Abstract
Objective
To investigate the possibility of combining tuberculosis (TB)-interferon (IFN)-γ release assays (IGRAs) with lymphocyte enumeration for diagnosis of Mycobacterium tuberculosis infection.
Methods
We performed a retrospective study of 166 TB patients [68 patients with pulmonary tuberculosis TB (PTB) and 98 patients with extra-pulmonary TB (EPTB)] diagnosed in our hospital between January 2016 and May 2018 along with 377 non-TB patients. The diagnostic performance of the TB-IGRA was evaluated using receiver operating characteristic (ROC) curves. Youden’s index was used to determine the optimal cut-off threshold.
Results
IFN-γ release in patients with PTB and EPTB were dramatically higher compared with non-TB patients (203.58±18.00 pg/mL, 201.83±14.56 pg/mL and 32.12±4.36 pg/mL, respectively). IFN-γ release was positively correlated with lymphocyte counts and percentages in patients with PTB (r = 0.252 and r = 0.278, respectively) and EPTB (r = 0.229 and r = 0.298, respectively). No correlation was observed in non-TB patients. The area under the ROC curve for TB-IGRA was 0.884. When the optimal cut-off value for IFN-γ (14 pg/mL, Youden’s index 0.661) was applied, the sensitivity was 88.6% and the specificity was 77.5%.
Conclusions
Combining TB-IGRA with lymphocyte enumeration was effective for diagnosis of early-stage Mycobacterium tuberculosis infection.
Keywords: Mycobacterium tuberculosis, TB-IGRA, lymphocyte subpopulations, interferon-γ, diagnostic performance, pulmonary TB, extra-pulmonary TB
Introduction
Mycobacterium tuberculosis (MTB) infection remains a major public health problem worldwide, and was the leading infectious cause of death as late as the 1910s.1 A systematic review conducted in 2011 found that about 70% of patients with sputum smear-positive pulmonary tuberculosis died within 10 years of infection without appropriate drug treatment.2 MTB represents a significant public health challenge in China because of poverty, delayed diagnosis and development of multi-drug resistance.3 In 2016, there were an estimated 10.4 million new cases of MTB worldwide, of which 9% occurred in China.4 Three potential clinical outcomes occur following exposure to MTB: clearance of the bacillus, development of resistance, or latent MTB infection (LTBI). Without prompt diagnosis and treatment, asymptomatic LTBI can persist for decades, eventually becoming symptomatic and leading to pulmonary tuberculosis (PTB) or extra-pulmonary tuberculosis (EPTB). Therefore, early and accurate diagnosis and appropriate treatment of LTBI are critical for achieving optimal patient outcomes.
Recently, interferon (IFN)-γ release assays for diagnosis of MTB infection (TB-IGRAs) have been developed as a promising diagnostic tool for PTB and EPTB.5,6 MTB antigen specific CD4+ T cells are isolated from whole blood of patients. Following stimulation with MTB antigens, TB-IGRAs directly detect IFN-γ release from treated CD4+ T cells to determine whether patients are infected with MTB.7 The MTB antigens used in TB-IGRAs include early secretory antigenic target-6 (ESAT-6) and culture filtrate protein-10 (CFP10). These two antigens are encoded within the RD-1 genomic region of MTB; all Bacillus Calmette–Guérin strains and most non-tuberculous mycobacteria lack these two antigens.8,9 Two methods are typically used for IGRAs: enzyme-linked immunosorbent assays (ELISAs) and enzyme-linked immune absorbent spot (ELISpot) assays. ELISA measures IFN–γ released by T cells in whole blood, while ELISpot detects the number of IFN-γ-secreting T cells responding to specific MTB antigens. Several studies have indicated that the TB-IGRA has higher specificity and sensitivity than sputum smears and tuberculin skin tests for diagnosis of MTB infection.10–12
TB-IGRAs are influenced by immune function and the inflammatory status of the body. Combined evaluation of immune status and TB-IGRAs can improve the reliability of TB-IGRAs and contribute to improved clinical diagnosis of MTB infection. Here, we investigated the cellular immune status associated with MTB infection using TB-IGRA and a series of clinical laboratory indexes. Our overall goal was to develop improved strategies for diagnosis of MTB infection by combining multiple parameters. We propose a new diagnostic threshold for TB-IGRA to diagnose MTB infection. Our results confirmed the necessity of conducting TB-IGRA during the early stages of MTB infection. These data may be helpful for future studies attempting to evaluate and diagnose elderly and immunocompromised patients with MTB infection as well as other patient groups and stages of infection that are currently difficult to diagnose.
