Abstract
Systemic lupus erythematosus (SLE) is a chronic multisystem autoimmune disease characterized by circulating autoantibodies, prevalent hypertension, renal injury, and cardiovascular disease. Onset of the disease often occurs in young women of childbearing age. Although kidney involvement is common to patients with SLE, little is known about temporal changes in renal hemodynamic function and its relationship to the pathogenesis of hypertension during autoimmune diseases. We hypothesized that the loss of immunological tolerance and subsequent production of autoantibodies in SLE leads to impaired renal hemodynamic function that precedes the development hypertension. Female NZBWF1 (SLE) mice and female NZW/LacJ (control) mice were instrumented with carotid artery and jugular vein catheters to determine mean arterial pressure (MAP) and glomerular filtration rate, respectively, at ages of 15, 20, 24, 28, 31, and 34 wk. In addition, urinary albumin excretion, blood urea nitrogen, circulating autoantibodies, and glomerulosclerosis were assessed at each age. Levels of circulating autoantibodies are increased between 24 and 28 wk of age in NZBWF1 mice and were significantly greater than in control mice. Glomerular filtration rate was significantly increased at 28 wk of age in NZBWF1 mice followed by a sharp decline at 34 wk of age. NZBWF1 mice had an increase in MAP that occurred by 34 wk of age. These data show that changes in circulating autoantibodies, renal hemodynamic function, and glomerular injury occur in NZBWF1 mice before changes in MAP, suggesting an important mechanistic role for autoimmunity to directly impair renal hemodynamic function and promote the development of hypertension.
Keywords: autoantibodies, autoimmunity, hypertension, renal hemodynamics, systemic lupus erythematosus
INTRODUCTION
Systemic lupus erythematosus (SLE) is an autoimmune disease that affects an estimated 1.5 million Americans and 5 million worldwide (32). Women are nine times more likely to develop SLE compared with men (48, 49, 59, 66) with the peak onset of disease occurring in adult women during childbearing years (48, 73). SLE increases the incidence of cardiovascular disease (25, 31, 38), which is the leading cause of mortality in patients with SLE (29, 44, 51). This increased risk among patients with SLE was recognized and reported in 1976 by Urowitz et al. (67). The most common manifestations of cardiovascular disease in SLE are premature atherosclerosis, myocardial infarction, stroke, transient ischemic attack, and ischemic peripheral vascular disease (10, 26). Whereas mortality from active lupus disease has declined over the years due to better drug management, cardiovascular disease-related mortality in SLE has not (19, 23, 31).
Hypertension is a major modifiable cardiovascular risk factor, of which the prevalence is significantly increased in patients with autoimmune diseases (43, 46) including SLE (1, 14, 23, 55, 58). Despite the prevalent hypertension in this patient population, the most recent American College of Cardiology/American Heart Association guidelines for treating high blood pressure in adults do not consider those with autoimmune diseases such as SLE (65, 74). Developing guidelines for patients with chronic autoimmune diseases is important because the hypertension (1, 14, 43, 46, 55, 58) is often difficult to manage (28) in this population and because of the significant cardiovascular related morbidity and mortality (25, 29, 31, 44, 51).
Work from our laboratory supports the concept that the loss of immunological self-tolerance and subsequent production of autoantibodies mechanistically contributes to the prevalent hypertension during SLE (33, 37, 63). Therapeutically targeting immune system dysfunction, inflammatory cytokines, and oxidative stress in experimental models of SLE not only attenuates hypertension but also reduces renal injury (33, 35, 37, 63, 68). Importantly, the incidence of renal disease is markedly increased in women with SLE (42) with reports of impaired renal function, including reduced glomerular filtration rate (GFR), reduced renal plasma flow (42), and increased plasma creatinine and proteinuria (2). However, these data are most often collected in patients who are experiencing a flare or who have active or advanced renal disease. Therefore, an important gap in knowledge remains as to whether changes in renal function and injury are occurring before the development of hypertension.
The present study was designed to test the hypothesis that the loss of immunological self-tolerance and subsequent production of autoantibodies in SLE leads to impaired renal hemodynamic function and renal injury that precede the development of hypertension. This was tested by measuring blood pressure, glomerular filtration rate (GFR), markers of renal injury, and autoantibody production in an established experimental female mouse model of SLE.
METHODS
Animal model.
Female NZBWF1 mice, an established murine model of lupus that develops renal injury and hypertension, which have been used to study SLE for more than 50 yr (53), and female control (NZW/LacJ) mice were obtained from The Jackson Laboratories (Bar Harbor, ME). Distinct groups of control and SLE mice were studied at six different ages (15, 20, 24, 28, 31, and 34 wk of age) with a total cohort of 103 (control) and 110 (SLE) mice. The data from each age comprises mice from multiple litters across a 2-yr period. Mice were housed with access to normal chow and water ad libitum and kept on a 12:12-h light-dark cycle at room temperatures between 21 and 23°C. All experiments were approved by the Institutional Animal Care and Use Committee of University of Mississippi Medical Center and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Autoantibody production.
Plasma samples were taken at each time point and analyzed for anti-dsDNA autoantibodies (dsDNA) IgG by ELISA (Alpha Diagnostic) as per the manufacturer’s instructions and as previously described by our laboratory (54, 70). Results are presented in kU/mL.
Urinary albumin.
Daily urinary albumin excretion rate (mg/day) was measured by ELISA (Alpha Diagnostics) using 24-h urine samples, as previously described by our laboratory (35, 70), at each time point.
Blood urea nitrogen.
Plasma blood urea nitrogen (BUN) was assessed at each time point by a Vet Axcel Chemistry Analyzer (Alfa Wasserman, Diagnostic Technologies) using 40 μL plasma.
Blood pressure and GFR measurements.
A subset of mice was instrumented with jugular vein and carotid artery catheters under isoflurane anesthesia for measurement of blood pressure and GFR in the same conscious animals. Mice were allowed to recover overnight, after which blood pressure was recorded in conscious, freely moving mice for 90 min using an eight-channel PowerLab/16SP (ADInstruments) blood pressure transducer. Data were recorded using Chart5 for Windows (ADInstruments).
GFR was measured in conscious, restrained mice by indwelling jugular vein catheterization and constant infusion of FITC-inulin using a Harvard pump (0.004 mL/min). FITC-inulin is freely filtered by the kidneys and is neither secreted nor reabsorbed, making it a suitable substance for studying GFR. Infusions were performed immediately after blood pressure recording. The carotid catheter was used to collect plasma samples at 4-, 4.5-, and 5-h time points to ensure a steady state was reached (defined as two consecutive samples with a FITC-inulin concentration within 10% of each other). At the end of the infusion protocol, 10 μL plasma from each time point was transferred to a 96-well clear bottom black plate and analyzed for fluorescence at 485-nm excitation and 530-nm emission. Plate readings (concentration, as indicated by brackets) were used to calculate GFR using the following formula:
Results were corrected for total kidney weight.
Glomerulosclerosis.
Glomerulosclerosis was assessed using a Masson’s trichrome staining protocol as previously described by our laboratory (37). Thirty glomeruli per mouse were scored blinded using a scale of 0−4, where 0 = no sclerosis, 1 = 0–25% of glomeruli have sclerosis, 2 = 25–50% of glomeruli have sclerosis, 3 = 50–75% of glomeruli have sclerosis, and 4 = 75–100% of glomeruli have sclerosis. A total glomerular score was tabulated by adding the number of 0s, 1s, 2s, 3s, and 4s multiplied by the number of glomeruli with that scale score and divided by the total number of glomeruli scored.
Western blots.
Kidney sections were homogenized in lysis buffer (20 mM Tris, 5 mM EDTA, 10 mM EGTA, 1 mM sodium orthovanadate, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 2 mM DTT, 0.1 mg/mL PMSF, 0.01 mg/mL leupeptin, and 0.01 mg/mL aprotinin) using the Next Advance Bullet Blender. Homogenates were run on Bio-Rad mini-Protean TGX Stain-free gels (4–15%) and transferred to a nitrocellulose membrane via the Bio-Rad TransBlot Turbo protocol. Total protein per lane was determined via Bio-Rad Stain-Free technology on a Chemidoc MP imager. Expression of nitric oxide (NO) synthase (NOS)1 and NOS3 was determined with BD Transduction Laboratory antibodies (catalog nos. 610309 and 610297, respectively) at a dilution of 1:250. Phosphorylation of NOS3 at serine residue 1177 (NOS3-pSer1177) was determined with Cell Signaling antibody (catalog no. 9571L) at a dilution of 1:250. TNF-α was assessed using a human/mouse TNF-α antibody from R&D Systems (AF-410-na) at a dilution of 1:500. Bio-Rad goat anti-mouse horseradish peroxidase conjugate, Bio-Rad goat anti-rabbit StarBright blue 700, and R&D Systems donkey anti-goat IgG horseradish peroxidase conjugate were used as secondary antibodies, each at a dilution of 1:1,000. Membranes were developed with enhanced chemiluminescent reagents (Thermo Scientific), imaged with the Bio-Rad Chemidoc MP, and quantified with Bio-Rad ImageLab software. The band densities for NOS1α, NOS1β, NOS3, and TNF-α were then divided by the total protein density to calculate the protein-to-total protein loaded ratio. The relative amount of NOS3-pSer1177 phosphorylation was determined by dividing the band density for NOS3-pSer1177 by the band density for NOS3. IC represents the internal control used to normalize values between blots.
