Abstract
Study Objectives
The primary aim of the present study was to estimate the incidence per annum of acute insomnia and to what extent those that develop acute insomnia recover good sleep or develop chronic insomnia. Unlike prior studies, a dense-sampling approach was used here (i.e. daily diaries) and this allowed for a more precise detection of acute insomnia and the follow-on states (the transitions to either recovery or chronic insomnia).
Methods
Good sleeper subjects (n = 1,248; 67% female) that were at least 35 years old participated in this prospective study on the natural history of insomnia. Subjects were recruited nationwide and completed online assessments for 1 year. The online measures consisted primarily of daily sleep diaries, as well as weekly/bi-weekly and monthly measures of sleep, stress, and psychological and physical health.
Results
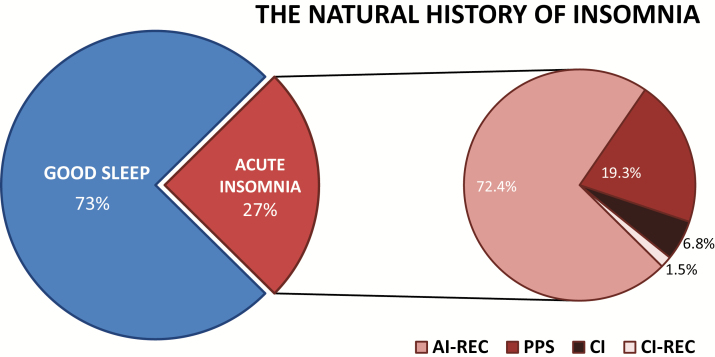
The 1-year incidence rate of acute insomnia was 27.0% (n = 337). The incidence rate of chronic insomnia was 1.8% (n = 23). Of those that developed acute insomnia, 72.4% (n = 244) went on to recover good sleep. 19.3% (n = 65) of the acute insomnia sample continued to experience persistent poor sleep, but did not meet criteria for chronic insomnia.
Conclusions
The incidence rate of acute insomnia (3 or more nights a week for between 2 and 12 weeks) is remarkably high. This said, most incident cases resolve within a few days to weeks. Incident chronic insomnia only occurs in about 2 in 100 individuals.
Keywords: insomnia, aging, natural history, acute insomnia, incidence
Statement of Significance.
While there are many studies on the prevalence of insomnia, only a few have evaluated new-onset incidence rates; none of which have adopted a high density sampling approach to determine the incidence of (1) good sleep to acute insomnia, (2) acute insomnia to the recovery of good sleep, and (3) acute insomnia to chronic insomnia. The present study addresses this gap. The remarkably high incidence rate of acute insomnia observed in this study suggests that this phenomenon, in contrast to chronic insomnia, may be normative (i.e. sleeplessness may be an inherent part of the fight-flight response). The next step is to evaluate the factors that mediate the transition from good sleep to acute insomnia and from acute to chronic insomnia.
Introduction
There is a wealth of data regarding insomnia prevalence [1–4]. In general, chronic insomnia (CI) has been found to occur in 6%–10% of the population. This said, prevalence rates as high as 30% have been reported when: duration of illness is not taken into account, qualitative criteria are used for insomnia frequency; and/or when daytime impairment criteria are not used to define “caseness” [2, 4]. Data on the prevalence of acute insomnia (AI) have only recently been published and suggest that the prevalence of AI is between 7.9% and 9.5% [5]. Little is known, however, about incidence rates for the transitions from Good Sleep (GS) to AI and from AI to either CI or Remission/Recovery. This lack of data makes it difficult to know (1) how common AI is, (2) empirically define the thresholds for AI/CI, and (3) identify the factors that mediate risk for (or protect from) developing CI.
