Abstract
Objective
The deficient placental blood perfusion caused by the attenuated infiltration of trophoblast cells is a key factor in the occurrence of preeclampsia (PE). Furthermore, the long noncoding (lnc)RNA SNHG12 (small nucleolar RNA host gene 12) can promote the proliferation and metastasis of multiple tumor cells. However, whether lncRNA SNHG12 affects proliferation and metastasis of trophoblast cells is unclear.
Methods
We examined the level of lncRNA SNHG12 in plasma and placenta of patients with PE and constructed trophoblast cells with overexpressed or knocked down SNHG12. CCK-8, wound healing, and Transwell assays were used to detect alterations in proliferation, migration, and invasion of trophoblast cells. Western blotting was used to detect proteins related to the epithelial–mesenchymal transition (EMT), and cell cycle assays clarified cell cycle distribution.
Results
LncRNA SNHG12 promoted the proliferation, migration, and invasion of trophoblast cells. The expression of matrix metalloproteinase-2 (MMP-2) and MMP-9, β-catenin, and vimentin were positively correlated with SNHG12, and expression of E-cadherin was negatively correlated with SNHG12. SNHG12 also promoted the transition of trophoblast cells from G0/G1 to S phase.
Conclusion
Overall, lncRNA SNHG12 promoted the migration and invasion of trophoblast cells by inducing the progression of EMT.
Keywords: Preeclampsia, trophoblast cells, lncRNA SNHG12, epithelial–mesenchymal transition (EMT), cell cycle, long noncoding RNA
Introduction
Preeclampsia (PE) is a common disease of pregnant women and a critical cause of death in women the perinatal period.1 The primary symptoms of PE include increasing levels of urinary protein and hypertension.2,3 If proper and effective treatment is not provided, severe symptoms such as kidney failure and hemolytic anemia ensue. Furthermore, infiltration of trophoblast cells into the muscle layer of uterine wall is an essential step in the normal development of placenta.4 The occurrence of PE is related to impaired invasion of trophoblast cells. Under normal physiological conditions, trophoblast cells invade the spiral artery in muscular layer of the uterine wall, resulting in remodeling of the spiral artery. In PE, invasion of trophoblast cells is weakened, which interferes with the process of remodeling, resulting in insufficient blood supply in the placenta.5 Therefore, impaired invasion of trophoblast cells is considered the mechanism underlying various placenta-related diseases.6
Long noncoding RNA (lncRNA) is a type of RNA molecule with a length >200 nucleotides.7 As a multifunctional transcript, lncRNAs play regulatory roles in many activities of the body. Research has revealed that the lncRNA SNHG12 (small nucleolar RNA host gene 12) can promote tumorigenesis of prostate cancer by sponging miR-133b.8 Furthermore, SNHG12 can enhance the proliferation and invasion of colorectal cancer by adsorbing microRNAs.9 In addition, some studies have revealed that SNHG12 leads to a poor prognosis in patients with gastric carcinoma and renal cancer.10,11 Moreover, inhibition of SNHG12 can suppress the proliferation, migration, and invasion of non-small-cell lung cancer, nasopharynx cancer, and cervical cells.12–14 However, whether SNHG12 can enhance the proliferation and infiltration of trophoblast cells remains unclear. A previous study showed that lncRNA SNHG5, which is in the same family as SNHG12, was downregulated in PE placenta tissues and promoted the proliferation, migration, and invasion of trophoblast cells by targeting miR-26a-5p.15 All of this evidence indicates that SNHG12 might promote the proliferation, migration, and invasion of trophoblast cells.
In this study, we collected clinical samples to verify the expression of SNHG12 in placental tissues of PE patients. Then we constructed stable trophoblast cell lines with overexpression or knockdown of SNHG12 to detect changes in proliferation, migration, and invasion. Finally, we detected epithelial–mesenchymal transition (EMT)-related proteins to further clarify the molecular mechanism in patients with PE.