Materials and methods
Subjects
MTB patients (PTB and EPTB patients) and non-TB patients visiting Ningbo City First Hospital between January 2016 and May 2018 from were enrolled in the study. MTB patients were diagnosed according to the Clinical Diagnosis Standards for TB in China.13 All patients were divided into three groups: (1) PTB, (2) EPTB, and (3) non-TB, which included patients with other diseases as diagnosed by imaging or pathological examination. PTB patients were subdivided into those with negative and positive sputum smears. Information on age, gender, erythrocyte sedimentation rate (ESR), and levels of C-reactive protein (CRP), white blood cells (WBCs), neutrophils, lymphocytes, total protein, albumin, immunoglobulin, complement, adenosine deaminase (ADA) and CA125 were obtained from medical records. Patients with hematological diseases, human immunodeficiency virus infection, or solid cancers as well as patients receiving immunosuppressive treatments were excluded from the study. The study was approved by the Ethical Committee of Ningbo City First Hospital and written informed consent was obtained from all participants.
TB-IGRA
The IGRA was carried out according to the manufacturer’s instructions (Wantai Biology Ltd., Beijing, China). In brief, 1 mL of heparinized whole blood was added to each of three tubes containing either specific MTB antigens (ESAT-6, CFP-10), no antigens (negative control) or phytohemagglutinin-P (positive control) within 2 hours of sampling. The tubes were incubated at 37°C for 24 hours and then centrifuged at 3,000 ×g for 10 minutes to harvest the supernatants. Subsequently, IFN-γ concentrations were determined by ELISA. First, 20 μL of sample were diluted and then 50 μL of sample plasma or calibration solution were added into sample or standard wells. The wells were gently mixed and incubated at 37°C for 60 minutes. Then, 50 μL of enzyme-labeled antibody was added to the sample and standard wells, gently mixed, and incubated at 37°C for 60 minutes. After washing five times, 50 μL of chromogenic solutions A and B were sequentially added into the wells and incubated for 15 minutes at 37°C. Finally, the reaction was stopped and absorbance was measured using a microplate reader at 450 nm. Standard curves were prepared for each experiment. According to the manufacturer’s recommendations, γ ≥ 0.98, IFN-γ ≥ 14 pg/mL was classified as positive. The linear range of detection was 2 pg/mL to 400 pg/mL.
Flow cytometry analysis
Lymphocyte subpopulations in whole blood were analyzed for a subset of PTB patients, EPTB patients and non-TB patients by flow cytometry. The following monoclonal antibodies were purchased from BD Biosciences (San Diego, CA, USA) and used for cell staining: CD3-fluorescein isothiocyanate, CD4-peridinin-chlorophyll-protein-cyanine 5, CD8-phycoerythrin (PE), CD19-allophycocyanin, and CD16/56-PE. Four-color flow cytometry was performed using a BD Canto II flow cytometer (BD Biosciences). Data were analyzed using FACSDiva software (BD Biosciences).
Statistical analyses
All statistical analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). Data were presented as means ± standard errors of the mean (SEMs). The Kruskal–Wallis H test was used to assess differences in age, CRP, ESR, WBCs, neutrophils, lymphocytes, total protein, albumin, immunoglobulin, complement, ADA and CA125. The chi-square (χ2) test was used to assess differences in gender proportion. Nonparametric correlation analysis was used to assess correlations between IFN-γ levels and frequencies or percentages of lymphocytes, CD4+ T cells, and CD8+ T cells. Receiver operating characteristic (ROC) curves were used to plot sensitivity versus 1-specificity (evaluated at several different diagnostic thresholds of IFN-γ concentrations). Youden’s index was used to determine the optimal cut-off threshold. Two-sided values of p < 0.05 were considered statistically significant.