As part of the National Institute of Mental Health Epidemiologic Catchment Area study, Ford and Kamerow were among the first to estimate the incidence of insomnia. They reported that the annual incidence rate of new-onset insomnia was 6.2% [6]. A decade later, a similar study in older adults (≥65 years old) suggested that the 3-year incidence rate of insomnia was 15% (or an annual incidence rate of approximately 5%) [7]. Aside from the age of the sample and the time interval between measurement points (i.e. 1 vs. 3 years), an important difference between this and the earlier study was the assessment of insomnia. While both studies used retrospective reports, one focused on duration of symptoms (e.g. “have you ever had a period of two weeks or more when you had trouble falling asleep, staying asleep, or with waking up too early?”) [6], whereas the other focused on frequency of symptoms (e.g. “how often do you have trouble falling asleep”) [7]. Two more recent studies provide convergent data suggesting that the annual incidence of insomnia is between 26% and 31% for the occurrence of insomnia “symptoms” and 7%–8% for insomnia “syndrome” [8, 9]. Subjects who were classified as “symptomatic” reported symptoms of initial, maintenance, or late insomnia at least three nights per week, without fulfilling all the diagnostic criteria of an insomnia syndrome (i.e. they could be satisfied with their sleep [not report distress or daytime consequences], or their sleep difficulties lasted for less than one month). Subjects who were classified as “syndromic” endorsed symptoms of insomnia at least three nights per week for a minimum duration of 1 month and reported dissatisfaction with their sleep. These rates decreased to 28.8% and 3.9% for those without a prior lifetime episode of insomnia [8].
To date, only one study has explicitly assessed the incidence rate of AI [5]. As part of a larger mixed model study, 412 “normal sleepers” from the United Kingdom (UK) were surveyed longitudinally to determine incidence, transition, and remission rates for AI. Individuals categorized as normal sleepers did not meet the minimum criterion for insomnia (i.e. did not report insomnia symptoms and sleep-related daytime impairment). Subjects completed three evaluations via telephone at baseline, 1 month, and 3 months. Sleep status was ascertained using the following questions: (1) “Have you ever had a problem with your sleep?”; (2) “Is this an ongoing problem at the moment?”; (3) “For how long has this been going on?”; and (4) “What is the nature of your sleep problem?.” The third question was used to differentiate AI (i.e. 3 days to 3 months) from CI (i.e. 3 months or longer). A final question pertained to whether subjects’ principal sleep complaint resulted in impairment in daytime functioning. According to the study findings, the annual incidence of AI was between 31% and 37% (depending on whether DSM-5 criteria with or without additional case criteria [SL or WASO of 30 min or longer and a self-reported increase in daytime impairment] was used).
In sum, the two early studies suggest that the annual incidence of insomnia ranges between 5% and 7% [6, 7]. The three later studies suggest that the annual incidence of insomnia ranges between 26% and 37% [5, 8, 9]. While the studies had different methods, measures, sampling rates and time frames, the difference between the early and late studies is potentially ascribable to how new onset insomnia was defined. The two early studies were describing the new onset of CI while the three latter studies were specifically profiling the incidence of acute and sub-CI. Of the studies that assessed recovery, it was found that 47%–78% of those that experience AI exhibit a resolution of their insomnia by the follow-up assessment [5, 7, 9]. While there are a number of limitations of the prior research (e.g. heterogeneity in sample demographics and methodology), the primary limitations (and those addressed by the present study) were that (1) the two to three time point assessment strategy lacks the temporal resolution needed to identify the onset and offset of AI; (2) only one of the studies adopted a quantitative approach to the assessment of sleep continuity (e.g. sleep latency [SL] and wake after sleep onset [WASO] defined in minutes); (3) the reported incident rates, for at least the two earlier studies [6, 7], are not truly the incidence of AI but rather the identification of new onset insomnia of any duration (includes both AI and CI).
The present study was undertaken as a natural history study of insomnia. Good sleeper subjects were recruited over three successive cohorts and were tracked for changes in sleep patterns for 1 year using online daily sleep diaries. The a priori aims for the study were to track incidence rates for transitions from GS to AI and from AI to either CI or Recovery back to GS (AI-REC). Please note this is the first of series of planned publications, which ultimately have the intent of (1) estimating the incidence rate of acute and CI, (2) defining, on an empirical basis, what is acute and CI, and what level of morbidity is associated with daytime dysfunction, and (3) exploration of what factors account for the transition from acute to CI.