Flow cytometry.
Blood was collected from the retroorbital plexus of control and SLE mice at 20, 28, and 34 wk of age. Cells were prepared as previously described by our laboratory (64), stained with anti-CD45R‐APC (clone RA3‐6B2), and analyzed using a Gallios flow cytometer (Becton Dickinson) at the University of Mississippi Medical Center flow cytometry core facility.
Statistical analysis.
Data are presented as means with 95% confidence intervals unless stated otherwise. Statistical analyses were performed using GraphPad Prism 6 (GraphPad Software). One-way ANOVA was used to determine differences within groups. Two-way ANOVA was used to determine differences between groups. Groups were compared using the appropriate multiple-comparison test. Values were considered statistically significant at P values of <0.05.
RESULTS
Autoantibodies.
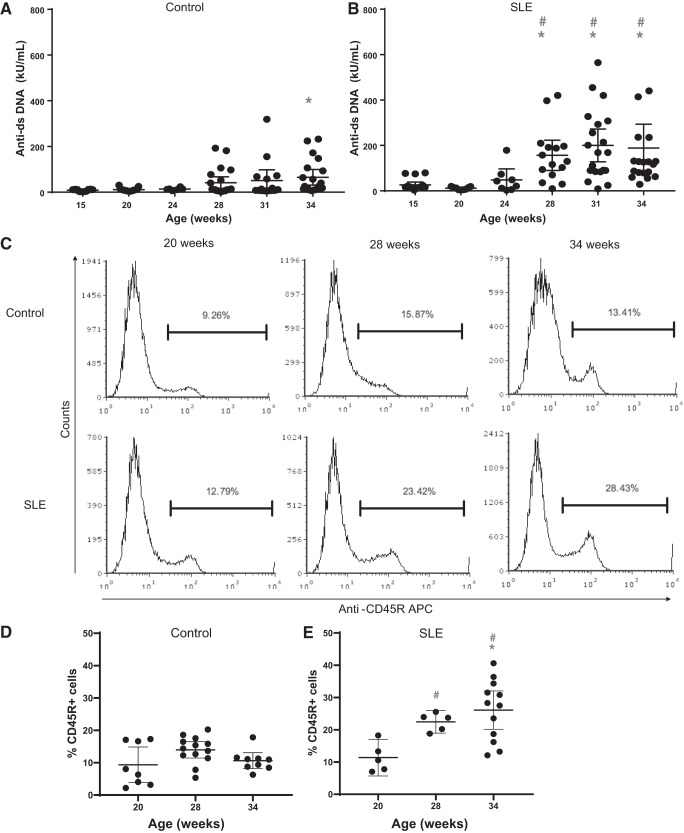
SLE is characterized by a loss of immune tolerance resulting in the production of autoantibodies that lead to disease manifestations. Anti-dsDNA autoantibodies are used diagnostically for SLE (27, 76). Between the ages of 24 and 28 wk, there was a significant increase in the levels of circulating autoantibodies compared with SLE mice at 15 wk of age and compared with age-matched control mice at 28, 31, and 34 wk of age. In age-matched controls, autoantibodies remained relatively low throughout the duration of the study; however, there was a statistically significant increase at 34 wk of age (Fig. 1, A and B).
Fig. 1.
Anti-dsDNA IgG is increased by 28 wk of age in female mice with systemic lupus erythematosus (SLE). A: anti-dsDNA IgG was increased at 34 wk of age (*P = 0.0234) compared with 15 wk of age in female control mice. B: anti-dsDNA IgG was significantly increased by 28 wk of age (*P = 0.0436), 31 wk of age (*P = 0.0012), and 34 wk of age (*P = 0.0032) compared with 15 wk of age in female mice with SLE (95% confidence interval: −258.5 to −2.428, −293.1 to −54.64, and −283 to −41.67, respectively). Anti-dsDNA IgG was significantly increased by 28 wk of age (#P = 0.0072), 31 wk of age (#P = 0.0002), and 34 wk of age (#P = 0.0016) compared with age-matched control mice (95% confidence interval: −208.5 to −22.14, −243.2 to −54.9, and −211.6 to −35.01, respectively). C: representative histograms from control and SLE mice at 20, 28, and 34 wk of age. Peripheral blood leukocytes were isolated and stained with anti-mouse CD45R PE-Cy7. D and E: quantitative representation of CD45R+ B cells at 20, 28, and 34 wk of age in control (D) and SLE (E) mice. E: the percentage of circulating CD45R+ B cells was increased by 34 wk of age (*P = 0.0030) compared with 20 wk in SLE mice (95% CI: −24.24 to −5.204). The percentage of circulating CD45R+ B cells was increased by 28 wk of age (#P = 0.0310) and 34 wk of age (#P < 0.0001) in female mice with SLE compared with age-matched controls (95% confidence interval: −16.35 to −0.6109 and −22.07 to −8.883, respectively). The percentage of CD45R+ B cells did not change in female control mice.
Flow cytometry.
B cells are essential mediators of humoral immunity, and they are responsible for the production of antibodies. Percentages of CD45R+ B cells were increased at 28 and 34 wk of age in SLE mice compared with control mice (Fig. 1, C–E). The percentage of CD45R+ B cells did not significantly change in control mice over time. The increase in CD45R+ B cells coincided with the increase in anti-dsDNA IgG levels in SLE mice.
Blood urea nitrogen.
Plasma BUN is a common clinical indicator of kidney function that increases in parallel with a decline in glomerular filtration. SLE mice developed significantly higher levels of plasma BUN by 31 wk of age and remained increased through 34 wk of age (Fig. 2) compared with control mice. BUN did not change in control mice, suggesting that renal function was normal.
Fig. 2.
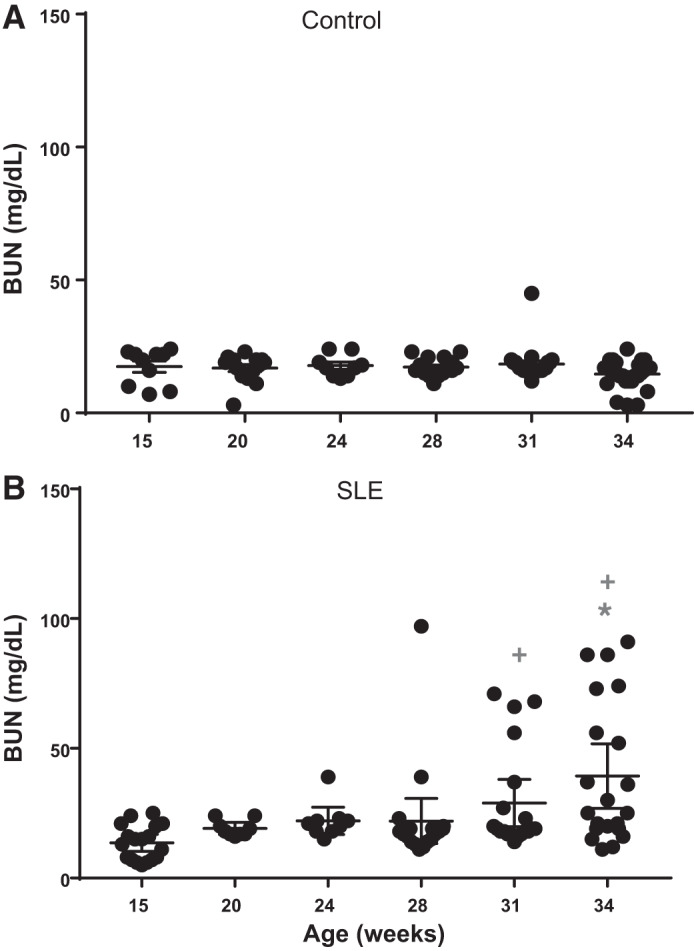
Plasma blood urea nitrogen (BUN) over time in female mice. A: BUN did not change in female control mice. B: BUN was significantly increased by 34 wk of age (; P = 0.0001) compared with 15 wk of age in female mice with systemic lupus erythematosus (SLE; 95% confidence interval: −41.03 to −10.43). BUN was significantly increased by 31 wk of age (+P = 0.0451) and 34 wk of age (+P < 0.0001) in mice with SLE compared with age-matched control mice (95% confidence interval: −21.46 to 0.1462 and −34.78 to −14.65, respectively).