Results
Comparison of clinical parameters in PTB, EPTB and non-TB patients
A total of 166 TB patients (68 PTB and 98 EPTB patients) and 377 non-TB patients were enrolled in the study between January 2016 and May 2018. Among PTB patients, 42 had negative sputum smears and 26 had positive sputum smears. The mean age of TB patients was 48.8 ± 1.50 years and the mean age of non-TB patients was 52.7 ± 0.96 years.
Levels of CRP, complement C3, ADA and CA125 in the sera of patients with PTB and EPTB were significantly elevated compared with non-TB patients (p = 0.019, p = 0.019, p = 0.009 and p < 0.001, respectively; Table 1). The total serum protein levels of patients with PTB were lower than those of patients with EPTB and those of non-TB patients (p = 0.004, Table 1). No significant differences in other parameters were identified among the three groups.
Table 1.
Demographic and clinical characteristics of TB and non-TB patients.
| Clinical parameters | PTB (n = 68) | EPTB (n = 98) | Non-TB (n = 377) | p value* |
|---|---|---|---|---|
| Age (years)§ | 49.1 ± 2.40 | 48.7 ± 2.02 | 52.7 ± 0.96 | 0.078 |
| Gender | ||||
| Male (n, %) | 35 (51.5%) | 49 (50%) | 177 (46.9%) | |
| Female (n, %) | 33 (48.5%) | 49 (50%) | 200 (53.1%) | 0.722 |
| CRP (mg/L)§ | 37.77 ± 5.05 | 39.67 ± 4.05 | 35.25 ± 2.76 | 0.019 |
| ESR (mm/h)§ | 45.9 ± 4.05 | 51.2 ± 2.68 | 49.0 ± 1.64 | 0.414 |
| Total protein (g/L)§ | 65.6 ± 0.94 | 68.8 ± 0.82 | 66.8 ± 0.41 | 0.004 |
| Albumin (g/L)§ | 37.2 ± 0.73 | 38.1 ± 0.50 | 37.3 ± 0.29 | 0.354 |
| IgG (mg/dL)§ | 1342.5 ± 50.86 | 1556.3 ± 74.55 | 1422.9 ± 23.36 | 0.175 |
| IgM (mg/dL)§ | 128.7 ± 8.80 | 120.9 ± 8.35 | 117.8 ± 3.29 | 0.185 |
| IgA (mg/dL)§ | 283.3 ± 16.79 | 310.3 ± 16.53 | 291.7 ± 7.46 | 0.286 |
| C3 (mg/dL)§ | 103.2 ± 3.55 | 107.9 ± 4.07 | 97.2 ± 1.54 | 0.019 |
| C4 (mg/dL)§ | 23.8 ± 1.13 | 25.0 ± 1.20 | 22.8 ± 0.45 | 0.146 |
| ADA (U/L)§ | 11.7 ± 0.78 | 12.4 ± 0.77 | 11.1 ± 0.37 | 0.009 |
| CA125 (U/mL)§ | 84.6 ± 20.23 | 121.8 ± 24.90 | 39.7 ± 4.82 | <0.001 |
Abbreviations: TB, tuberculosis; PTB, pulmonary tuberculosis; EPTB, extra-pulmonary tuberculosis; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; IgG, immunoglobulin G; IgM, immunoglobulin M; IgA, immunoglobulin A; ADA, adenosine deaminase.
§Data are shown as means ± SEMs.
*From Chi-square (χ2) test and Kruskal–Wallis H test.
Comparison of lymphocyte subpopulations in PTB, EPTB and non-TB patients
Lymphocyte subpopulations in whole blood were analyzed for 37 PTB patients, 30 EPTB patients and 336 non-TB patients by flow cytometry There was no significant difference in WBC and neutrophil counts among PTB, EPTB and non-TB patients (p = 0.145 and p = 0.392, respectively, Table 2). However, the lymphocyte counts of patients with PTB and EPTB were lower than those of non-TB patients (p = 0.004, Table 2). No significant differences were observed in the proportions of CD3+ T cells, CD4+ T cells, CD8+ T cells, CD19+ B cells or CD16+56+ NK cells among PTB, EPTB and non-TB patients.
Table 2.