Methods
Subjects and procedure
Adult good sleeper subjects (>35 years of age) were recruited from two nationwide platforms over three recruitment intervals, separated by approximately 1 year’s time. Recruitment did not include individuals from 18 to 35 years of age because the study was focused on insomnia in middle-aged and older adults. Subjects were recruited from Zogby Analytics [10] (an international polling agency) and ResearchMatch [11]. The study was conducted in two phases, described as follows.
Phase-1
For subjects recruited by Zogby, age-appropriate good sleepers were identified and screened via a preliminary survey administered to panel members. Appropriate individuals were referred on to the study website. For ResearchMatch, age-appropriate individuals without sleep disorders were identified via an internal poll. Interested subjects were then referred to a screening questionnaire hosted on a Redcap server. In both cases (Zogby and ResearchMatch) potential study candidates provided a yes response to the following statement: “Are you a good sleeper? That is, do you reliably (5 or more nights per week) take less than 15 min to fall asleep and are awake during the night for less than 15 min? Has this been true for you for at least the last 6 months?.” The screening criteria (i.e. 15 min or less) was rigorous to enhance the likelihood that the study recruited enduringly good sleepers. No other inclusion or exclusion criteria were applied. Eligible subjects who expressed an interest in participating in the study were then referred to the study website where they (1) reviewed HIPAA forms and provided their informed consent, (2) completed an intake survey (profiling sleep, health, and mental health status and history), and (3) completed 2 weeks of online sleep diaries (baseline assessment) to corroborate their status as good sleepers.
Phase-2
Subjects that entered Phase-2 were monitored for a year and completed a number of assessments via the study website. The online questionnaires included: daily morning and evening sleep diaries; weekly and bi-weekly instruments (e.g. medical symptoms checklist, perceived stress scale, etc.); and monthly instruments (including one additional instrument regarding menstruation for women). Subjects that transitioned to acute or CI also completed an additional measure that was specific to insomnia (i.e. insomnia severity index). Instruments that have or are based on specific time frames (e.g. “in the last week, did you…”) were given according to the prescribed time frames. Instruments that were retrospective but without a specific time frame were given according to a schedule that we developed; where the guiding principle was to reduce subject costs by administering the instrument less versus more frequently. Accordingly, the majority of instruments were given monthly in order to assess changes over the 1-year study period. See Table 1 for a list of all the questionnaires included in this study. While a comprehensive list of measures is included here, between-subjects analyses on all of these questionnaires is beyond the scope of this paper and will be the focus of future work. Note, as participation in this study was reliant on the completion of online measures, participants were withdrawn from the study for noncompliance if their adherence rate dropped below 60% across 14 days at any point during the study (see Subject Attrition section). This adherence cut-off was empirically determined to maximize the number of subjects that could be retained for the final analyses.
Table 1.
Study instruments
| Schedule | Instrument |
|---|---|
| Baseline only* | Dysfunctional Beliefs and Attitudes About Sleep Scale (DBAS-16)[12] |
| Ford Insomnia Response to Stress Test (FIRST) [13] | |
| Daily | AM daily sleep diary |
| PM daily sleep diary | |
| Weekly | Health and Medical Symptoms Checklist (MHS-CL) |
| Daily Hassles Scale (DHS) [14] | |
| Perceived Stress Scale (PSS) [15] | |
| Social Readjustment Rating Scale (SRRS) [16] | |
| Brief Fatigue Inventory (BFI) [17] | |
| Epworth Sleepiness Scale (ESS) [18] | |
| Insomnia Severity Index (ISI) [19, 20] | |
| Glasgow Sleep Effort Scale (GSES) [21] | |
| Sleep Preoccupation Scale (SPS) [22] | |
| Sleep Hygiene Index (SHI) [23] | |
| Biweekly | Patient Health Questionnaire (PHQ-9)[24] |
| Monthly | Medical History Form (MHF) |
| Sleep Medication History Form | |
| Psychiatric Health Screen | |
| Alcohol Use Disorders Identification Test (AUDIT) [25] | |
| Drug Abuse Screening Test (DAST-20)[26] | |
| Menstrual Cycle Questionnaire | |
| Sleep Associated Monitoring Index (SAMI) [27] |
*It was the case that “baseline only” measures were only given at baseline, and that the daily, weekly, etc. measures were given at baseline and according to their corresponding schedule.