Albuminuria.
Renal injury, measured by albuminuria, is a common finding in patients with SLE (2, 12, 58). Approximately 30% of the mice with SLE had increased urinary albumin excretion by 34 wk of age (Fig. 3). This is consistent with the prevalence of albuminuria reported in our previous studies with this model (35, 68, 70). Control mice did not develop albuminuria at any of the ages studied.
Fig. 3.
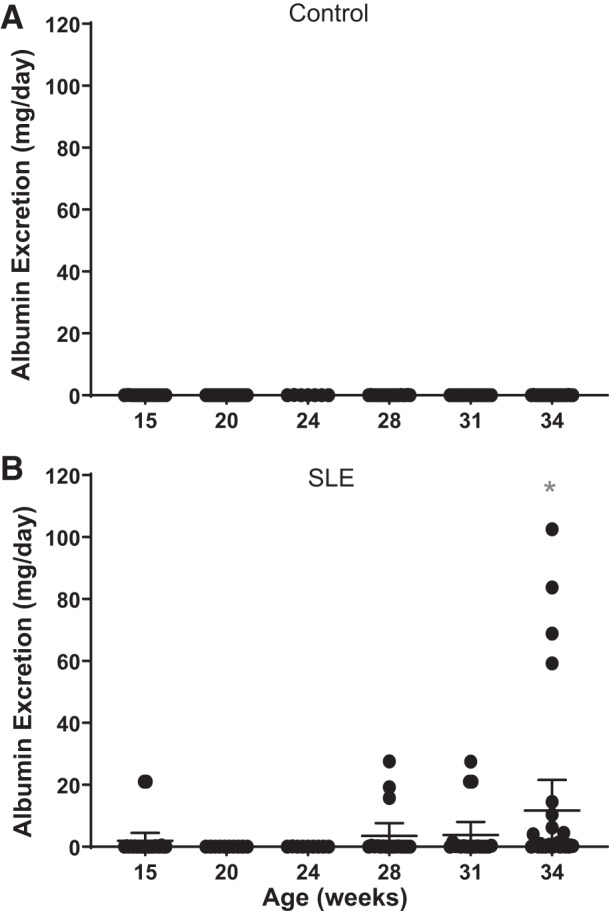
Urinary albumin excretion over time in female mice. A: albumin excretion did not change in female control mice. B: albumin excretion was increased by 34 wk of age compared with 15 wk of age in female mice with systemic lupus erythematosus (SLE). Albumin excretion was significantly increased by 34 wk of age (*P = 0.0263) in mice with SLE compared with age-matched control mice (95% confidence interval: −22.40 to −0.8994).
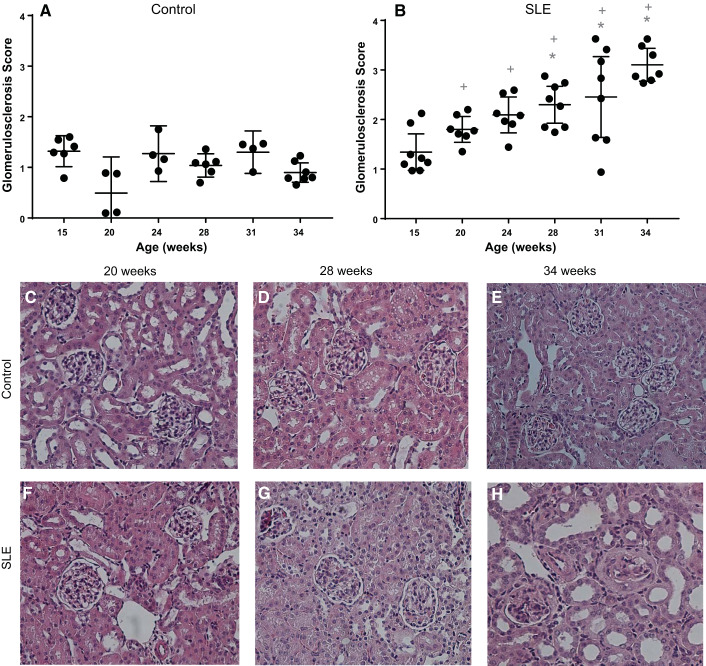
Glomerulosclerosis.
Glomerular scoring was assessed for control and SLE mice at each time point. Whereas glomerular scores were unchanged in control mice across the study, glomerular injury became evident by 28 wk of age in SLE mice (Fig. 4, A–H) and remained significantly increased compared with control mice for the duration of the study.
Fig. 4.
A and B: glomerulosclerosis was present in female mice with systemic lupus erythematosus (SLE) by 28 wk of age. A: glomerular scores did not increase in female control mice. B: glomerular scores were significantly increased by 28 wk of age (*P = 0.0058), 31 wk of age (*P = 0.0011), and 34 wk of age (*P < 0.0001) compared with 15 wk of age in female mice with SLE (95% confidene interval: −1.695 to −0.218, −1.849 to −0.3727, and −2.526 to −0.998, respectively). Glomerular scores were significantly increased at 20 wk of age (+P = 0.0002), 24 wk of age (+P = 0.0361), 28 wk of age (+P < 0.0001), 31 wk of age (+P = 0.0008), and 34 wk of age (+P < 0.0001) in mice with SLE compared with age-matched control mice (95% confidence interval: −2.095 to −0.5209, −1.609 to −0.03508, −1.938 to −0.5817, −1.923 to −0.3850, and −2.879 to −1.536, respectively). C−H: representative pictures of glomerulosclerosis (×40) from paraffin-embedded kidneys stained with hematoxylin and eosin.
Glomerular filtration rate.
Low GFR is commonly reported in patients with SLE (42); however, the temporal changes in GFR that occur during this disorder have not been previously examined. In the present study, GFR remained unchanged in mice with SLE until 28 wk of age, at which time GFR was significantly increased compared with 15 wk of age and compared with age-matched control mice. This was followed by a sharp decline in mice with SLE by 34 wk of age (Fig. 5). In control mice, GFR remained relatively constant throughout the study. However, an increase in GFR was observed at 28 wk of age that returned to normal at 31 and 34 wk of age.
Fig. 5.
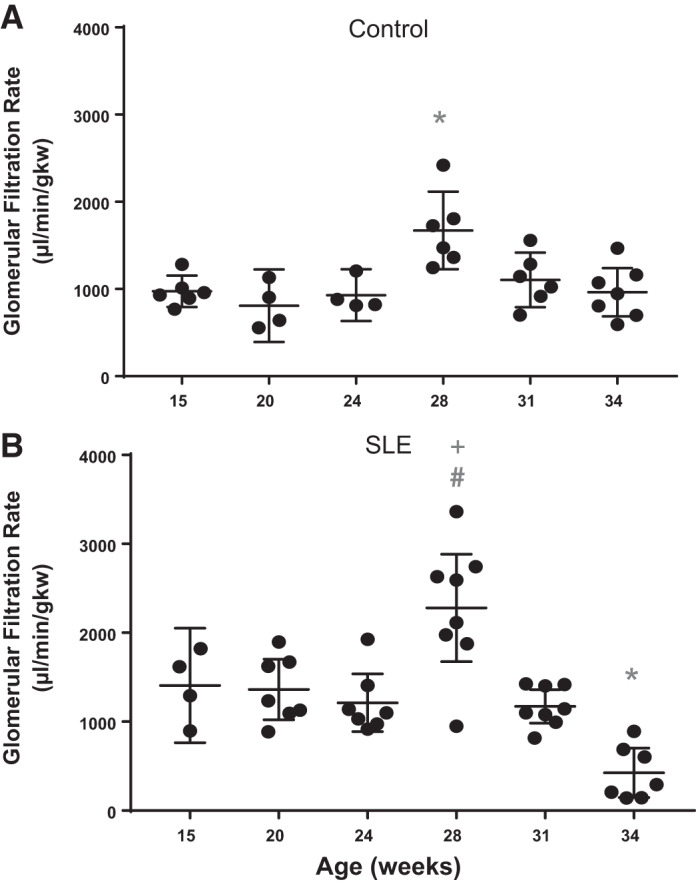
Glomerular filtration rate (GFR) is altered by 28 wk of age in female mice with systemic lupus erythematosus (SLE). A: GFR was increased in female control mice by 28 wk of age (*P = 0.0017) compared with 15 wk of age (95% confidence interval: −1169 to −226.3). B: GFR was significantly increased by 28 wk of age (#P = 0.0140) and significantly decreased by 34 wk of age (*P = 0.0057) compared with 15 wk of age in female mice with SLE (95% confidence interval: −1,615 to 131.5 and 223.6 to 1,742, respectively) and significantly increased at 28 wk (+P = 0.0254) in female mice with SLE compared with age-matched control mice (95% confidence interval: −1,133 to −50.97).