Analysis of lymphocyte subpopulations in TB and non-TB patients.
| Cells | PTB (n = 37) | EPTB (n = 30) | Non-TB (n = 336) | p value* |
|---|---|---|---|---|
| WBCs (*109/L)ψ | 7.31 ± 0.36 | 6.44 ± 0.21 | 7.39 ± 0.19 | 0.145 |
| Neutrophils (*109/L)ψ | 5.29 ± 0.35 | 4.50 ± 0.20 | 5.19 ± 0.17 | 0.392 |
| Lymphocytes (*109/L)ψ | 1.31 ± 0.08 | 1.23 ± 0.06 | 1.48 ± 0.05 | 0.004 |
| CD3 (%)ψ | 72.47 ± 1.56 | 66.60 ± 2.13 | 70.35 ± 0.59 | 0.093 |
| CD4 (%)ψ | 41.90 ± 1.34 | 37.86 ± 2.22 | 40.05 ± 0.60 | 0.335 |
| CD8 (%)ψ | 27.41 ± 1.41 | 26.46 ± 1.34 | 28.11 ± 0.63 | 0.967 |
| CD4/CD8 ratioψ | 1.74 ± 0.14 | 1.64 ± 0.20 | 1.79 ± 0.07 | 0.678 |
| CD16 + 56 (%)ψ | 13.38 ± 1.31 | 18.34 ± 1.95 | 15.09 ± 0.48 | 0.104 |
| CD19 (%)ψ | 13.09 ± 0.83 | 13.39 ± 1.53 | 13.03 ± 0.39 | 0.776 |
Abbreviations: TB, tuberculosis; PTB, pulmonary tuberculosis; EPTB, extra-pulmonary tuberculosis; WBC, white blood cell.
ψData are shown as means ± SEMs.
*From Kruskal-Wallis H test.
Comparison of IFN-γ release levels in PTB, EPTB and non-TB patients
The levels of IFN-γ release in PTB, EPTB and non-TB patients were 203.58±18.00 pg/mL, 201.83±14.56 pg/mL and 32.12±4.36 pg/mL, respectively. PTB and EPTB patients showed clear signs of higher IFN-γ release than non-TB patients (both p < 0.001, Table 3).
Table 3.
Comparison of IFN-γ levels in PTB, EPTB and non-TB patients.
| Variables | PTB | EPTB | Non-TB |
|---|---|---|---|
| IFN-γ (pg/mL)θ | 203.58 ± 18.00 | 201.83 ± 14.56 | 32.12 ± 4.36 |
| p value* | <0.001 | <0.001 | — |
θData are shown as means ± SEMs.
*Comparison of IFN-γ level in PTB and EPTB patients with the level in non-TB patients by Mann–Whitney U test.
Spearman correlation between IFN-γ release and lymphocytes in PTB, EPTB and non-TB patients
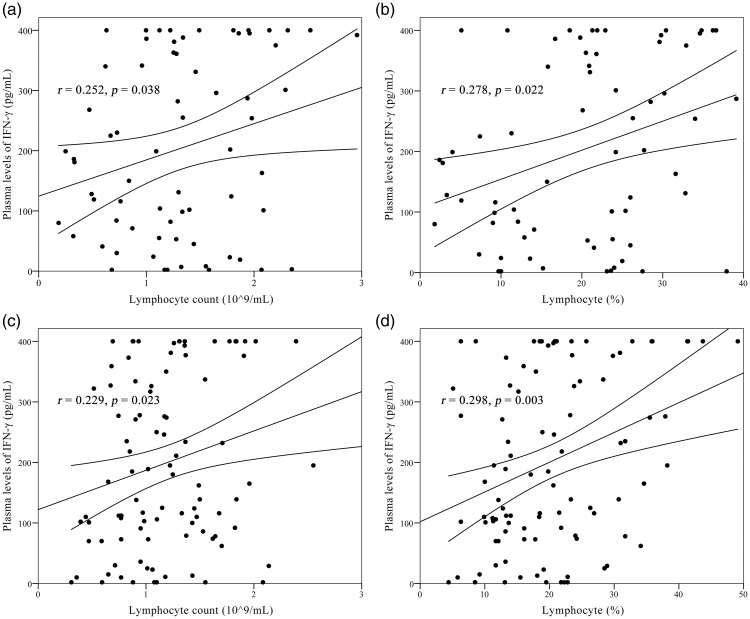
IFN-γ release levels were positively correlated with lymphocyte counts (r = 0.252, p = 0.038) and lymphocyte percentages (r = 0.278, p = 0.022) in patients with PTB. Similarly, lymphocyte counts (r = 0.229, p = 0.023) and lymphocyte percentages (r = 0.298, p = 0.003) were positively correlated with IFN-γ concentrations in patients with EPTB (Figure 1). However, IFN-γ levels showed no correlation with lymphocyte counts and percentages in non-TB patients (p = 0.820 and p = 0.396, respectively). No correlations between IFN-γ release and frequencies or percentages of CD4+ T cells or CD8+ T cells were identified among the three groups (data not shown).