Daily sleep diary
The prospective assessment of sleep continuity disturbance (i.e. difficulty initiating or maintaining sleep) was conducted via online daily sleep diaries through a dedicated web-portal (all questionnaires were completed on this study-specific site). Items included in the online sleep diary were based on the Consensus Sleep Diary [28]. Primarily, the diary was used to quantify daily variations in SL, wake after sleep onset (WASO), early morning awakenings (EMA), nocturnal awakenings (NWAK), total sleep time (TST), and time in bed (TIB). Participant received daily email reminders to complete their entries. Participants were also emailed if they missed a diary entry and send a “warning” email if their adherence dropped below 60% across 14 days at any point during the study.
Subject compensation
In order to encourage high adherence rates (i.e. the completion of daily sleep diaries), a novel compensation strategy was used: a lottery. All study participants were automatically enrolled in the study lottery. The lottery was conducted once a month where each questionnaire that a subject completed was automatically counted as an “entry” into the lottery. Each subject accumulated entries over the course of each month. At the end of each month, a drawing was conducted where winners were randomly selected from all the submitted entries. Each subject was eligible to win one prize per month, and one prize of each dollar value over the year. The prizes for the first cohort were as follows: 2 of $750, 4 of $500, 10 of $250, and 20 of $100 (total of 36 awards/month). At the end of the year, a final lottery was conducted for all the participants that completed the study. In this case, 22 awards of $1000 were randomly awarded. Identification of Cohort. While the study recruited good sleepers by self-report (5 or more nights per week taking 15 min or less to fall asleep and awake during the night for 15 min or less [including EMA]), extra steps were taken to confirm stable GS in the analysis stage. First, using moving 7-day windows (successive, overlapping 7-day segments; i.e. first window consists of days 1–7, the second window consists of days 2–8, the third window consists of days 3–9, etc.) each week was determined to be a good sleeping week or poor sleeping week. A poor sleeping week consisted of 3 or more nights with SL ≥ 30 min and/or WASO ≥ 30 min and/or EMA ≥ 30 min. Notably, while frequency and chronicity are defined in the diagnostic criteria, severity is not. It is, however, common in research criteria to use 30 min as a severity threshold (e.g. a SL ≥ 30 min is considered clinically significant), and therefore this is the criteria used here [5, 29]. Provided diary responses were given for at least 4 days of the week, the remaining weeks were good sleeping weeks. A stable good sleeper had to have 10 of the first 12 weeks classified as good sleeping weeks.
Identification of transitions
Each subjects’ sleep diary data were used to identify instances of sleep initiation and/or maintenance difficulties and to determine if such difficulties persisted. Acute insomnia was defined as two consecutive weeks with a frequency of at least 3 nights per week of sleep initiation and/or maintenance complaints. For the definitions of CI and recovery, we chose 3 months since it is consistent with current diagnostic criteria for defining a new and enduring state. Because we are applying these rules to prospective, high frequency sampled data (i.e. daily sleep diaries), one has to adopt a more quantitatively precise definition. The current definition was selected to allow for some normal variation in good and bad sleep (takes into account that insomnia severity varies from night-to-night and week-to-week), but to also be, by anyone’s definition, consistent with what most would define as CI. The definition for recovery from CI was more lenient (greater than 50% “good sleep” during a 12-week period) in order to “catch” people early in recovery, but we also wanted to be sure that this definition captured an enduring state change (hence, requiring the last 4 weeks be “good sleep”). See also Table 2 for specific definitions). Additionally, standard quantitative criteria for insomnia severity (≥30 min) were used to identify excessively long SL and/or WASO and/or early morning awakening (EMA) times. In contrast to DSM-5’s criteria for Insomnia Disorder, qualitative assessments of distress and/or impairment in daytime functioning were not included in these definitions so that post hoc analyses could be conducted to empirically determine what levels of sleep continuity severity, frequency and chronicity are associated with daytime complaints (to be reported elsewhere). It is possible or even likely that, by excluding impairments in daytime functioning from our definition of insomnia, the incidence rate of insomnia may be overestimated. For example, Ohayon reported that the prevalence of insomnia, when only considering quantitative criteria or nocturnal symptoms, was 30%–48%. The prevalence rate dropped to 9%–15% when daytime consequences were included in the criteria [4]. This said, the present study assesses incidence not prevalence and uses a more dense sampling approach (i.e. daily diaries), which may maximize the ability to detect new onset cases.