Mean arterial pressure.
Hypertension is prevalent in patients with SLE (1, 14, 43, 46, 55, 58). SLE mice had significantly higher mean arterial pressure (MAP) by 34 wk of age compared with 15 wk of age and significantly higher MAP at 15, 28, 31, and 34 wk of age compared with age-matched control mice (Fig. 6). Blood pressure remained constant at all ages in control mice.
Fig. 6.
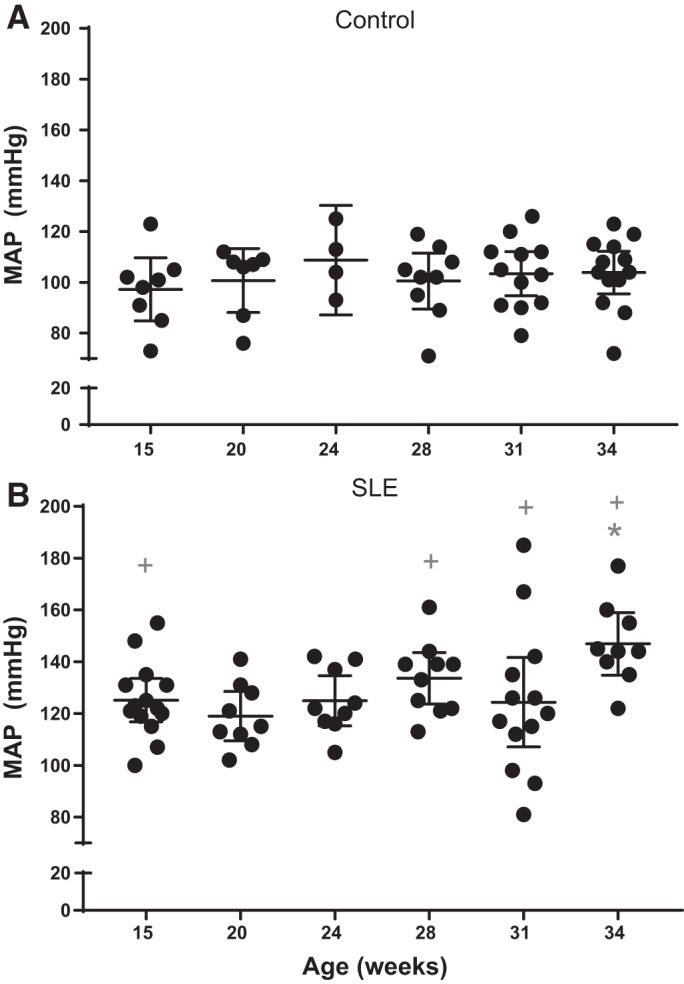
Mean arterial pressure (MAP) is increased by 34 wk of age in female mice with systemic lupus erythematosus (SLE). A: MAP remained unchanged in female control mice. B: MAP was significantly increased by 34 wk of age (*P = 0.0318) compared with 15 wk of age in female mice with SLE (95% confidence interval: −42.2 to −1.292). MAP was significantly increased by 15 wk of age (+P = 0.0012), 28 wk of age (+P = 0.0002), 31 wk of age (+P = 0.0105), and 34 wk (+P < 0.0001) compared with age-matched control mice (95% confidence interval: −47.28 to −8.506, −53.14 to −12.95, −38.48 to −3.457, and −62.01 to −24.07, respectively).
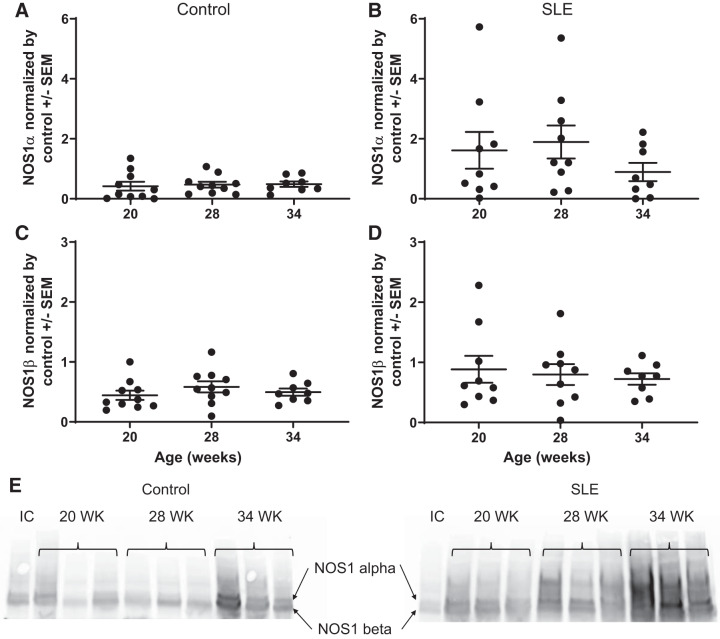
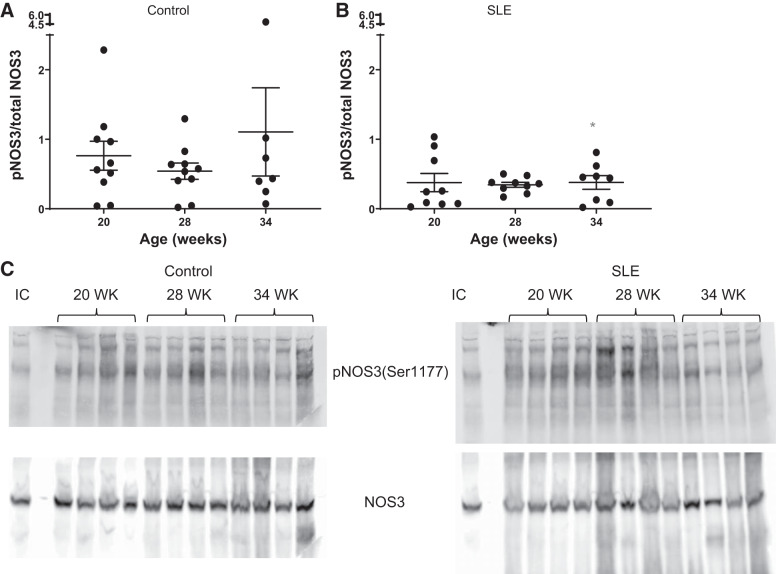
Renal NOS.
Whole kidney NOS1α protein expression was unchanged in control mice. In mice with SLE, there was an apparent increase in NOS1α between 20 and 28 wk of age followed by a decline at 34 wk of age. NOS1β, a marker of renal injury (61), remained unchanged in control mice throughout the study. Consistent with renal injury, NOS1β expression appeared higher in SLE mice throughout the study, although it did not reach statistical signifcance (Fig. 7). Whole kidney NOS3 expression was also assessed. NOS3-pSer1177 (expressed as the ratio of NOS3-pSer1177 to total NOS3) remained unchanged throughout the study in control mice. This was lower in kidneys from mice with SLE throughout the study and was significantly decreased in SLE mice at 34 wk of age compared with age-matched controls (Fig. 8).
Fig. 7.
Nitric oxide synthase (NOS)1α and NOS1β over time in female mice. A: NOS1α was not changed in control mice. B: NOS1α was higher but not changed in female mice with systemic lupus erythematosus (SLE). C: NOS1β did not change in female control mice. D: NOS1β was higher but not changed in female mice with SLE. E: representative blots for NOS1α and NOS1β. IC, internal control.
Fig. 8.
Phosphorylation of nitric oxide synthase (NOS3) at serine residue 1177 [pNOS3(Ser1177) to total NOS3] over time in female mice. A: phosphorylation of NOS3 at serine residue 1177 was not changed in control mice. B: phosphorylation of NOS3 at serine residue 1177 was lower in female mice with systemic lupus erythematosus (SLE). Phosphorylation of NOS3 at serine residue 1177 was significantly lower at 34 wk of age (*P = 0.0440) in female mice with SLE compared with age-matched control mice (95% confidence interval: 0.03876 to 3.489). C: representative blots for phosphorylation of NOS3 at serine residue 1177 and total NOS3. IC, internal control.
