following:
A potential caveat is, of course, if the kidney disease is so severe or advanced that it is not possible to isolate cells or glomeruli with at least some degree of intactness and/or functionality.
Our approach maintains the PT fragments on the solid plastic surface of the XFe24 cell culture plate, which will limit some functional assays to be carried out in conjunction with respiratory measurements. This is because solid support will only allow access to one side of the cells.
It should be recognized that there are species differences in glomeruli isolation; rat glomeruli preparation is very clean and free of nonglomerular nephron fragments, whereas with mice the investigator has to be careful and check the purity of the sample before using it for experiments.
To summarize, we described here a rapid method to assess mitochondrial respiratory function in fresh PT fragments or glomeruli, in two rodent models of our choice. Cells from these freshly isolated segments are not cultured further to ensure a closer to in vivo scenario and native substrate preference and allows for studying mitochondria in the PT or glomeruli in their original intracellular environment. The method provides the basis for a reproducible and reliable approach for kidney disease research, where alterations in mitochondrial function during pathogenesis are of significance.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant R01-DK-115749-01A1 (to K. Stadler) and by NIDDK Grant R00-DK-105160, the Dialysis Clinic Incorporated Reserve Fund, and an American Physiological Society Research Career Enhancement award (to D. V. Ilatovskaya).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.V.I. and K.S. conceived and designed research; A.M., M.D., R.F.S., and D.V.I. performed experiments; M.D., D.V.I., R.F.S., and K.S. analyzed data; A.M., D.V.I., and K.S. interpreted results of experiments; A.M., D.V.I., and K.S. prepared figures; K.S. drafted manuscript; D.V.I. and K.S. edited and revised manuscript; All authors approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Gyda Beeson (Medical University of South Carolina Metabolomics/Seahorse Biosciences Development Core, South Carolina COBRE Grant P20-GM-103542 in Oxidants, Redox Balance, and Stress Signaling). We acknowledge Nancy Smythe (Medical University of South Carolina Research Electron Microscopy Service Laboratory) for assistance with the preparation of samples and transmission electron microscopy imaging.
REFERENCES
- 1.Brand MD. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic Biol Med 100: 14–31, 2016. doi: 10.1016/j.freeradbiomed.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J 435: 297–312, 2011. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinkkoetter PT, Bork T, Salou S, Liang W, Mizi A, Ozel C, Koehler S, Hagmann HH, Ising C, Kuczkowski A, Schnyder S, Abed A, Schermer B, Benzing T, Kretz O, Puelles VG, Lagies S, Schlimpert M, Kammerer B, Handschin C, Schell C, Huber TB. Anaerobic glycolysis maintains the glomerular filtration barrier independent of mitochondrial metabolism and dynamics. Cell Rep 27: 1551–1566.e5, 2019. doi: 10.1016/j.celrep.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cameron RB, Peterson YK, Beeson CC, Schnellmann RG. Structural and pharmacological basis for the induction of mitochondrial biogenesis by formoterol but not clenbuterol. Sci Rep 7: 10578, 2017. doi: 10.1038/s41598-017-11030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daehn I, Casalena G, Zhang T, Shi S, Fenninger F, Barasch N, Yu L, D’Agati V, Schlondorff D, Kriz W, Haraldsson B, Bottinger EP. Endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis. J Clin Invest 124: 1608–1621, 2014. doi: 10.1172/JCI71195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Domondon M, Polina I, Nikiforova AB, Sultanova RF, Kruger C, Vasileva VY, Fomin MV, Beeson GC, Nieminen AL, Smythe N, Maldonado EN, Stadler K, Ilatovskaya DV. Renal glomerular mitochondria function in salt-sensitive hypertension. Front Physiol 10: 1588, 2020. doi: 10.3389/fphys.2019.01588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickman KG, Mandel LJ. Glycolytic and oxidative metabolism in primary renal proximal tubule cultures. Am J Physiol 257: C333–C340, 1989. doi: 10.1152/ajpcell.1989.257.2.C333. [DOI] [PubMed] [Google Scholar]