Table 2.
Definitions for state transitions*
| Acute insomnia (AI) | Two or more consecutive weeks with a frequency of ≥3 nights/week of sleep latency and/or wake after sleep onset (WASO) severity ≥30 min. |
| Recovery (REC) | Within a 12-week period, 7 or more weeks of good sleep after AI and/or CI episodes where the final 4 weeks in the period must be designated as good sleep. |
| Persistent poor sleep (PPS) | Recurring bouts of AI without transition to CI or REC |
| Chronic insomnia (CI) | 10 or more weeks in a 12-week period with same frequency and severity criteria as AI |
*Participants that did not meet either transition criteria were considered continuous good sleepers.
Statistical analyses
Demographic characteristics were compared between the good sleeper cohort and those excluded from the analysis using t-tests, Wilcoxon rank-sum tests for skewed data, or Fisher’s exact tests for binary variables. Statistical analyses were performed in SAS v9.4 (SAS; Institute Cary, NC) and Stata v15.1 (College Station, TX).
Results
Subject attrition
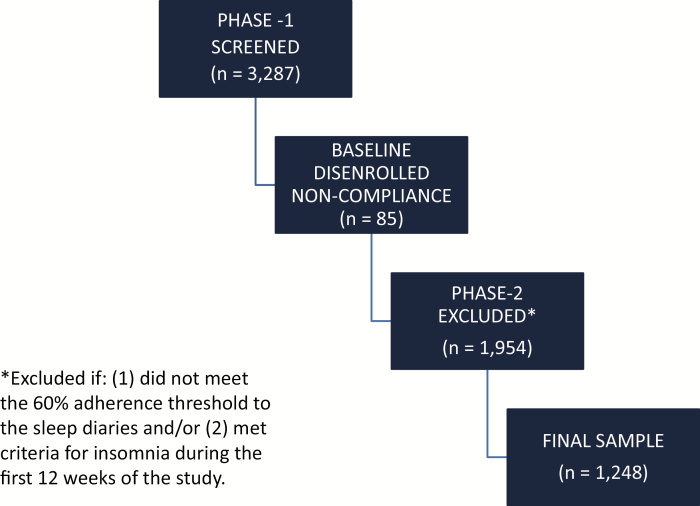
A total of 3,287 subjects were positively screened for GS and entered into Phase-1 (consent, baseline questionnaires and two weeks of daily sleep diaries). Eighty-five subjects (2.6%) were disenrolled due to non-compliance during the 2-week baseline assessment (daily diaries). Of the remaining 3,202 subjects that completed the baseline assessment and entered into Phase-2 of the study, 1,954 subjects (59.4%) were excluded from our final analyses if the subject (1) did not meet the 60% adherence threshold to the daily sleep diaries (minimum threshold to confirm GS and assess incidence over time) and/or (2) met criteria for AI during the first 12 weeks of the study (i.e. had 2 or more consecutive poor sleeping weeks during this baseline period; see Figure 1 for subject flow). Of the 1,954 subjects that were excluded in Phase-2, 926 subjects were excluded for not meeting the 60% adherence threshold and 1,028 subjects were excluded for meeting criteria for at least AI (i.e. they did not enter the study as good sleepers). A total of 1,248 subjects (38.0%) entered into, and completed Phase-2. Table 3 provides a statistical comparison of the final sample (n = 1,248; i.e. included) relative to those subjects that were excluded (n = 2,039). The final sample was slightly older, had lower BMIs, and had a greater proportion of subjects who were white and male. While significant differences between the samples were observed, the magnitudes of the differences were small (see Table 3). For example, the included sample had a mean age of 53.2 years, while the excluded sample had a mean age of 52.2 years (p = 0.02). The final sample also reported lower scores on baseline sleep and clinical profile measures.