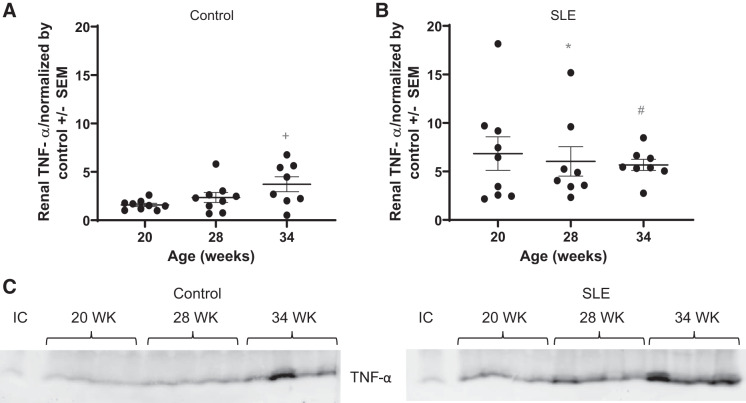
Renal TNF-α.
Western blot analysis of the inflammatory cytokine TNF-α in the kidney showed that expression was higher in mice with SLE compared with control mice (Fig. 9). This is consistent with previously published work from our laboratory showing significantly higher levels of renal cortical TNF-α at 34 wk of age in mice with SLE (68) and others showing increased levels of circulating and renal TNF-α with increasing age (18, 41).
Fig. 9.
Whole kidney TNF-α expression is higher in mice with systemic lupus erythematosus (SLE). A: renal TNF-α was significantly increased by 34 wk of age (+P = 0.0158) compared with 20 wk of age in female control mice (SE of difference = 0.7419=). B: renal TNF-α expression was significantly higher at 20 wk of age (*P = 0.0019) and 28 wk of age (#P = 0.0476) in mice with SLE compared with age-matched control mice (SE of difference = 1.430 and 1.474, respectively). C: representative blots for TNF-α expression. IC, internal control.
DISCUSSION
Patients with SLE develop autoantibodies to nuclear components, renal impairment, and prevalent hypertension. A previous study in our laboratory has shown that female NZBWF1 mice have impaired renal hemodynamic function during active renal disease (69), similar to what has been published in humans (12). However, the impact of autoimmunity on renal hemodynamic function early in the course of the disease, before obvious renal injury, has not been investigated. Understanding these changes has important implications for understanding the factors that promote the prevalent hypertension during SLE and will address a common question as to whether the hypertension is driving the renal injury. In the present study, we hypothesized that the development of autoantibodies associated with autoimmune disease precedes changes in renal hemodynamic function that lead to hypertension in female mice with SLE. This study advances the field by 1) further linking autoantibody production with the prevalent renal dysfunction and hypertension during SLE, 2), demonstrating that hypertension is not the major factor leading to renal injury in this model of SLE, 3) demonstrating that impaired renal function precedes the development of hypertension in female mice with SLE, and 4) providing evidence that NOS and TNF-α expression are changing in the kidneys of mice with SLE in a manner consistent with increased renal injury/inflammation and generalized renal vascular dysfunction.
Patients with SLE commonly develop autoantibodies to nuclear components, with anti-dsDNA IgG being highly specific for SLE (50, 77). While the presence of autoantibodies correlates with disease activity in humans (16, 77), the impact of autoantibodies on renal hemodynamic function and blood pressure remains unclear. This study affirms that autoantibodies progressively increase in female mice with SLE (7), whereas autoantibody levels remain relatively constant in age-matched controls. The data reported here show that anti-dsDNA IgG increases between 24 and 28 wk of age in mice with SLE. This is consistent with our previous work (24) showing an increase in this specific antibody at the same age. Others have reported the detection of autoantibodies at even earlier time points in this model (3, 7, 13). By 34 wk of age, there is a moderate but statistically significant increase in circulating anti-dsDNA IgG in control mice. Despite the small increase in circulating autoantibodies, control mice did not exhibit signs of autoimmune disease at the ages studied. This is consistent with the known phenotype of the NZW/LacJ strain (47, 71). The increased circulating CD45R+ B cells in mice with SLE are consistent with the production of autoantibodies and further support the concept that autoantibodies precede the development of disease phenotypes (4), including renal injury, changes in renal function, and hypertension.
It is estimated that up to 50−60% of patients with SLE exhibit signs of renal involvement (11, 56, 77), including immune complex deposition, glomerulonephritis, glomerular and interstitial scarring, and tubular lesions (39), leading to an increased incidence of chronic kidney disease. Consistent with SLE in humans, we and others have previously shown that female NZBWF1 mice develop glomerulosclerosis, tubulointerstitial fibrosis, and albuminuria (7, 17, 22, 35, 37, 45, 54, 64, 78). Patients with SLE also have prevalent hypertension (1, 14, 43, 46, 55, 58), and, like humans, female NZBWF1 mice develop hypertension (52, 54). Although SLE is an autoimmune disorder and it is known that circulating autoantibodies promote immune complex-mediated renal disease, the question about the impact of renal injury on blood pressure during SLE has not been directly addressed. Clinical data suggest that many patients with SLE develop renal injury including albuminuria, glomerulosclerosis, and nephritis before, after, or without developing hypertension (2, 12, 58). In addition, clinical studies have suggested that higher “normal” levels of albuminuria have predictive value for identifying those at higher risk for the development of hypertension (20, 72, 75). Therefore, in humans, evidence suggests that renal injury is not dependent on the development of hypertension. Additionally, markers of renal inflammation, such as the cytokine TNF-α, have been shown to be elevated in humans with SLE (6), and TNF-α correlates with human hypertension as well (9). The increased renal TNF-α expression in the present study is consistent with human SLE and also consistent with our previously published work (68). Importantly, blockade of TNF-α partially attenuates the hypertension in this model. The present study provides new insights because renal TNF-α expression is increased as early as 20 wk of age in SLE mice compared with controls, which also precedes the development of hypertension.
Like humans, female mice with SLE develop glomerulosclerosis and albuminuria, whereas age-matched control mice do not. The development of albuminuria, and severity of albuminuria, is variable in this model. The data in the present study are consistent with previously published studies in this model (34, 70) with ~40% of the mice developing albuminuria by 34 wk of age. Importantly, albuminuria was unchanged in control animals across the entire study, whereas albumin excretion began to change by 28 wk of age in SLE mice. This is consistent with human data in which rates of albuminuria vary, particularly in patients without established renal disease (8). It is a significant finding of this study that glomerular injury precedes the development of hypertension in mice with SLE. Similarly, other markers of renal injury, including BUN, also began to significantly change by 31 wk of age in mice with SLE before the development of hypertension. BUN levels have been previously reported by others to be increased by 8 mo of age in female NZBWF1 mice (15, 62). These data provide new evidence demonstrating that hypertension is not the major factor causing renal injury in this model. These data are also consistent with previous work from our laboratory suggesting that glomerulosclerosis scores or albumin excretion may not correlate with hypertension in NZBWF1 mice (63). This does not exclude the possibility that established hypertension exacerbates existing renal injury, but it does clearly demonstrate that the development of renal injury is not dependent upon elevated blood pressure.
Given that renal injury is occurring by 28 wk of age, it is reasonable to conclude that the circulating autoantibodies and subsequent renal injury are mechanistically contributing to changes in renal hemodynamic function associated with SLE. While it is clear that patients with SLE with active disease exhibit impaired GFR and renal blood flow during active flares (42), there is a paucity of data related to how renal hemodynamic function changes in the long term and how it changes with relationship to the development of hypertension in these patients. This study shows the course of renal hemodynamic changes throughout the lifespan of female mice with SLE. Our findings demonstrate that GFR is significantly increased at 28 wk of age compared with 15 wk of age in SLE mice followed by a sharp decline after autoantibodies are present and before changes in MAP.
The reason behind the increased GFR at 28 wk of age is unclear. We interpret the significant increase in GFR at 28 wk of age to be an indicator of renal function change that occurs in both groups of mice. However, GFR returns to normal levels in control mice but falls dramatically in SLE mice so that GFR is significantly lower at 34 wk of age compared with control mice. These data reflect the changing renal hemodynamic function before the development of hypertension. In an attempt to gain insight into the possible factors that might contribute to the changing GFR, we assessed protein expression of renal NOS1 and NOS3. NOS1 and NOS3 are expressed throughout the kidney (21, 40), where they catalyze NO using l-arginine and molecular oxygen to regulate hemodynamics and vascular tone, respectively (21, 30). NO has been reported to protect kidney function in a healthy animal; however, under conditions of oxidative stress and renal injury, that protection is lost (5). Renal NO has also been shown to contribute to renal hemodynamic regulation, pressure natriuresis, attenuation of tubuloglomerular feedback, renal sympathetic nerve activity, tubular Na+ reabsorption inhibition, and hypertension (5, 40). We assessed two splice variants of NOS1, NOS1α and NOS1β, and the amount of phosphorylated NOS3 (ratio of NOS3-pSer1177 to total NOS3). Although there is biological variability, the results suggest that NOS1α is increased at 28 wk of age in SLE mice, which may contribute to the increased GFR reported at that age. Similarly, renal NOS1β expression appears higher in SLE kidneys at all ages examined, consistent with the developing renal injury associated with this model. Neither NOS1α nor NOS1β expression changed in female control mice. NOS3-pSer1177 is associated with endothelial function in the vasculature (21), and NOS1 has been reported to help maintain vasodilation when NOS3 is dysfunctional (57).