Materials and methods
Cell culture
The HTR-8/SVneo human trophoblast cell line was obtained from the Shanghai Institutes for Biological Sciences (Shanghai, China). The cells were cultured in RPMI 1640 medium containing 10% fetal bovine serum (FBS; Gibco/Thermo Fisher Scientific, Waltham, MA, USA) and placed in a humid atmosphere with 5% CO2 at 37°C.
Collection of patient samples
Samples of placental tissue and plasma were collected from healthy individuals (10 samples) and patients with PE (10 samples). This research was authorized by the ethics committee of The Affiliated Huaian No. 1 People’s Hospital of Nanjing Medical University. All patients and healthy volunteers consented to publication of this paper.
Cell transfection
Small interfering RNA (siRNA) for lncRNA SNHG12 was purchased from GenePharma (Shanghai, China). The sequence of si-SNHG12 was as follows: sense 5′-GCAGUGUGCUACUGAACUUTT-3′ and antisense 5′-AAGUUCAGUAGCACACUGCTT-3′. In addition, to establish HTR-8/SVneo cells that stably overexpressed SNHG12, we constructed lentiviral vectors and transfected the cells. The lentiviral particles were purchased from Genechem Shanghai (Shanghai, China). All procedure during the experiment were carried out according to the manufacturer’s instructions. Then, real-time quantitative PCR was performed to determine the efficacy of transfection.
CCK-8 assays
The cells of different groups were seeded into three 96-well plates. After the cells adhered to the bottom of the plates, absorbance values of the first 96-well plate were detected by using cell counting kit-8 (CCK-8; Dojindo, Japan) methods at 24 hours. The other two plates were detected at 48 and 72 hours, respectively.
Wound healing assays
The cells were planted into 6-well plates. After the cells adhered to the bottom of the plates, they were cultured in medium without FBS. Then, the cell layer was scratched using the tip of a 200-µL pipette. The width of the blank area (where cells were scratched off) was photographed and measured after 12, 24, and 48 hours.
Transwell assays
Transwell assays were performed using an 8-µm Boyden chamber (Corning Inc., Corning, NY, USA) to detect changes in cell invasion. The Matrigel was diluted with medium without FBS and then layered onto the upper surface of the chamber. Medium containing FBS was added to the lower chamber. After 24 hours, cells on the opposite of the bottom membrane were stained with crystal violet (Sigma Chemical Co., St. Louis, MO, USA). Finally, the stained cells were photographed and counted under a microscope.
Cell cycle
Propidium iodide (Beyotime, Beijing, China) staining was used to detect the cell cycle distribution of the cells. The cells were collected into centrifuge tubes and fixed with 70% ice ethanol for 4 hours at 4°C. Then, cells were washed with PBS three times, and incubated with RNase A and propidium iodide at 37°C for 30 min. This process took place completely in the dark. Finally, the cell cycle was analyzed by flow cytometry.
RT-PCR
The Trizol method (Thermo Fisher Scientific, Waltham, MA, USA) was used to extract total mRNA of the cells. Procedures were conducted in accordance with the manufacturer’s instructions. The level of lncRNA SNHG12 was detected by SYBR-Green real-time quantitative PCR (qPCR; Applied Biosystems, Foster City, CA, USA), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the loading control. The primers of SNHG12 and GAPDH were as follows: SNHG12 forward primer: 5′-TCTGGTGATCGAGGACTTCC-3′ reverse primer: 5′-ACCTCCTCAGTATCACACACT-3′; GAPDH forward primer: 5′-AGAAGGCTGGGGCTCATTTG-3′ reverse primer: 5′-AGGGGCCATCCACAGTCTTC-3′. Finally, the 2−ΔΔCT method was used to determine the levels of SNHG12. The Applied Biosystem 7500 real-time PCR system was used to conduct real-time (RT)-PCR.