Figure 1.
Subject flow.
Table 3.
Between-participants comparisons between the final sample and the excluded sample on baseline measures
| Excluded (N = 2039) | Included (N = 1248) | ||||
|---|---|---|---|---|---|
| Variable | Mean or % | SD | Mean or % | SD | p* |
| Age (years) | 52.2 | 11.6 | 53.2 | 11.0 | 0.02 |
| BMI (kg/m2) | 29.6 | 7.6 | 28.9 | 7.4 | <0.01 |
| AUDIT | 9.0 | 3.6 | 8.9 | 3.6 | 0.52 |
| DBAS-16 | 43.1 | 19.0 | 38.6 | 17.9 | <0.001 |
| PSS | 14.0 | 6.1 | 12.4 | 5.6 | <0.001 |
| % Education (>HS) | 86.4 | 87.9 | 0.24 | ||
| % Female | 75.8 | 67.4 | <0.001 | ||
| % Income (≥$30,000) | 75.0 | 77.8 | 0.07 | ||
| % Ethnic minority | 23.1 | 17.5 | <0.001 | ||
| % PHQ-9 ≥ 5 | 25.2 | 15.2 | <0.001 | ||
| Variable | Median | Min, Max | Median | Min, Max | p† |
| DHS | 27.0 | 0, 310 | 20.0 | 0, 296 | <0.001 |
| FIRST | 18.0 | 9, 36 | 16.0 | 9, 36 | <0.001 |
| SAMI | 21.0 | 11, 55 | 19.0 | 11, 55 | <0.001 |
| SPS | 24.0 | 10, 64 | 21.0 | 10, 63 | <0.001 |
| ESS | 6.0 | 0, 24 | 6.0 | 0, 24 | <0.001 |
AUDIT = Alcohol Use Disorders Identification Test, BMI = body mass index, DBAS-16 = Dysfunctional Beliefs and Attitudes about Sleep Scale, DHS = Daily Hassles Scale, ESS = Epworth Sleepiness Scale, FIRST = Ford Insomnia Response to Stress Test, PHQ-9 = Patient Health Questionnaire, PSS = Perceived Stress Scale, SAMI = Sleep Associated Monitoring Index, SPS = Sleep Preoccupation Scale.
*ANOVA (or Fisher’s exact) p-value from test of equal means (or %) across groups. †All of the variables arrayed above as median values were arrayed in this manner owing to skewed distributions with p-values from Kruskal–Wallis test.
Incidence rates
As can be seen in Figure 2, the 1-year incidence rate of AI was 27.0% (n = 337). Of these, 72.4% (n = 244) of subjects recovered GS (AI-REC), and 6.8% (n = 23) developed CI. Notably, 19.3% (n = 65) neither recovered nor went on to develop CI. This group exhibited what might be best referred to as persistent poor sleep (PPS; problems with sleep initiation or maintenance [SL or WASO or EMA > 30 min] that did not meet or exceed frequency (3 or more days per week) or chronicity criteria [3 or more months in duration]). Of those that developed CI, 5 subjects (1.5% of AI) recovered GS (CI-REC). Note: the definition of new onset insomnia (AI or CI) was based solely on quantitative criteria for severity, frequency and chronicity. As noted above, daytime impairment was not included as a criterion but rather was assessed as a dependent variable.
Figure 2.
One-year incident rate of acute insomnia (AI), persistent poor sleep (PPS), chronic insomnia (CI), and recovery (REC; from both AI and CI).
Discussion
The data from the present study suggest that AI is common (affects about 27% of the population per annum) and that for most people, sleep continuity disturbance is self-limiting (about 72% of those with incident insomnia recover). Of those that develop AI, only about 7% developed CI, but it appears that up to another 19% exhibit an intermediate form of insomnia (i.e. persistent poor sleep or a form of poor sleep that is neither acute nor chronic).