Our data show that the amount of phoshphorylated NOS3 (NOS3-pSer1177 to total NOS3) did not change in control mice across the study. Importantly, renal NOS3-pSer1177 was lower in mice with SLE compared with control mice. The decrease in NOS3-pSer1177 may be reflective of renal vascular dysfunction. This would be consistent with our previous study showing that mice with SLE exhibit systemic vascular dysfunction by 20 wk of age (before the onset of hypertension) (54). The lower renal NOS3-pSer1177, coupled with the increased NOS1β, may also be reflective of, and consistent with, our previously published work showing that there is increased oxidative stress in the kidneys of mice with SLE (35).
Irrespective of the factors that are causing the changes in GFR, the decrease in GFR that occurs after 28 wk of age provides mechanistic insights into the development of hypertension during SLE. A reduction in GFR can cause hypertension by reducing medullary blood flow that leads to a decrease in renal interstitial hydrostatic pressure at the vasa recta promoting an increase in tubular Na+ reabsorption. The changing renal hemodynamics reported in the present study are consistent with our previously published work demonstrating that mice with SLE have a rightward, hypertensive shift in the pressure natriuresis relationship (36).
Taken together, our data support the concept that autoantibodies, which are known to promote renal injury during SLE, ultimately lead to changes in renal hemodynamic function that mechanistically contribute to the prevalent hypertension. In humans, the Prevention of Renal and Vascular End State Disease study showed a significant interaction between estimated GFR or albuminuria and the subsequent development of hypertension (60). Therefore, our data in mice more clearly demonstrate the link between renal injury, impaired renal hemodynamics, and their relationship to the subsequent development of hypertension during SLE. Ultimately, this study makes a significant new advance by providing a temporal analysis of SLE disease characteristics and how they relate the development of hypertension, a prevalent feature of patients with SLE.
GRANTS
This work was supported by Veterans Administration Merit Award BX002604-01A2 (to M. J. Ryan) and National Institutes of Health (NIH) Grants PO1-HL-051971 and P20-GM-104357 to the University of Mississippi Medical Center (UMMC) Department of Physiology and Biophysics. This work was also supported by NIH Grants HL-134711 (to J. M. Sasser) and HL-137673 (to M. Garrett) in UMMC Physiology and Toxicology. Flow cytometry experiments were performed at the UMMC Cancer Center and Research Institute Flow Cytometry Core Facility.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
E.L.D. and J.M.S. performed experiments; E.L.D., E.B.T., and J.M.S. analyzed data; E.L.D., E.B.T., J.M.S., and M.J.R. interpreted results of experiments; E.L.D. prepared figures; E.L.D. drafted manuscript; E.L.D., E.B.T., J.M.S., and M.J.R. edited and revised manuscript; E.L.D., E.B.T., J.M.S., and M.J.R. approved final version of manuscript; M.J.R. conceived and designed research.
ACKNOWLEDGMENTS
The authors thank the University of Mississippi Medical Center (UMMC) Analytic Assay Core, UMMC Department of Physiology and Biophysics, UMMC Histology Core, and Dr. M. Garrett and Dr. W. Wu (UMMC Pharmacology and Toxicology).
REFERENCES
- 1.Al-Herz A, Ensworth S, Shojania K, Esdaile JM. Cardiovascular risk factor screening in systemic lupus erythematosus. J Rheumatol 30: 493–496, 2003. [PubMed] [Google Scholar]
- 2.Anders HJ, Weening JJ. Kidney disease in lupus is not always ‘lupus nephritis’. Arthritis Res Ther 15: 108, 2013. doi: 10.1186/ar4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews BS, Eisenberg RA, Theofilopoulos AN, Izui S, Wilson CB, McConahey PJ, Murphy ED, Roths JB, Dixon FJ. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med 148: 1198–1215, 1978. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 349: 1526–1533, 2003. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 5.Arellano-Mendoza MG, Vargas-Robles H, Del Valle-Mondragon L, Rios A, Escalante B. Prevention of renal injury and endothelial dysfunction by chronic l-arginine and antioxidant treatment. Ren Fail 33: 47–53, 2011. doi: 10.3109/0886022X.2010.541583. [DOI] [PubMed] [Google Scholar]
- 6.Aringer M, Feierl E, Steiner G, Stummvoll GH, Höfler E, Steiner CW, Radda I, Smolen JS, Graninger WB. Increased bioactive TNF in human systemic lupus erythematosus: associations with cell death. Lupus 11: 102–108, 2002. doi: 10.1191/0961203302lu160oa. [DOI] [PubMed] [Google Scholar]
- 7.Bassi N, Luisetto R, Ghirardello A, Gatto M, Valente M, Della Barbera M, Nalotto L, Punzi L, Doria A. 17-β-Estradiol affects BLyS serum levels and the nephritogenic autoantibody network accelerating glomerulonephritis in NZB/WF1 mice. Lupus 24: 382–391, 2015. doi: 10.1177/0961203314559636. [DOI] [PubMed] [Google Scholar]
- 8.Batlle-Gualda E, Martínez AC, Guerra RA, Pascual E. Urinary albumin excretion in patients with systemic lupus erythematosus without renal disease. Ann Rheum Dis 56: 386–389, 1997. doi: 10.1136/ard.56.6.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bautista LE, Vera LM, Arenas IA, Gamarra G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J Hum Hypertens 19: 149–154, 2005. doi: 10.1038/sj.jhh.1001785. [DOI] [PubMed] [Google Scholar]
- 10.Benvenuti F, Gatto M, Larosa M, Iaccarino L, Punzi L, Doria A. Cardiovascular risk factors, burden of disease and preventive strategies in patients with systemic lupus erythematosus: a literature review. Expert Opin Drug Saf 14: 1373–1385, 2015. doi: 10.1517/14740338.2015.1073259. [DOI] [PubMed] [Google Scholar]
- 11.Boumpas DT, Austin HA III, Fessler BJ, Balow JE, Klippel JH, Lockshin MD. Systemic lupus erythematosus: emerging concepts. Part 1: renal, neuropsychiatric, cardiovascular, pulmonary, and hematologic disease. Ann Intern Med 122: 940–950, 1995. doi: 10.7326/0003-4819-122-12-199506150-00009. [DOI] [PubMed] [Google Scholar]
- 12.Budman DR, Steinberg AD. Hypertension and renal disease in systemic lupus erythematosus. Arch Intern Med 136: 1003–1007, 1976. doi: 10.1001/archinte.1976.03630090033009. [DOI] [PubMed] [Google Scholar]
- 13.Burnett R, Ravel G, Descotes J. Clinical and histopathological progression of lesions in lupus-prone (NZB x NZW) F1 mice. Exp Toxicol Pathol 56: 37–44, 2004. doi: 10.1016/j.etp.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Chung CP, Avalos I, Oeser A, Gebretsadik T, Shintani A, Raggi P, Stein CM. High prevalence of the metabolic syndrome in patients with systemic lupus erythematosus: association with disease characteristics and cardiovascular risk factors. Ann Rheum Dis 66: 208–214, 2007. doi: 10.1136/ard.2006.054973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corna D, Morigi M, Facchinetti D, Bertani T, Zoja C, Remuzzi G. Mycophenolate mofetil limits renal damage and prolongs life in murine lupus autoimmune disease. Kidney Int 51: 1583–1589, 1997. doi: 10.1038/ki.1997.217. [DOI] [PubMed] [Google Scholar]
- 16.Cozzani E, Drosera M, Gasparini G, Parodi A. Serology of lupus erythematosus: correlation between immunopathological features and clinical aspects. Autoimmune Dis 2014: 321359, 2014. doi: 10.1155/2014/321359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davidson A, Bethunaickan R, Berthier C, Sahu R, Zhang W, Kretzler M. Molecular studies of lupus nephritis kidneys. Immunol Res 63: 187–196, 2015. doi: 10.1007/s12026-015-8693-6. [DOI] [PubMed] [Google Scholar]