Western blotting
The immunoblotting assay was used to extract the proteins, and the concentration of total proteins was measured by the bicinchoninic acid (Beyotime) method. Then, equal amounts of proteins were loaded onto a 10% SDS-PAGE gel (Beyotime). Total proteins were separated and transferred to a polyvinylidene fluoride (PVDF) membrane (Thermo Fisher Scientific). The membrane was blocked with 5% skim milk (BD Company, Franklin Lakes, NJ, USA), which was dissolved in PBS-Tween (PBST). The membrane was then incubated with primary antibodies (all from Cell Signaling Technology, Danvers, MA, USA): CDK-2 (#2546S), cyclin D1 (#55506), P21 (#2947S), MMP-2 (#40994S), MMP-9 (#13667), E-cadherin (#3195S), vimentin (#5741S) and β-catenin (#8480S). The membrane was washed with PBST three times on the second day. The membrane was then incubated with the secondary antibody (rabbit IgG, #3423, Cell Signaling Technology) for 2 hours at room temperature, washed with PBST three times, and exposed in the machine.
Statistical analysis
The statistical analysis of this paper was performed using GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA, USA). All the data were presented as means ±standard deviations. Student’s t-test method was used to judge the differences between different groups. P < 0.05 was considered statistically significant. All experiments were repeated three times.
Results
Higher levels of SNHG12 enhanced proliferation, migration, and invasion of trophoblasts
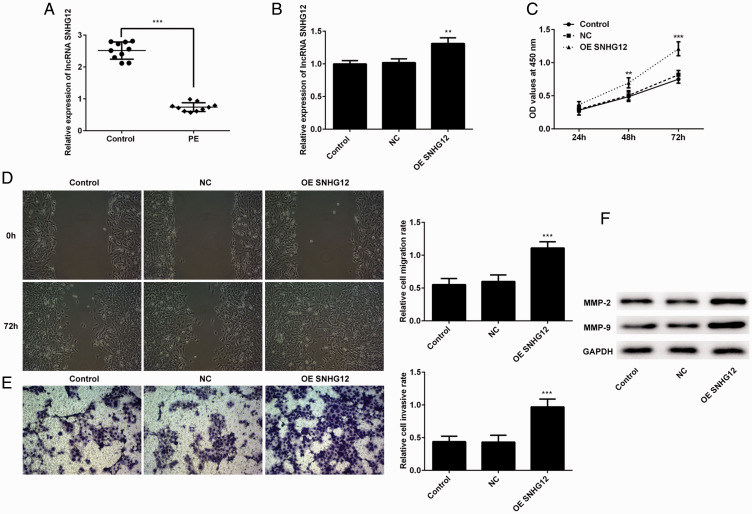
The levels of SNHG12 in placenta and plasma of PE patients was detected by qPCR. As shown in Figure 1A, the expression of SNHG12 was decreased in placenta and plasma of PE patients. To verify the efficacy of SNHG12 on the proliferation, migration, and invasion of trophoblast cells, lentivirus was used to establish HTR-8/SVneo cells in which SNHG12 was overexpressed. Then, qPCR was used to confirm the efficacy of transfection. The level of SNHG12 in the overexpression group was higher than that in the negative control group (Figure 1B). Then, the CCK-8 assay was performed to detect changes in cell proliferation. According to the results (Figure 1C), proliferation of the overexpression group of cells was strengthened compared with the negative control group at 48 and 72 hours. We assessed the migration and invasion abilities of these cells using the wound healing and Transwell assays, respectively. The results (Figure 1D and Figure 1E) showed that overexpression of SNHG12 enhanced the migration and invasion of HTR-8/SVneo cells. Furthermore, the migration- and invasion-related proteins MMP-2 and MMP-9 were upregulated after overexpression of SNHG12 (Figure 1F). Based on these results, we confirmed that overexpression of SNHG12 promoted the proliferation, migration, and invasion of trophoblast cells.
Figure 1.