Figure 1.
Analysis of correlations between plasma IFN-γ levels and lymphocyte counts and percentages in PTB and EPTB patients. (a) Correlation between plasma IFN-γ levels and absolute numbers of lymphocytes in PTB patients. (b) Correlation between plasma IFN-γ levels and lymphocyte proportions in PTB patients. (c) Correlation between plasma IFN-γ levels and absolute numbers of lymphocytes in EPTB patients. (d) Correlations between plasma IFN-γ levels and lymphocyte proportions in EPTB patients.
IFN, interferon; PTB, pulmonary tuberculosis; EPTB, extra-pulmonary tuberculosis.
Comparison of IFN-γ release levels in PTB patients with positive and negative sputum smears
Levels of IFN-γ release in PTB patients with positive sputum smears were 136.81±28.49 pg/mL, lower than those of patients with negative sputum smears (244.91±21.02; p = 0.004).
ROC curve analysis of IFN-γ release level for diagnosing MTB infection
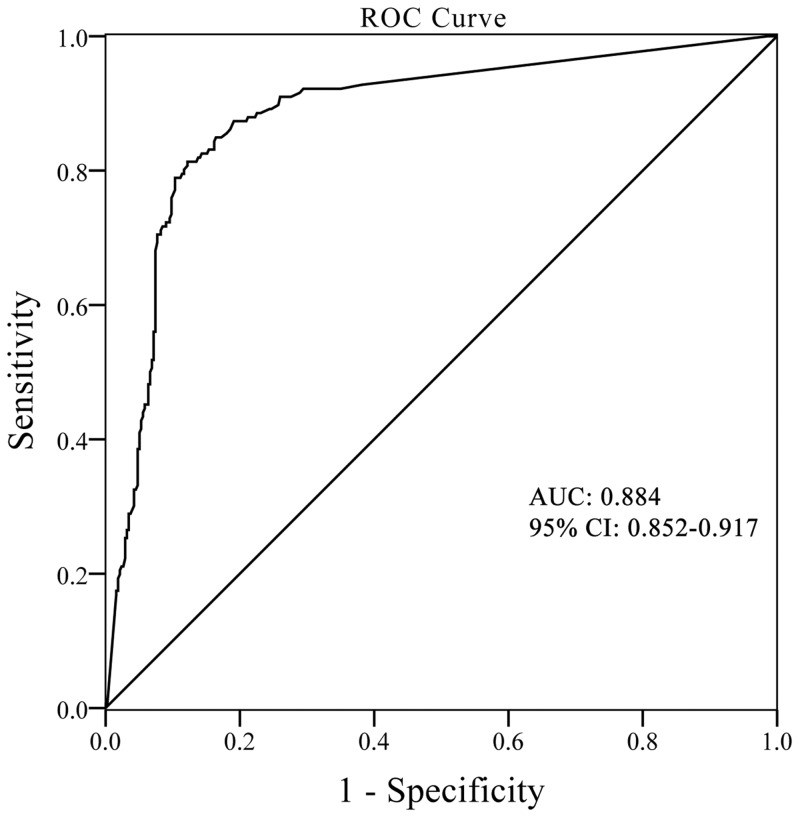
Analysis of the ROC curve for IFN-γ showed that the AUC for MTB diagnosis was 0.884, with a sensitivity of 88.6% and specificity of 77.5% when the recommended cut-off value of 14 pg/mL (Youden’s index 0.661) was applied (Figure 2). If a cut-off value of 28 pg/mL was used, the sensitivity was 84.9% and the specificity was 83.6%, although Youden’s index was slightly decreased to 0.685.