- 18.Dayan M, Segal R, Globerson A, Habut B, Shearer GM, Mozes E. Effect of aging on cytokine production in normal and experimental systemic lupus erythematosus-afflicted mice. Exp Gerontol 35: 225–236, 2000. doi: 10.1016/S0531-5565(00)00076-0. [DOI] [PubMed] [Google Scholar]
- 19.Doria A, Iaccarino L, Ghirardello A, Zampieri S, Arienti S, Sarzi-Puttini P, Atzeni F, Piccoli A, Todesco S. Long-term prognosis and causes of death in systemic lupus erythematosus. Am J Med 119: 700–706, 2006. doi: 10.1016/j.amjmed.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 20.Forman JP, Fisher ND, Schopick EL, Curhan GC. Higher levels of albuminuria within the normal range predict incident hypertension. J Am Soc Nephrol 19: 1983–1988, 2008. doi: 10.1681/ASN.2008010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J 33: 829–837, 2012. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frese-Schaper M, Zbaeren J, Gugger M, Monestier M, Frese S. Reversal of established lupus nephritis and prolonged survival of New Zealand black x New Zealand white mice treated with the topoisomerase I inhibitor irinotecan. J Immunol 184: 2175–2182, 2010. doi: 10.4049/jimmunol.0903153. [DOI] [PubMed] [Google Scholar]
- 23.Giannelou M, Mavragani CP. Cardiovascular disease in systemic lupus erythematosus: a comprehensive update. J Autoimmun 82: 1–12, 2017. doi: 10.1016/j.jaut.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert EL, Ryan MJ. Impact of early life ovariectomy on blood pressure and body composition in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol 307: R990–R997, 2014. doi: 10.1152/ajpregu.00038.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gustafsson JT, Herlitz Lindberg M, Gunnarsson I, Pettersson S, Elvin K, Öhrvik J, Larsson A, Jensen-Urstad K, Svenungsson E. Excess atherosclerosis in systemic lupus erythematosus−a matter of renal involvement: case control study of 281 SLE patients and 281 individually matched population controls. PLoS One 12: e0174572, 2017. doi: 10.1371/journal.pone.0174572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gustafsson JT, Svenungsson E. Definitions of and contributions to cardiovascular disease in systemic lupus erythematosus. Autoimmunity 47: 67–76, 2014. doi: 10.3109/08916934.2013.856005. [DOI] [PubMed] [Google Scholar]
- 27.Han S, Zhuang H, Shumyak S, Yang L, Reeves WH. Mechanisms of autoantibody production in systemic lupus erythematosus. Front Immunol 6: 228, 2015. doi: 10.3389/fimmu.2015.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikdahl E, Wibetoe G, Rollefstad S, Salberg A, Bergsmark K, Kvien TK, Olsen IC, Soldal DM, Bakland G, Lexberg Å, Fevang BTS, Gulseth HC, Haugeberg G, Semb AG. Guideline recommended treatment to targets of cardiovascular risk is inadequate in patients with inflammatory joint diseases. Int J Cardiol 274: 311–318, 2019. doi: 10.1016/j.ijcard.2018.06.111. [DOI] [PubMed] [Google Scholar]
- 29.Knight JS, Kaplan MJ. Cardiovascular disease in lupus: insights and updates. Curr Opin Rheumatol 25: 597–605, 2013. doi: 10.1097/BOR.0b013e328363eba3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Komers R, Lindsley JN, Oyama TT, Allison KM, Anderson S. Role of neuronal nitric oxide synthase (NOS1) in the pathogenesis of renal hemodynamic changes in diabetes. Am J Physiol Renal Physiol 279: F573–F583, 2000. doi: 10.1152/ajprenal.2000.279.3.F573. [DOI] [PubMed] [Google Scholar]
- 31.Lewandowski LB, Kaplan MJ. Update on cardiovascular disease in lupus. Curr Opin Rheumatol 28: 468–476, 2016. doi: 10.1097/BOR.0000000000000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lupus Foundation of America Lupus facts and statistics. 2012. [Google Scholar]
- 33.Mathis KW, Taylor EB, Ryan MJ. Anti-CD3 antibody therapy attenuates the progression of hypertension in female mice with systemic lupus erythematosus. Pharmacol Res 120: 252–257, 2017. doi: 10.1016/j.phrs.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathis KW, Venegas-Pont M, Flynn ER, Williams JM, Maric-Bilkan C, Dwyer TM, Ryan MJ. Hypertension in an experimental model of systemic lupus erythematosus occurs independently of the renal nerves. Am J Physiol Regul Integr Comp Physiol 305: R711–R719, 2013. doi: 10.1152/ajpregu.00602.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mathis KW, Venegas-Pont M, Masterson CW, Stewart NJ, Wasson KL, Ryan MJ. Oxidative stress promotes hypertension and albuminuria during the autoimmune disease systemic lupus erythematosus. Hypertension 59: 673–679, 2012. doi: 10.1161/HYPERTENSIONAHA.111.190009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathis KW, Venegas-Pont M, Masterson CW, Wasson KL, Ryan MJ. Blood pressure in a hypertensive mouse model of SLE is not salt-sensitive. Am J Physiol Regul Integr Comp Physiol 301: R1281–R1285, 2011. doi: 10.1152/ajpregu.00386.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mathis KW, Wallace K, Flynn ER, Maric-Bilkan C, LaMarca B, Ryan MJ. Preventing autoimmunity protects against the development of hypertension and renal injury. Hypertension 64: 792–800, 2014. doi: 10.1161/HYPERTENSIONAHA.114.04006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mok CC, Poon WL, Lai JP, Wong CK, Chiu SM, Wong CK, Lun SW, Ko GT, Lam CW, Lam CS. Metabolic syndrome, endothelial injury, and subclinical atherosclerosis in patients with systemic lupus erythematosus. Scand J Rheumatol 39: 42–49, 2010. doi: 10.3109/03009740903046668. [DOI] [PubMed] [Google Scholar]
- 39.Moulton VR, Suarez-Fueyo A, Meidan E, Li H, Mizui M, Tsokos GC. Pathogenesis of human systemic lupus erythematosus: a cellular perspective. Trends Mol Med 23: 615–635, 2017. doi: 10.1016/j.molmed.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mount PF, Power DA. Nitric oxide in the kidney: functions and regulation of synthesis. Acta Physiol (Oxf) 187: 433–446, 2006. doi: 10.1111/j.1748-1716.2006.01582.x. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura T, Ebihara I, Fukui M, Osada S, Tomino Y, Masaki T, Goto K, Furuichi Y, Koide H. Renal expression of mRNAs for endothelin-1, endothelin-3 and endothelin receptors in NZB/W F1 mice. Ren Physiol Biochem 16: 233–243, 1993. doi: 10.1159/000173768. [DOI] [PubMed] [Google Scholar]
- 42.Nakano M, Ueno M, Hasegawa H, Watanabe T, Kuroda T, Ito S, Arakawa M. Renal haemodynamic characteristics in patients with lupus nephritis. Ann Rheum Dis 57: 226–230, 1998. doi: 10.1136/ard.57.4.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol 55: 829–835, 2006. doi: 10.1016/j.jaad.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 44.Nossent J, Cikes N, Kiss E, Marchesoni A, Nassonova V, Mosca M, Olesinska M, Pokorny G, Rozman B, Schneider M, Vlachoyiannopoulos PG, Swaak A. Current causes of death in systemic lupus erythematosus in Europe, 2000−2004: relation to disease activity and damage accrual. Lupus 16: 309–317, 2007. doi: 10.1177/0961203307077987. [DOI] [PubMed] [Google Scholar]
- 45.Okudaira H, Terada E, Okudaira K. Animal models utilized in the research of autoimmune disease control: experimental therapy of glomerulonephritis in NZB/W F1 mice. Prog Clin Biol Res 229: 157–174, 1987. [PubMed] [Google Scholar]
- 46.Panoulas VF, Douglas KM, Milionis HJ, Stavropoulos-Kalinglou A, Nightingale P, Kita MD, Tselios AL, Metsios GS, Elisaf MS, Kitas GD. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford) 46: 1477–1482, 2007. doi: 10.1093/rheumatology/kem169. [DOI] [PubMed] [Google Scholar]