Incidence of (and recovery from) AI
The observed incidence of AI is remarkably high, although not as common as was found by Ellis and colleagues (31%–37%) [5]. The difference in estimates is possibly ascribable to methodological differences between the studies, if not cultural differences between the study samples. For example, differences may exist in terms of occupational response to insomnia—people from the UK may be more likely to be absent from work whereas people in the United State (US) more likely to exhibit “presenteeism.” As such, it may be that those in the UK ascribe a specific sequelae event to their insomnia, aiding recall, whereas those in the US may not. This is, however, just one example of a potential cultural difference. The methodological differences include: (1) the current cohort did not contain subjects between 18 and 35 years of age (the mean age of the UK sample was 27.7 + 11.4 years); (2) The UK sample was not assessed with prospective sampling (daily sleep diaries [arguably a more refined method for the detection AI]); (3) the current study used quantitative criteria for the identification of insomnia (i.e. 3 or more nights with SL ≥ 30 min and/or WASO ≥ 30 min and/or EMA ≥ 30 min). This said, the annual incidence rate of insomnia in both studies (27%–37%) is so common that it suggests that the occurrence of AI may not be, as is the case with CI, necessarily pathological (i.e. of or relating to a true disease or disorder). More than this, as previously noted by Ellis and colleagues [30], it is possible that AI may be normative (i.e. expected as part of the natural rhythm of sleep/insomnia) , if not adaptive. One way this might be true is that the AI that occurs with stress may be an unrecognized part of the fight-flight response; a necessary override to the normal two process regulation of sleep timing, depth and/or duration [31]. Put differently, stress induced insomnia may prohibit the systematic imperative for sleep under unsafe conditions. While this idea awaits empirical validation, it has been proffered on multiple occasions [30, 32, 33]. This perspective was presaged by Spielman and colleagues when they suggested that “No matter how important sleep may be, it was adaptively deferred when the mountain lion entered the cave” (p.3) [34] A similar perspective was advanced by others, including D. Handley (personal communication, 2005) “we live with insomnia today, because at some point in our evolutionary history, insomnia allowed us to live.” Conceived of this way, it is not surprising that at least 27% of the population experience AI per year. Given this perspective, it is also not surprising that 72% of the population do not go on to develop either “persistent poor sleep” or CI. That is, as the threat (or stress) abates, the AI resolves. The question that arises here is “what factors mediate the transition from AI to GS?.” While it is possible that this occurs simply with the absence of behavioral adaptations to sleep loss (e.g. sleep extension), it is also possible that there are factors that are uniquely prophylactic. While these issues are speculative and under further investigation, possible mediators include individual differences with respect to: basal sleep need (i.e. whether incident insomnia results in TSTs that are below sleep need) [35]; resilience (i.e. how robust the individual’s capacity is to avoid sleep loss) [13]; psychosocial demand (i.e. the intensity of daytime work and/or social requirements) [36]; and context (i.e. the significance of sleep loss in the context of the individual’s cumulative medical, psychological, daily hassles, and/or life stress impacts).
Incidence of CI
In marked contrast to the incidence of AI, a remarkably small number of subjects transition from AI to CI on an annual basis (~7% of AI subjects and 1.8% of the initial good sleeper sample). While the exact explanation(s) for this transition are unknown, it is possible that, for individuals that do transition from AI to CI, behavioral adaptations to sleep loss and/or nocturnal wakefulness (e.g. sleep extension) may serve to perpetuate insomnia (mediate the transition from AI to CI) [37]. Alternatively, the transition from AI to CI may be mediated by other factors, some of which may be related to more cognitively driven phenomenon (such as sleep reactivity, monitoring, and/or preoccupation) [13, 38, 39], the failure to engage in naturalistic sleep restriction (i.e. if one can only sleep 6 h, then only try for 6 h) [34, 37], conditioned aberrant neurobiologic control and regulation of sleep ability (i.e. conditioned local wakefulness during non-REM sleep) [40, 41], or inherent and enduring decrements to sleep ability (e.g. permanent alterations in dopaminergic, orexinergic and/or GABAnergic tone) [42, 43]. Moreover, it is possible that all of these factors to one extent or another contribute to the development of CI, and therefore, should be studied further.
