Abstract
Estrogen exposure is one of the strongest risk factors for breast cancer development. Chemoprevention with selective estrogen receptor modulators (SERM), such as tamoxifen and raloxifene, has been shown in randomized controlled trials to reduce breast cancer incidence by up to 50% among high-risk women. Despite the strength of this evidence, there is significant underutilization of chemoprevention. Given the relatively few modifiable breast cancer risk factors, SERM use provides an important strategy for the primary prevention of this disease. Understanding factors which influence chemoprevention decision-making will inform efforts to implement breast cancer risk assessment and increase chemoprevention uptake in clinical practice.
Unlike cardiovascular disease, there are limited medical options for the primary prevention of cancer. Results of the Breast Cancer Prevention Trial (BCPT) and Study of Tamoxifen and Raloxifene (STAR) trial demonstrated a significant reduction in breast cancer incidence among high-risk women with the selective estrogen receptor modulators (SERM), tamoxifen and raloxifene (1, 2). This led to the approval of both drugs by the FDA for the primary prevention of breast cancer. Based upon the strength of this evidence, several professional organizations, including the US Preventive Services Task Force (USPSTF), recommend that healthcare providers (HCP) discuss chemoprevention with high-risk women (3). An estimated 10 million women in the United States meet high-risk criteria for breast cancer and are potentially eligible for chemoprevention; however, less than 10% of high-risk women offered a SERM initiate therapy (4).
Barriers to chemoprevention uptake include lack of routine breast cancer risk assessment in the primary care setting and limited knowledge about SERMs among high-risk women and primary care providers. Other factors influencing uptake include time constraints during the clinical encounter for counseling about chemoprevention, competing comorbidities, and concerns about rare but serious side effects of SERMs, including thromboembolism and endometrial cancer.
In this issue of the journal, Holmberg and colleagues reported the results of the NRG Oncology/NSABP Protocol DMP-1, a multicenter survey study among high-risk women counseled about breast cancer chemoprevention. The objective of the study was to assess social, cultural, and psychological factors that affect decision-making about chemoprevention among high-risk women. Among predominantly white women recruited from mainly cancer centers and community clinical oncology programs throughout the United States, over 40% of high-risk women decided to take a SERM for breast cancer risk reduction. The authors found that personal beliefs, including attitudes toward medications and breast cancer worry, and experiences with SERMs were associated with decisions to take or not take these chemopreventive agents. These findings may inform future interventions to educate high-risk women about the risks and benefits of chemoprevention. For example, emphasis may be placed on correlating chemoprevention with use of medications for other chronic conditions, such as cardiovascular disease or osteoporosis, or improving accurate perceptions of breast cancer risk, as well as the risk of side effects from SERMs. Another strategy to increase chemoprevention uptake is patient selection to target those women who have a favorable risk-benefit profile from SERM use, including (i) younger age, with a lower risk of serious side effects; (ii) prior hysterectomy; (iii) atypical hyperplasia or lobular carcinoma in situ; and (iv) BRCA2 mutation carriers who tend to develop estrogen receptor-positive breast cancers.
Holmberg and colleagues found that the strongest predictor of a decision to take a SERM among high-risk women was provider recommendation (odds ratio, 14.0; 95% confidence interval, 8.39–23.37). This is consistent with prior studies that demonstrated that HCP recommendation strongly influenced chemoprevention uptake. Providers who were less informed about SERMs were half as likely to prescribe these medications compared to those who felt sufficiently trained (5). The authors found that over 90% of high-risk women who decided to take a SERM reported that their HCP recommended the medication. The professional characteristics, including medical specialty, of the HCPs involved in this DMP-1 study were not well-defined. We recently reported that medical oncology referral was associated with increased chemoprevention uptake among women with high-risk breast lesions (6). Among 1719 women with atypical hyperplasia, lobular, or ductal carcinoma in situ, about a third were referred to a medical oncologist, which was associated with a greater than 5-fold increase in chemoprevention uptake compared with those who were not referred (Fig. 1). Although medical oncologists may be well-versed at prescribing hormonal therapy for breast cancer treatment, they may not be ideal candidates for increasing chemoprevention uptake more broadly.
Figure 1.
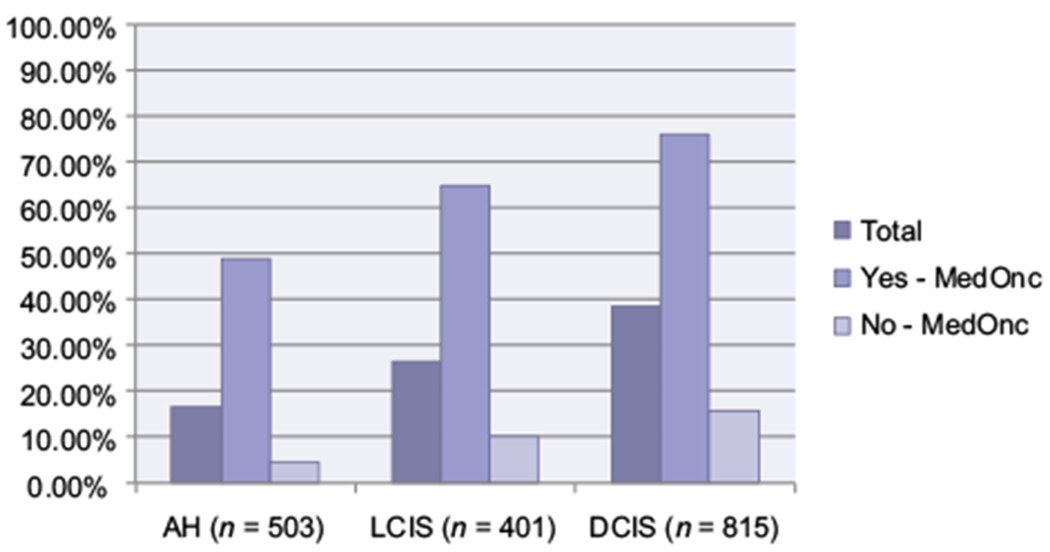
Chemoprevention uptake by breast histology and medical oncology referral. Abbreviations: AH, atypical hyperplasia; DCIS, ductal carcinoma in situ; LCIS, lobular carcinoma in situ. Data from Trivedi et al. (6).