Figure 2.
ROC curves of IFN-γ level for MTB diagnosis and corresponding area under the ROC curve (AUC) values in MTB patients.
ROC, receiver operating characteristics; IFN, interferon; MTB, mycobacterium tuberculosis; CI, confidence interval.
Discussion
Over time, the symptoms of MTB infection become more and more insidious, and the signs become more and more atypical. Clinical diagnosis of MTB infection is still a major challenge because existing diagnostic methods have shortcomings. TB-IGRA is widely used for diagnosis of MTB infection, but this method is influenced by immune function and the inflammatory status of the body. Combined evaluation of immune status and TB-IGRA can improve the reliability of TB-IGRA for clinical diagnosis of MTB infection.
In the current study, we evaluated the diagnostic performance of TB-IGRAs in 68 PTB, 98 EPTB and 377 non-TB patients. We found that IFN-γ release was significantly higher in PTB and EPTB patients than in non-TB patients. IFN-γ release in PTB patients and EPTB patients was similar. Serbina et al.14 first found that IFN-γ was produced by CD8+ T cells through studies of CD4+ T cell-deficient mice. Another study showed that mycobacterium-specific CD8+ T cells can secrete IFN-γ without help from CD4+ T cells.15 Our results are in agreement with those of previous studies,16–18 which indicated that IFN-γ release was clearly elevated during MTB infection. Furthermore, we found that 28 pg/mL of IFN-γ had advantages over the recommended cut-off value of 14 pg/mL for TB-IGRA. The specificity (83.6%) at the cut-off value of 28 pg/mL was higher than that (77.5%) at the recommended value of 14 pg/mL. This suggests that the cut-off value for TB-IGRA should be reconsidered. Our results are consistent with those of previous studies,11,19 which indicated that TB-IGRA was a reliable diagnostic tool for MTB infection.
We also found that IFN-γ release was lower in PTB patients with positive sputum smears compared with PTB patients with negative sputum smears. However, Kim and colleagues found no difference between the IFN-γ levels of active TB and LTBI patients (p = 0.639).20 Another study showed that IFN-γ levels in LTBI patients were higher compared with those in patients with active TB, although this difference was not statistically significant.21 Pathan et al.16 found that frequencies of circulating ESAT-6 peptide-specific IFN-γ-secreting CD4+ T cells were higher in LTBI patients than in patients with active TB. This population of Th1-type ESAT-6 peptide-specific CD4+ T cells in LTBI patients could secrete high levels of IFN-γ to restrict MTB in vivo,16 which supports our results. Both activated CD4+ T cells and CD8+ T cells are primed for IFN-γ production, but CD4+ T cells are responsible for the majority of IFN-γ production during the active TB phase.22 We found no statistical difference in the counts and percentages of CD4+ or CD8+ T cells between PTB patients with positive sputum smears and PTB patients with negative sputum smears.
Spearman correlation analysis suggested that IFN-γ release in PTB and EPTB patients was positively correlated with counts and proportion of lymphocytes. However, WBC and neutrophils counts did not show obvious relationships with IFN-γ release levels. Our data agree with those of Jun et al.23 and imply that lymphocytes are the primary participants in defense against MTB infection. Studies in vitro have suggested that CD4+ T cells mediate potent immunity against MTB infection by secreting cytokines and eliminating infected macrophages.24,25 Transgenic mice lacking CD4+ T cells are also susceptible to MTB infection.26 CD8+ T cells may also secrete IFN-γ and kill mycobacteria via cytotoxic mechanisms.22,27 However, the absolute counts and proportions of CD3+ T cells, CD4+ T cells, CD8+ T cells, CD19+ B cells, and CD16+56+ NK cells among PTB, EPTB and non-TB did not differ significantly. Leung and colleagues also found that the absolute counts of CD3+ T cells, CD4+ T cells, CD8+T cells, CD19+ B cells, and CD16+56+ NK cells did not differ between TB patients and healthy controls.22 However, a study by Guglielmetti et al. showed that absolute numbers of CD4+ and CD8+ T cells in active PTB patients were lower than in controls.28 In addition, they found that the proportions of CD4+DR+ and CD8+ T cells were elevated, while the proportions of CD5+CD19+ and CD27+CD19+ cells were decreased in active PTB patients compared with healthy controls.28 The studies above suggested that PTB, especially active PTB, is associated with altered lymphocyte homeostasis. Our results showed no statistical differences in lymphocyte subpopulations among PTB, EPTB and non-TB patients. This finding may have several explanations. First, patients with PTB and EPTB could have been in the active phase or latent phase of disease at the time of testing, which may have influenced lymphocyte subpopulation counts. Second, non-TB patients may suffer from other diseases that influence lymphocyte populations. Third, the number of PTB and EPTB patients studied was not large enough to identify more subtle differences between these groups.