Overexpression of lncRNA SNHG12 enhanced the proliferation, migration, and invasion of HTR-8/SVneo cells. (A) lncRNA SNHG12 was downregulated in the plasma and placenta of patients with PE. (B) The levels of lncRNA SNHG12 in the control (normal cells), NC (transfected with vector), and overexpression group (OE) were validated by the qPCR. (C) Viability of cells was detected with the CCK-8 method at 24, 48, and 72 hours after the seeding of these cells and measured as optical density (OD) at 450 nm. (D) The images of the scratch were taken at 72 hours. (E) Representative photos of the cells on the bottom of the chamber were taken after 72 hours. (F) Expression of MMP-2 and MMP-9 was detected by western blotting. *P < 0.05, **P < 0.01, ***P < 0.001. lncRNA, long noncoding RNA; SNHG12, small nucleolar RNA host gene 2; qPCR, real-time quantitative PCR; PE, preeclampsia; CCK-8, cell counting kit-8; MMP, matrix metalloproteinase.
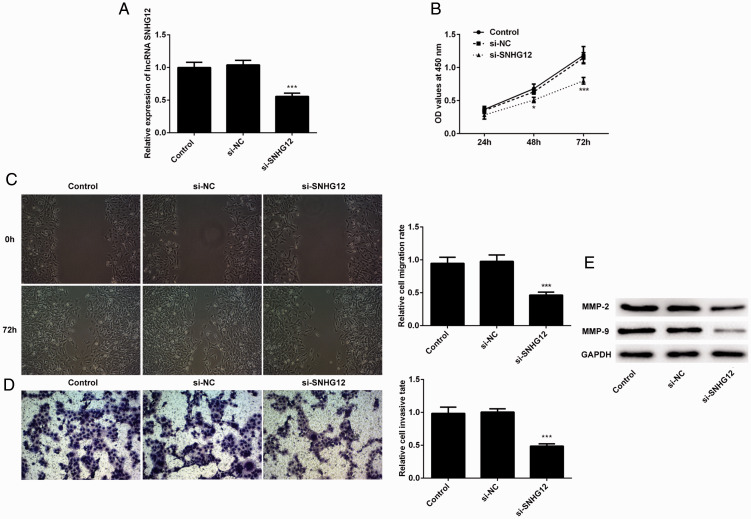
Knockdown of SNHG12 suppressed proliferation, migration, and invasion of trophoblasts
After demonstrating the efficacy of SNHG12 overexpression, we designed short interfering (si)RNA to knockdown SNHG12 and observe subsequent changes in proliferation, migration, and invasion. We validated the effect of siRNA using qPCR (Figure 2A) and assessed proliferation of these cells by CCK-8 assay. The results (Figure 2B) showed that knockdown of SNHG12 inhibited the proliferation of HTR-8/SVneo cells at 48 and 72 hours after cells adhered. Next, we determined the effect of knockdown of SNHG12 on the migration and invasion of HTR-8/SVneo cells. As shown in Figure 2C and 2D, the migration and invasion of these cells was repressed after the knockdown. In addition, the levels of MMP-2 and MMP-9 declined after knockdown of SNHG12 (Figure 2E). These results revealed that the knockdown of SNHG12 suppressed the proliferation, migration, and invasion of trophoblast cells.
Figure 2.
Knockdown of lncRNA SNHG12 suppressed the proliferation, migration, and invasion of HTR-8/SVneo cells. (A) The level of lncRNA SNHG12 was validated by qPCR in the knockdown group. (B) Viability of cells was detected with the CCK-8 method at 24, 48, and 72 hours and measured as optical density (OD) at 450 nm. (C) Representative photographs were taken at 72 hours after the scratching. (D) The images of the Transwell assay were taken at 72 hours after the seeding of these cells. (e) Levels of MMP-2 and MMP-9 were detected by western blotting. *P < 0.05, **P < 0.01, ***P < 0.001. lncRNA, long noncoding RNA; SNHG12, small nucleolar RNA host gene 2; si, short interfering; control, normal cells; NC, negative control, transfected with vector; qPCR, real-time quantitative PCR; CCK-8, cell counting kit-8; MMP, matrix metalloproteinase.