Increasing knowledge about breast cancer risk assessment and the risks and benefits of SERMs among HCPs in the primary care setting has the potential to more widely diffuse chemoprevention into clinical practice. Possible implementation strategies include building breast cancer risk calculators into the electronic health record and integrating clinical decision support tools on chemoprevention for patients and HCPs into clinic workflow. Compared to cancer risk assessment and prevention, primary care providers are generally more comfortable with screening and prescribing medications for other chronic conditions, such as hypertension, hyperlipidemia, diabetes, osteoporosis, and depression. HCPs learning about a handful of drugs that have proven efficacy for cancer prevention is not unreasonable.
Another barrier to SERM uptake is the lack of short-term surrogate outcomes for breast cancer risk assessment or predicting response to chemoprevention. HCPs often follow objective surrogate clinical outcomes on physical examination (i.e., blood pressure for hypertension), laboratory testing (i.e., serum cholesterol for hyperlipidemia or HgA1c for diabetes), and imaging studies (i.e., DEXA scans for osteoporosis) to assess response to therapy. Currently, no objective measure of response or benefit from SERMs is used in the clinical setting. Mammographic density, which represents the amount of fibroglandular tissue in the breast relative to fat, is a strong breast cancer risk factor and has been shown to be modified by SERM use. Cuzick and colleagues demonstrated that at least a 10% reduction in mammographic density with 12 to 18 months of tamoxifen use was associated with a 63% relative risk reduction in breast cancer incidence (7). Given that high-risk women are routinely getting annual screening mammography, this may be a useful indicator of response to SERMs for breast cancer chemoprevention if automated methods for measuring mammographic density can be implemented in the clinical setting.
Strengths of the DMP-1 study by Holmberg and colleagues include the relatively large sample size, large number of participating sites, and use of validated survey measures, which increase the generalizability of their findings. The main limitation of the study is the assessment of chemoprevention decision-making rather than actual SERM uptake. In order to realize the benefits of breast cancer chemoprevention, actual uptake and long-term adherence to SERMs for up to 5 years need to be assessed.
The DMP-1 study was conducted from 2011 to 2013, just prior to the publication of the randomized controlled trials of aromatase inhibitors (AI) for breast cancer chemoprevention among high-risk postmenopausal women (8, 9). It remains to be seen whether there will be greater acceptance of AIs in the primary prevention setting. High-risk postmenopausal women now have multiple options with SERMs and AIs for breast cancer chemoprevention. In terms of bone health and cardiovascular disease risk, SERMs decrease fracture risk and serum cholesterol levels, whereas AIs have the opposite effects (1, 2, 8, 9). Therefore, competing comorbidities may inform the choice of chemopreventive agents.
In summary, patient preferences and provider recommendations are guiding factors influencing chemoprevention decisionmaking. Increasing uptake of breast cancer chemoprevention among high-risk women has the potential to significantly reduce the public health burden of this disease and perhaps the cost of cancer care (10). Facilitating discussions about chemoprevention between patients and providers and enhancing informed, shared decision-making also has merits and is in accordance with recommendations from the USPSTF and other professional organizations.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998;90:1371–88. [DOI] [PubMed] [Google Scholar]
- 2.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Effects of tamoxifen vs. raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 2006;295:2727–41. [DOI] [PubMed] [Google Scholar]
- 3.Nelson HD, Smith ME, Griffin JC, Fu R. Use of medications to reduce risk for primary breast cancer: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2013;158:604–14. [DOI] [PubMed] [Google Scholar]
- 4.Smith SG, Sestak I, Forster A, Partridge A, Side L, Wolf MS, et al. Factors affecting uptake and adherence to breast cancer chemoprevention: a systematic review and meta-analysis. Ann Oncol 2016;27:575–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan CP, Haas JS, Perez-Stable EJ, Des Jarlais G, Gregorich SE. Factors affecting breast cancer risk reduction practices among California physicians. Prev Med 2005;41:7–15. [DOI] [PubMed] [Google Scholar]
- 6.Trivedi MS, Coe AM, Vanegas A, Kukafka R, Crew KD. Chemoprevention uptake among women with atypical hyperplasia and lobular and ductal carcinoma in situ. Cancer Prev Res (Phila) 2017;10:434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuzick J, Warwick J, Pinney E, Duffy SW, Cawthorn S, Howell A, et al. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J Natl Cancer Inst 2011; 103:744–52. [DOI] [PubMed] [Google Scholar]
- 8.Cuzick J, Sestak I, Forbes JF, Dowsett M, Knox J, Cawthorn S, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet 2014;383:1041–8. [DOI] [PubMed] [Google Scholar]
- 9.Goss PE, Ingle JN, Ales-Martinez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 2011;364:2381–91. [DOI] [PubMed] [Google Scholar]
- 10.Hershman D, Sundararajan V, Jacobson JS, Heitjan DF, Neugut AI, Grann VR. Outcomes of tamoxifen chemoprevention for breast cancer in very high-risk women: a cost-effectiveness analysis. J Clin Oncol 2002;20:9–16. [DOI] [PubMed] [Google Scholar]