Taken together, the results of the present study demonstrate that the IFN-γ release in patients with PTB and EPTB was significantly elevated compared with non-TB patients. IFN-γ release in patients with positive sputum smears was lower compared with patients with negative sputum smears. The counts and proportions of CD4+ and CD8+ T cells in patients with PTB and EPTB were not significantly different than those of non-TB patients. The TB-IGRA can be used as a reliable diagnostic tool for MTB infection with high sensitivity and specificity. A cut-off value of 28 pg/mL IFN-γ may offer improved MTB diagnosis with higher specificity. Further studies are required to confirm the optimal cut-off value. TB-IGRA is useful to diagnose MTB infection, especially in the early stages of LTBI.
Acknowledgements
We thank Dr. Qifa Song for his assistance and guidance in conducting this study. We gratefully acknowledge the staff of the Haematology Laboratory of Ningbo City First Hospital for their assistance.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
Grants from Ningbo Municipal Bureau of Science and Technology, Grant/Award Number: 2014C50069
ORCID iD
Jinguo Chu https://orcid.org/0000-0002-5353-3420
References
- 1.World Health Organization. Global tuberculosis report 2017. Geneva: World Health Organization, 2017. [Google Scholar]
- 2.Tiemersma EW, van der Werf MJ, Borgdorff MW, et al. Natural history of tuberculosis: duration and fatality of untreated pulmonary tuberculosis in HIV negative patients: a systematic review. PLoS One 2011; 6: e17601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bei C, Fu M, Zhang Y, et al. Mortality and associated factors of patients with extensive drug-resistant tuberculosis: an emerging public health crisis in China. BMC Infect Dis 2018; 18: 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Floyd K, Glaziou P, Zumla A, et al. The global tuberculosis epidemic and progress in care, prevention, and research: an overview in year 3 of the End TB era. Lancet Respir Med 2018; 6: 299–314. [DOI] [PubMed] [Google Scholar]
- 5.Nishimura T, Hasegawa N, Mori M, et al. Accuracy of an interferon-gamma release assay to detect active pulmonary and extra-pulmonary tuberculosis. Int J Tuberc Lung Dis 2008; 12: 269–274. [PubMed] [Google Scholar]
- 6.Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 2008; 149: 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pai M, Behr M. Latent Mycobacterium tuberculosis infection and interferon-gamma release assays. Microbiol Spectr 2016; 4: TBTB2-0023-2016. [DOI] [PubMed] [Google Scholar]
- 8.Chee CBE, Reves R, Zhang Y, et al. Latent tuberculosis infection: opportunities and challenges. Respirology 2018; 23: 893–900. [DOI] [PubMed] [Google Scholar]
- 9.Brock I, Munk ME, Kok-Jensen A, et al. Performance of whole blood IFN-gamma test for tuberculosis diagnosis based on PPD or the specific antigens ESAT-6 and CFP-10. Int J Tuberc Lung Dis 2001; 5: 462–467. [PubMed] [Google Scholar]
- 10.Zellweger JP, Zellweger A, Ansermet S, et al. Contact tracing using a new T-cell-based test: better correlation with tuberculosis exposure than the tuberculin skin test. Int J Tuberc Lung Dis 2005; 9: 1242–1247. [PubMed] [Google Scholar]
- 11.Higuchi K, Kawabe Y, Mitarai S, et al. Comparison of performance in two diagnostic methods for tuberculosis infection. Med Microbiol Immunol 2009; 198: 33–37. [DOI] [PubMed] [Google Scholar]
- 12.Diel R, Goletti D, Ferrara G, et al. Interferon-gamma release assays for the diagnosis of latent Mycobacterium tuberculosis infection: a systematic review and meta-analysis. Eur Respir J 2011; 37: 88–99. [DOI] [PubMed] [Google Scholar]
- 13.Chinese Medical Association. Clinical Diagnosis Standard of TB for Clinical Technology Operation (TB volumes). People’s Medical Publishing House, 2005; PMPH; ISBN 9787117065108.