- 47.Perry D, Sang A, Yin Y, Zheng YY, Morel L. Murine models of systemic lupus erythematosus. J Biomed Biotechnol 2011: 271694, 2011. doi: 10.1155/2011/271694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pons-Estel GJ, Alarcón GS, Scofield L, Reinlib L, Cooper GS. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin Arthritis Rheum 39: 257–268, 2010. doi: 10.1016/j.semarthrit.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rees F, Doherty M, Grainge MJ, Lanyon P, Zhang W. The worldwide incidence and prevalence of systemic lupus erythematosus: a systematic review of epidemiological studies. Rheumatology (Oxford) 56: 1945–1961, 2017. doi: 10.1093/rheumatology/kex260. [DOI] [PubMed] [Google Scholar]
- 50.Reveille JD. Predictive value of autoantibodies for activity of systemic lupus erythematosus. Lupus 13: 290–297, 2004. doi: 10.1191/0961203303lu1015oa. [DOI] [PubMed] [Google Scholar]
- 51.Roman MJ, Salmon JE. Cardiovascular manifestations of rheumatologic diseases. Circulation 116: 2346–2355, 2007. doi: 10.1161/CIRCULATIONAHA.106.678334. [DOI] [PubMed] [Google Scholar]
- 52.Rudofsky UH, Dilwith RL, Roths JB, Lawrence DA, Kelley VE, Magro AM. Differences in the occurrence of hypertension among (NZB X NZW)F1, MRL-lpr, and BXSB mice with lupus nephritis. Am J Pathol 116: 107–114, 1984. [PMC free article] [PubMed] [Google Scholar]
- 53.Rudofsky UH, Lawrence DA. New Zealand mixed mice: a genetic systemic lupus erythematosus model for assessing environmental effects. Environ Health Perspect 107, Suppl 5: 713–721, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ryan MJ, McLemore GR Jr. Hypertension and impaired vascular function in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol 292: R736–R742, 2007. doi: 10.1152/ajpregu.00168.2006. [DOI] [PubMed] [Google Scholar]
- 55.Sabio JM, Vargas-Hitos JA, Navarrete-Navarrete N, Mediavilla JD, Jiménez-Jáimez J, Díaz-Chamorro A, Jiménez-Alonso J. Prevalence of and factors associated with hypertension in young and old women with systemic lupus erythematosus. J Rheumatol 38: 1026–1032, 2011. doi: 10.3899/jrheum.101132. [DOI] [PubMed] [Google Scholar]
- 56.Saxena R, Mahajan T, Mohan C. Lupus nephritis: current update. Arthritis Res Ther 13: 240, 2011. doi: 10.1186/ar3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwarz PM, Kleinert H, Förstermann U. Potential functional significance of brain-type and muscle-type nitric oxide synthase I expressed in adventitia and media of rat aorta. Arterioscler Thromb Vasc Biol 19: 2584–2590, 1999. doi: 10.1161/01.ATV.19.11.2584. [DOI] [PubMed] [Google Scholar]
- 58.Shaharir SS, Mustafar R, Mohd R, Mohd Said MS, Gafor HA. Persistent hypertension in lupus nephritis and the associated risk factors. Clin Rheumatol 34: 93–97, 2015. doi: 10.1007/s10067-014-2802-0. [DOI] [PubMed] [Google Scholar]
- 59.Siegel M, Lee SL. The epidemiology of systemic lupus erythematosus. Semin Arthritis Rheum 3: 1–54, 1973. doi: 10.1016/0049-0172(73)90034-6. [DOI] [PubMed] [Google Scholar]
- 60.Smink PA, Lambers Heerspink HJ, Gansevoort RT, de Jong PE, Hillege HL, Bakker SJ, de Zeeuw D. Albuminuria, estimated GFR, traditional risk factors, and incident cardiovascular disease: the PREVEND (Prevention of Renal and Vascular Endstage Disease) study. Am J Kidney Dis 60: 804–811, 2012. doi: 10.1053/j.ajkd.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 61.Smith C, Merchant M, Fekete A, Nyugen HL, Oh P, Tain YL, Klein JB, Baylis C. Splice variants of neuronal nitric oxide synthase are present in the rat kidney. Nephrol Dial Transplant 24: 1422–1428, 2009. doi: 10.1093/ndt/gfn676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Song YW, Kim HA, Baek HJ, Lee EB, Chung ES, Hong KM. Paclitaxel reduces anti-dsDNA antibody titer and BUN, prolonging survival in murine lupus. Int J Immunopharmacol 20: 669–677, 1998. doi: 10.1016/S0192-0561(98)00057-5. [DOI] [PubMed] [Google Scholar]
- 63.Taylor EB, Barati MT, Powell DW, Turbeville HR, Ryan MJ. Plasma cell depletion attenuates hypertension in an experimental model of autoimmune disease. Hypertension 71: 719–728, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taylor EB, Ryan MJ. Immunosuppression with mycophenolate mofetil attenuates hypertension in an experimental model of autoimmune disease. J Am Heart Assoc 6: e005394, 2017. doi: 10.1161/JAHA.116.005394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tselios K, Koumaras C, Urowitz MB, Gladman DD. Do current arterial hypertension treatment guidelines apply to systemic lupus erythematosus patients? a critical appraisal. Semin Arthritis Rheum 43: 521–525, 2014. doi: 10.1016/j.semarthrit.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 66.Tucker LB, Menon S, Schaller JG, Isenberg DA. Adult- and childhood-onset systemic lupus erythematosus: a comparison of onset, clinical features, serology, and outcome. Br J Rheumatol 34: 866–872, 1995. doi: 10.1093/rheumatology/34.9.866. [DOI] [PubMed] [Google Scholar]
- 67.Urowitz MB, Bookman AA, Koehler BE, Gordon DA, Smythe HA, Ogryzlo MA. The bimodal mortality pattern of systemic lupus erythematosus. Am J Med 60: 221–225, 1976. doi: 10.1016/0002-9343(76)90431-9. [DOI] [PubMed] [Google Scholar]
- 68.Venegas-Pont M, Manigrasso MB, Grifoni SC, LaMarca BB, Maric C, Racusen LC, Glover PH, Jones AV, Drummond HA, Ryan MJ. Tumor necrosis factor-alpha antagonist etanercept decreases blood pressure and protects the kidney in a mouse model of systemic lupus erythematosus. Hypertension 56: 643–649, 2010. doi: 10.1161/HYPERTENSIONAHA.110.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Venegas-Pont M, Mathis KW, Iliescu R, Ray WH, Glover PH, Ryan MJ. Blood pressure and renal hemodynamic responses to acute angiotensin II infusion are enhanced in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol 301: R1286–R1292, 2011. doi: 10.1152/ajpregu.00079.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Venegas-Pont M, Sartori-Valinotti JC, Maric C, Racusen LC, Glover PH, McLemore GR Jr, Jones AV, Reckelhoff JF, Ryan MJ. Rosiglitazone decreases blood pressure and renal injury in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol 296: R1282–R1289, 2009. doi: 10.1152/ajpregu.90992.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wallace D, Hahn B.. Animal models in lupus. In: Dubois’ Lupus Erythematosus and Related Syndromes. Amsterdam: Elsevier, 2019, p. 164–215. [Google Scholar]
- 72.Wang TJ, Evans JC, Meigs JB, Rifai N, Fox CS, D’Agostino RB, Levy D, Vasan RS. Low-grade albuminuria and the risks of hypertension and blood pressure progression. Circulation 111: 1370–1376, 2005. doi: 10.1161/01.CIR.0000158434.69180.2D. [DOI] [PubMed] [Google Scholar]
- 73.Weckerle CE, Niewold TB. The unexplained female predominance of systemic lupus erythematosus: clues from genetic and cytokine studies. Clin Rev Allergy Immunol 40: 42–49, 2011. doi: 10.1007/s12016-009-8192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 71: 1269–1324, 2018. doi: 10.1161/HYP.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 75.Xu H, Huang X, Risérus U, Cederholm T, Lindholm B, Ärnlöv J, Carrero JJ. Urinary albumin excretion, blood pressure changes and hypertension incidence in the community: effect modification by kidney function. Nephrol Dial Transplant 29: 1538–1545, 2014. doi: 10.1093/ndt/gfu057. [DOI] [PubMed] [Google Scholar]
- 76.Yu C, Gershwin ME, Chang C. Diagnostic criteria for systemic lupus erythematosus: a critical review. J Autoimmun 48-49: 10–13, 2014. doi: 10.1016/j.jaut.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 77.Yung S, Chan TM. Autoantibodies and resident renal cells in the pathogenesis of lupus nephritis: getting to know the unknown. Clin Dev Immunol 2012: 139365, 2012. doi: 10.1155/2012/139365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yung S, Yap DY, Chan TM. Recent advances in the understanding of renal inflammation and fibrosis in lupus nephritis. F1000 Res 6: 874, 2017. doi: 10.12688/f1000research.10445.1. [DOI] [PMC free article] [PubMed] [Google Scholar]