- 8.Eirin A, Lerman A, Lerman LO. Enhancing mitochondrial health to treat hypertension. Curr Hypertens Rep 20: 89, 2018. doi: 10.1007/s11906-018-0889-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Funk JA, Odejinmi S, Schnellmann RG. SRT1720 induces mitochondrial biogenesis and rescues mitochondrial function after oxidant injury in renal proximal tubule cells. J Pharmacol Exp Ther 333: 593–601, 2010. doi: 10.1124/jpet.109.161992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Funk JA, Schnellmann RG. Persistent disruption of mitochondrial homeostasis after acute kidney injury. Am J Physiol Renal Physiol 302: F853–F864, 2012. doi: 10.1152/ajprenal.00035.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ilatovskaya DV, Levchenko V, Lowing A, Shuyskiy LS, Palygin O, Staruschenko A. Podocyte injury in diabetic nephropathy: implications of angiotensin II-dependent activation of TRPC channels. Sci Rep 5: 17637, 2015. doi: 10.1038/srep17637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ilatovskaya DV, Palygin O, Levchenko V, Endres BT, Staruschenko A. The role of angiotensin ii in glomerular volume dynamics and podocyte calcium handling. Sci Rep 7: 299, 2017. doi: 10.1038/s41598-017-00406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ilatovskaya DV, Palygin O, Levchenko V, Staruschenko A. Single-channel analysis and calcium imaging in the podocytes of the freshly isolated glomeruli. J Vis Exp 2015: e52850, 2015. doi: 10.3791/52850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ilatovskaya DV, Staruschenko A. Single-channel analysis of TRPC channels in the podocytes of freshly isolated glomeruli. Methods Mol Biol 998: 355–369, 2013. doi: 10.1007/978-1-62703-351-0_28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Itani HA, Dikalova AE, McMaster WG, Nazarewicz RR, Bikineyeva AT, Harrison DG, Dikalov SI. Mitochondrial cyclophilin D in vascular oxidative stress and hypertension. Hypertension 67: 1218–1227, 2016. doi: 10.1161/HYPERTENSIONAHA.115.07085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jesinkey SR, Funk JA, Stallons LJ, Wills LP, Megyesi JK, Beeson CC, Schnellmann RG. Formoterol restores mitochondrial and renal function after ischemia-reperfusion injury. J Am Soc Nephrol 25: 1157–1162, 2014. doi: 10.1681/ASN.2013090952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, Chugh S, Danesh FR. Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med (Maywood) 233: 4–11, 2008. doi: 10.3181/0705-MR-134. [DOI] [PubMed] [Google Scholar]
- 18.Lahera V, de Las Heras N, López-Farré A, Manucha W, Ferder L. Role of mitochondrial dysfunction in hypertension and obesity. Curr Hypertens Rep 19: 11, 2017. doi: 10.1007/s11906-017-0710-9. [DOI] [PubMed] [Google Scholar]
- 19.Li SY, Susztak K. The role of peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) in kidney disease. Semin Nephrol 38: 121–126, 2018. doi: 10.1016/j.semnephrol.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nourbakhsh N, Singh P. Role of renal oxygenation and mitochondrial function in the pathophysiology of acute kidney injury. Nephron Clin Pract 127: 149–152, 2014. doi: 10.1159/000363545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nowak G, Schnellmann RG. Improved culture conditions stimulate gluconeogenesis in primary cultures of renal proximal tubule cells. Am J Physiol Cell Physiol 268: C1053–C1061, 1995. doi: 10.1152/ajpcell.1995.268.4.C1053. [DOI] [PubMed] [Google Scholar]