- 14.Serbina NV, Lazarevic V, Flynn JL. CD4(+) T cells are required for the development of cytotoxic CD8(+) T cells during Mycobacterium tuberculosis infection. J Immunol 2001; 167: 6991–7000. [DOI] [PubMed] [Google Scholar]
- 15.Carter JR. Centers for Disease Control and Prevention. Human immunodeficiency virus (HIV), sexually transmitted diseases (STDs), and tuberculosis (TB) related applied research projects. Fed Regist 1998; 63: 18427–18430. [PubMed] [Google Scholar]
- 16.Pathan AA, Wilkinson KA, Klenerman P, et al. Direct ex vivo analysis of antigen-specific IFN-gamma-secreting CD4 T cells in Mycobacterium tuberculosis-infected individuals: associations with clinical disease state and effect of treatment. J Immunol 2001; 167: 5217–5225. [DOI] [PubMed] [Google Scholar]
- 17.Carrere-Kremer S, Rubbo PA, Pisoni A, et al. High IFN-gamma release and impaired capacity of multi-cytokine secretion in IGRA supernatants are associated with active tuberculosis. PLoS One 2016; 11: e0162137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao X, Liu Y, Liu Y, et al. Multiplex analysis of plasma cytokines/chemokines showing different immune responses in active TB patients, latent TB infection and healthy participants. Tuberculosis (Edinb) 2017; 107: 88–94. [DOI] [PubMed] [Google Scholar]
- 19.Qiu Y, Wang Y, Lin N, et al. Multicenter clinical evaluation of three commercial reagent kits based on the interferon-gamma release assay for the rapid diagnosis of tuberculosis in China. Int J Infect Dis 2015; 40: 108–112. [DOI] [PubMed] [Google Scholar]
- 20.Kim CH, Choi KJ, Yoo SS, et al. Comparative analysis of whole-blood interferon-gamma and flow cytometry assays for detecting post-treatment immune responses in patients with active tuberculosis. Cytometry B Clin Cytom 2014; 86: 236–243. [DOI] [PubMed] [Google Scholar]
- 21.Kampmann B, Whittaker E, Williams A, et al. Interferon-gamma release assays do not identify more children with active tuberculosis than the tuberculin skin test. Eur Respir J 2009; 33: 1374–1382. [DOI] [PubMed] [Google Scholar]
- 22.Leung WL, Law KL, Leung VS, et al. Comparison of intracellular cytokine flow cytometry and an enzyme immunoassay for evaluation of cellular immune response to active tuberculosis. Clin Vaccine Immunol 2009; 16: 344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu JC, Li ZY, Chen XN, et al. More significance of TB-IGRA except for the diagnose of tuberculosis. J Clin Lab Anal 2018; 32: e22183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corominas M, Cardona V, Gonzalez L, et al. B-lymphocytes and co-stimulatory molecules in Mycobacterium tuberculosis infection. Int J Tuberc Lung Dis 2004; 8: 98–105. [PubMed] [Google Scholar]
- 25.Boom WH. The role of T-cell subsets in Mycobacterium tuberculosis infection. Infect Agents Dis 1996; 5: 73–81. [PubMed] [Google Scholar]
- 26.Caruso AM, Serbina N, Klein E, et al. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J Immunol 1999; 162: 5407–5416. [PubMed] [Google Scholar]
- 27.Serbina NV, Flynn JL. Early emergence of CD8(+) T cells primed for production of type 1 cytokines in the lungs of Mycobacterium tuberculosis-infected mice. Infect Immun 1999; 67: 3980–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guglielmetti L, Cazzadori A, Conti M, et al. Lymphocyte subpopulations in active tuberculosis: association with disease severity and the QFT-GIT assay. Int J Tuberc Lung Dis 2013; 17: 825–828. [DOI] [PubMed] [Google Scholar]