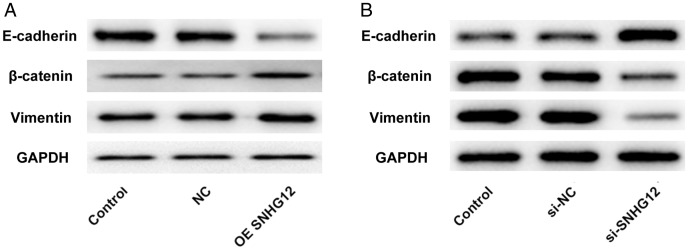
SNHG12 promoted the development of EMT
According to our results, we found that SNHG12 enhanced the proliferation, migration, and invasion of trophoblast cells. Given that EMT is associated with changes in proliferation, migration, and invasion of these cells, we detected levels of the EMT-related proteins E-cadherin, β-catenin, and vimentin. The level of E-cadherin, a marker protein of epithelial cells, was downregulated after overexpression of SNHG12. However, expression of β-catenin and vimentin, marker proteins of mesenchymal cells, was enhanced (Figure 3A). Further, the variation trend of these proteins was opposite when SNHG12 was knocked down in HTR-8/SVneo cells (Figure 3B). Therefore, these results confirmed that SNHG12 could facilitate the EMT.
Figure 3.
The lncRNA SNHG12 promoted the development of EMT to strengthen the migration and invasion of HTR-8/SVneo cells. (A) Expression of EMT-related proteins (E-cadherin, β-catenin, and vimentin) was detected by western blotting after the overexpression of lncRNA SNHG12. (B) Levels of E-cadherin, β-catenin, and vimentin were detected by western blotting after knockdown of lncRNA SNHG12. *P < 0.05, **P < 0.01, ***P < 0.001. lncRNA, long noncoding RNA; SNHG12, small nucleolar RNA host gene 2; EMT, epithelial–mesenchymal transition.
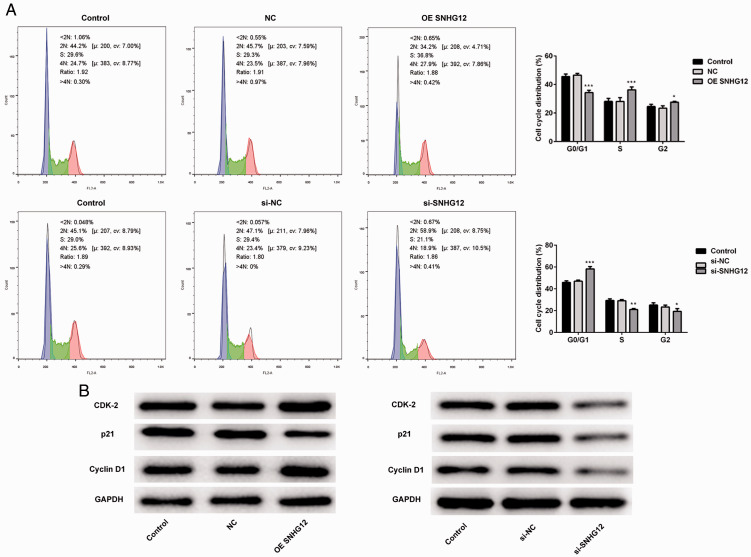
SNHG12 induced the transition from G1 to S phase
Results from the CCK-8 assays revealed that SNHG12 could enhance the proliferation of HTR-8/SVneo cells. Therefore, we performed cell cycle assays to clarify changes in cell cycle distribution after overexpression or knockdown of SNHG12. According to the results (Figure 4A), the proportion of HTR-8/SVneo cells in S phase increased after overexpression of SNHG12, and the ratios of cells in G0/G1 phase declined. In contrast, after knockdown of SNHG12, cells in S phase decreased and cells in G0/G1 phase increased significantly. Next, western blotting was performed to detect the cell cycle–related proteins P21, cyclinD1, and CDK-2. As shown in Figure 4B, the expression of CDK-2 and cyclinD1 increased after overexpression of SNHG12, whereas the level of P21 decreased compared with that of the negative control group. In contrast, after knockdown of SNHG12, levels of CDK-2 and cyclinD1 decreased compared with the negative control group and expression of P21 was also repressed. Overall, these results indicated that SNHG12 could enable trophoblast cells to avoid G0/G1 phase arrest and accelerate the rate of proliferation.