- 22.Nowak G, Schnellmann RG. l-Ascorbic acid regulates growth and metabolism of renal cells: improvements in cell culture. Am J Physiol Cell Physiol 271: C2072–C2080, 1996. doi: 10.1152/ajpcell.1996.271.6.C2072. [DOI] [PubMed] [Google Scholar]
- 23.Palygin O, Spires D, Levchenko V, Bohovyk R, Fedoriuk M, Klemens CA, Sykes O, Bukowy JD, Cowley AW Jr, Lazar J, Ilatovskaya DV, Staruschenko A. Progression of diabetic kidney disease in T2DN rats. Am J Physiol Renal Physiol 317: F1450–F1461, 2019. doi: 10.1152/ajprenal.00246.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park J, Shrestha R, Qiu C, Kondo A, Huang S, Werth M, Li M, Barasch J, Suszták K. Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science 360: 758–763, 2018. doi: 10.1126/science.aar2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rasbach KA, Schnellmann RG. PGC-1alpha over-expression promotes recovery from mitochondrial dysfunction and cell injury. Biochem Biophys Res Commun 355: 734–739, 2007. doi: 10.1016/j.bbrc.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 26.Rasbach KA, Schnellmann RG. Signaling of mitochondrial biogenesis following oxidant injury. J Biol Chem 282: 2355–2362, 2007. doi: 10.1074/jbc.M608009200. [DOI] [PubMed] [Google Scholar]
- 27.Ruggiero C, Ehrenshaft M, Cleland E, Stadler K. High-fat diet induces an initial adaptation of mitochondrial bioenergetics in the kidney despite evident oxidative stress and mitochondrial ROS production. Am J Physiol Endocrinol Metab 300: E1047–E1058, 2011. doi: 10.1152/ajpendo.00666.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sas KM, Kayampilly P, Byun J, Nair V, Hinder LM, Hur J, Zhang H, Lin C, Qi NR, Michailidis G, Groop PH, Nelson RG, Darshi M, Sharma K, Schelling JR, Sedor JR, Pop-Busui R, Weinberg JM, Soleimanpour SA, Abcouwer SF, Gardner TW, Burant CF, Feldman EL, Kretzler M, Brosius FC III, Pennathur S. Tissue-specific metabolic reprogramming drives nutrient flux in diabetic complications. JCI Insight 1: e86976, 2016. doi: 10.1172/jci.insight.86976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma K. Mitochondrial dysfunction in the diabetic kidney. Adv Exp Med Biol 982: 553–562, 2017. doi: 10.1007/978-3-319-55330-6_28. [DOI] [PubMed] [Google Scholar]
- 30.Sharma K. Mitochondrial hormesis and diabetic complications. Diabetes 64: 663–672, 2015. doi: 10.2337/db14-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szeto HH. Pharmacologic approaches to improve mitochondrial function in AKI and CKD. J Am Soc Nephrol 28: 2856–2865, 2017. doi: 10.1681/ASN.2017030247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szeto HH, Liu S, Soong Y, Alam N, Prusky GT, Seshan SV. Protection of mitochondria prevents high-fat diet-induced glomerulopathy and proximal tubular injury. Kidney Int 90: 997–1011, 2016. doi: 10.1016/j.kint.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 33.Tang MJ, Suresh KR, Tannen RL. Carbohydrate metabolism by primary cultures of rabbit proximal tubules. Am J Physiol Cell Physiol 256: C532–C539, 1989. doi: 10.1152/ajpcell.1989.256.3.C532. [DOI] [PubMed] [Google Scholar]
- 34.Terryn S, Jouret F, Vandenabeele F, Smolders I, Moreels M, Devuyst O, Steels P, Van Kerkhove E. A primary culture of mouse proximal tubular cells, established on collagen-coated membranes. Am J Physiol Renal Physiol 293: F476–F485, 2007. doi: 10.1152/ajprenal.00363.2006. [DOI] [PubMed] [Google Scholar]
- 35.Vinay P, Gougoux A, Lemieux G. Isolation of a pure suspension of rat proximal tubules. Am J Physiol Renal Physiol 241: F403–F411, 1981. doi: 10.1152/ajprenal.1981.241.4.F403. [DOI] [PubMed] [Google Scholar]