Figure 4.
LncRNA SNHG12 induced the HTR-8/SVneo cells transition from G0/G1 phase to S phase, thereby avoiding G0/G1 arrest. (A) The proportion of cells in S phase was increased after overexpression (OE) of lncRNA SNHG12. (B) The knockdown (si-SNHG12) of lncRNA SNHG12 induced the G0/G1 arrest of HTR-8/SVneo cells. (C) Expression of CDK-2, cyclin D1, and P21 was detected by western blotting after the overexpression (OE) or knockdown (si-) of lncRNA SNHG12. *P < 0.05, **P < 0.01, ***P < 0.001. lncRNA, long noncoding RNA; SNHG12, small nucleolar RNA host gene 2; si-SNHG12, short interfering SNHG12.
Discussion
PE is the main reason for infant death, fetal body dysplasia, and damage of different maternal organs.16–18 In a general sense, the development of PE is associated with pregnancy-induced hypertension and renal failure.19 Therefore, it is important to clarify the specific molecular mechanism underlying the occurrence of PE and identify the molecules that regulate the development of PE. Many factors are thought to be associated with the pathogenesis of PE. Trophoblast cells invade the uterine spiral artery and regulate the remodeling of the spiral artery, which leads to a decrease in peripheral vascular pressure.4 Remodeling of the uterine spiral artery, as regulated by trophoblast cells, allows abundant maternal blood flow in the placenta, providing nutrients for the growth of the fetus.20 However, insufficient invasion of trophoblast cells can lead to disrupted uterine spiral artery remodeling and ultimately result in PE.5
Small nucleolar RNA host gene 12 (SNHG12) is a lncRNA. Research has shown that the level of lncRNA SNHG12 was increased in colorectal cancer cells and could promote the proliferation and invasion of these cells by targeting miR-16.9 Moreover, a study also reported that SNHG12 could enhance the migration and proliferation of glioma cells, and a higher level of SNHG12 was associated with a decrease in the 5-year survival rate.21 In addition to the enhanced efficacy of proliferation, migration, and invasion of cancer cells, SMHG12 could also induce drug resistance in osteosarcoma.22 Our results showed that overexpression of SNHG12 could enhance the proliferation, migration, and invasion of trophoblast cells, whereas knockdown of SNHG12 could inhibit these indicators of trophoblast cells. These results are similar to the findings in cancer cells, but the enhancement of trophoblast cell migration and invasion is beneficial to the development of embryo.
EMT is a process that enables epithelial cells to acquire the characteristics of mesenchymal cells, which ultimately leads to enhanced migration and invasion. A study pointed out that SNHG12 promoted the metastasis of nasopharyngeal carcinoma cells by facilitating the development of EMT.13 In our study, we found enhanced migration and invasion. We detected the levels of E-cadherin, β-catenin, and vimentin in trophoblast cells that overexpressed or knocked down SNHG12. Expression of E-cadherin was negatively correlated with levels of SNHG12, whereas expression of vimentin and β-catenin was positively correlated with levels of SNHG12. These results implied that lncNA SNHG12 could promote migration and invasion by inducing the occurrence and development of EMT.
In this research, SNHG12 not only enhanced the metastasis of trophoblast cells, but also significantly accelerated proliferative capacity. Some studies have revealed that lncRNA can promote the proliferation of carcinoma cells by avoiding cell cycle arrest.23,24 Furthermore, SNHG5, which is homologous gene to SNHG12, could induce a smooth transition of osteosarcoma cells from G1 phase to S phase, which accelerates proliferation of these cells.25 Similarly, in our study, knockdown of SNHG12 led to G0/G1 arrest, thereby inhibiting the proliferation of trophoblast cells. However, after overexpression of SNHG12, cells in G0/G1 phase were decreased and cell proliferation was promoted. Furthermore, cyclin D1 and CDK-2 could induce transition of cells from G1 phase to S phase, thus avoiding G0/G1 arrest.26 However, the expression of cell cycle-dependent kinase inhibitor P21 could induce G2 arrest and affect the proliferation of cells.27 In this research, the expression of CDK-2 and Cyclin D1 was positively correlated with SNHG12, whereas the level of P21 was negatively related with SNGH12. Thus, we can conclude that SNHG12 can prevent G0/G1 arrest in these cells, facilitating proliferation of trophoblast cells.
In summary, we revealed the effect of SNHG12 on the proliferation, migration, and invasion of trophoblast cells. SNHG12 was downregulated in plasma and placenta of PE patients. In addition, SNHG12 could enhance the proliferation and metastasis of trophoblast cells by inducing the transition from G1 phase to S phase and the occurrence of EMT, respectively. Therefore, we showed that SNHG12 could be used as a marker in the treatment of PE, which is caused by weaker infiltration of trophoblast cells. Furthermore, this research provides a new strategy for the clinical treatment of PE.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Hairong Wang https://orcid.org/0000-0002-3947-0830
References
- 1.Roberts JM, Mascalzoni D, Ness RB, et al. Collaboration to understand complex diseases: preeclampsia and adverse pregnancy outcomes. Hypertension 2016; 67: 681–687. [DOI] [PubMed] [Google Scholar]
- 2.Duhig K, Vandermolen B, Shennan A. Recent advances in the diagnosis and management of pre-eclampsia. F1000Res 2018; 7: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ukah UV, De Silva DA, Payne B, et al. Prediction of adverse maternal outcomes from pre-eclampsia and other hypertensive disorders of pregnancy: a systematic review. Pregnancy Hypertens 2018; 11: 115–123. [DOI] [PubMed] [Google Scholar]
- 4.He N, van Iperen L, de Jong D, et al. Human extravillous trophoblasts penetrate decidual veins and lymphatics before remodeling spiral arteries during early pregnancy. PLoS One 2017; 12: e0169849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Red-Horse K, Zhou Y, Genbacev O, et al. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J Clin Invest 2004; 114: 744–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang P. Decidual vasculopathy in preeclampsia and spiral artery remodeling revisited: shallow invasion versus failure of involution. AJP Rep 2018; 8: e241–e246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yunusov D, Anderson L, DaSilva LF, et al. HIPSTR and thousands of lncRNAs are heterogeneously expressed in human embryos, primordial germ cells and stable cell lines. Sci Rep 2016; 6: 32753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng G, Song Z, Liu Y, et al. Long noncoding RNA SNHG12 indicates the prognosis of prostate cancer and accelerates tumorigenesis via sponging miR-133b. J Cell Physiol 2019. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Zhou J, Wang S, et al. Long non-coding RNA SNHG12 promotes proliferation and invasion of colorectal cancer cells by acting as a molecular sponge of microRNA-16. Exp Ther Med 2019; 18: 1212–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Q, Zhou W, Du SQ, et al. Overexpression of SNHG12 regulates the viability and invasion of renal cell carcinoma cells through modulation of HIF1alpha. Cancer Cell Int 2019; 19: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao G, Wang S, Liang X, et al. Oncogenic role of long non-coding RNA SNHG12 in gastric cancer cells by targeting miR-16. Exp Ther Med 2019; 18: 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin XJ, Chen XJ, Zhang ZF, et al. Long noncoding RNA SNHG12 promotes the progression of cervical cancer via modulating miR-125b/STAT3 axis. J Cell Physiol 2019; 234: 6624–6632. [DOI] [PubMed] [Google Scholar]
- 13.Liu ZB, Tang C, Jin X, et al. Increased expression of lncRNA SNHG12 predicts a poor prognosis of nasopharyngeal carcinoma and regulates cell proliferation and metastasis by modulating Notch signal pathway. Cancer Biomark 2018; 23: 603–613. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Liang S, Yu Y, et al. Knockdown of SNHG12 suppresses tumor metastasis and epithelial-mesenchymal transition via the Slug/ZEB2 signaling pathway by targeting miR-218 in NSCLC. Oncol Lett 2019; 17: 2356–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y, Xi L, Ma Y, et al. The lncRNA small nucleolar RNA host gene 5 regulates trophoblast cell proliferation, invasion, and migration via modulating miR-26a-5p/N-cadherin axis. J Cell Biochem 2019; 120: 3173–3184. [DOI] [PubMed] [Google Scholar]
- 16.Majak GB, Reisaeter AV, Zucknick M, et al. Preeclampsia in kidney transplanted women; Outcomes and a simple prognostic risk score system. PLoS One 2017; 12: e0173420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pritchard N, Kaitu’u-Lino TJ, Gong S, et al. ELABELA/APELA levels are not decreased in the maternal circulation or placenta among women with preeclampsia. Am J Pathol 2018; 188: 1749–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rolnik DL, Wright D, Poon LC, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med 2017; 377: 613–622. [DOI] [PubMed] [Google Scholar]
- 19.Amaral LM, Wallace K, Owens M, et al. Pathophysiology and current clinical management of preeclampsia. Curr Hypertens Rep 2017; 19: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lala PK, Nandi P. Mechanisms of trophoblast migration, endometrial angiogenesis in preeclampsia: the role of decorin. Cell Adh Migr 2016; 10: 111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei W, Wang ZL, Feng HJ, et al. Long non-coding RNA SNHG12 promotes the proliferation and migration of glioma cells by binding to HuR. Int J Oncol 2018; 53: 1374–1384. [DOI] [PubMed] [Google Scholar]
- 22.Zhou B, Li L, Li Y, et al. Long noncoding RNA SNHG12 mediates doxorubicin resistance of osteosarcoma via miR-320a/MCL1 axis. Biomed Pharmacother 2018; 106: 850–857. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Li B, Wang C, et al. Long noncoding RNA FOXD2-AS1 promotes glioma cell cycle progression and proliferation through the FOXD2-AS1/miR-31/CDK1 pathway. J Cell Biochem 2019; 120: 19784–19795. [DOI] [PubMed] [Google Scholar]
- 24.Zheng C, Xiao Y, Li Y, et al. Knockdown of long non-coding RNA PVT1 inhibits the proliferation of Raji cells through cell cycle regulation. Oncol Lett 2019; 18: 1225–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z, Wang Z, Liu J, et al. Long non-coding RNA SNHG5 sponges miR-26a to promote the tumorigenesis of osteosarcoma by targeting ROCK1. Biomed Pharmacother 2018; 107: 598–605. [DOI] [PubMed] [Google Scholar]
- 26.Gerard C, Goldbeter A. The balance between cell cycle arrest and cell proliferation: control by the extracellular matrix and by contact inhibition. Interface Focus 2014; 4: 20130075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koyano T, Namba M, Kobayashi T, et al. The p21 dependent G2 arrest of the cell cycle in epithelial tubular cells links to the early stage of renal fibrosis. Sci Rep 2019; 9: 12059. [DOI] [PMC free article] [PubMed] [Google Scholar]