Incidence of persistent poor sleep
While presaged by other’s prior work with the natural history of insomnia (i.e. the identification of a subsyndromal insomnia) [8], it remains surprising that more than 19% of individuals that experience AI neither recover nor develop CI; they simply exhibit a variable (by day and week), but nonetheless persistent (over the courses of months), level of sleep continuity disturbance. To date, this group was not found to differ on demographic or baseline variables (with the exception of having slightly higher BMIs). While it is possible the unique factors account for this “middle of the road” outcome, it is also possible that the same factors (but to different degrees) that are responsible for pathological outcomes are also responsible for prophylaxis. For example, those that engage in sleep extension develop CI, those that maintain stable times in bed develop persistent poor sleep (i.e. intermediate insomnia), and those that naturally engage in sleep restriction (e.g. awaken early and start their day) recover. Alternatively, those with persistent poor sleep may be on the way to developing CI but are doing so at a slower rate of progression. An extended follow-up or repeat assessment would be required to address this issue.
Study limitations and strengths
The current study had both important limitations and strengths. The primary limitation was the high exclusion rate (i.e. loss of subjects owing to ineligibility, non-compliance, subject withdrawal, and disenrollment). This issue is important as it may potentially limit the generalizability of the findings. More, while we believe that the results are generalizable to the population at large, our sample was marginally different than the general population (based on 2018 U.S. Census data), such that, our sample had a greater proportion of persons that identified as female (67% as compared to 51%) and white (82.5% as compared to 76.5%). Perhaps the larger concern pertains to the limitations on our ability to detect new-onset CI, and this limitation will adversely affect (in terms of statistical power) future efforts to assess the factors that mediate the transition to CI. In addition, it is important to note that the present study did not include adults between the ages of 18 and 35 years. The incidence rates reported here may therefore not generalize to this age group. More, as mentioned above, the definition of insomnia used in the present study included nocturnal symptoms only, and it’s possible that the annual incidence rate of insomnia with daytime consequences is lower. It’s also possible that, given the prospective assessment of sleep continuity disturbance (i.e. daily sleep diaries), the incidence rates of insomnia reported here are more accurate, particularly for AI. Traditional diagnostic criteria (i.e. DSM-5 and ICSD-3) do not require daily diaries and rely on retrospective assessments of sleep continuity, which are less burdensome to the client but are less likely to capture brief episodes of sleep continuity disturbance due to cyclical nature of insomnia. Along these lines, while the present study took multiple steps to exclude individuals with a prior history of insomnia (i.e. at recruitment, include only those with GS during the past 6 months, require a 12-week baseline without insomnia to continue in the study), it is still possible that some participants did have a prior history of insomnia and were in a good phase when the baseline assessment was completed. It is important that future studies take into consideration the tendency for insomnia symptoms to fluctuate over time and how these fluctuations may influence the estimation of new-onset acute or CI incidence rates.
The present study also had a number of important strengths. To our knowledge, this is the first study to prospectively assess the incidence of acute and CI using a dense-sampling approach (i.e. daily sleep diaries) in a large sample of good sleepers. This assessment strategy was particularly advantageous because it offered (1) the opportunity to more accurately determine whether a transition occurred and (2) the temporal resolution to identify when the transition(s) occurred.
Conclusion
Additional analyses are ongoing to evaluate which demographic and clinical variables predict the various transitions, particularly whether pre-existing insomnia history, pre-morbid dysfunctional beliefs and practices, and/or the phenomenon of sleep extension mediate/moderate the observed occurrences of CI or recovery.
Acknowledgments
This study was conceived of in collaboration with Dr. Jason Ellis. Further, the concept served as a springboard for several investigations, which were partially supported two grants—one awarded to Dr. Ellis and one awarded to Dr. Perlis (Ellis: Economic and Social Research Council [RES-061-25-0120-A]; Perlis: National Institutes of Health [R01AG041783]). Dr. Perlis’ academic work is also supported by a K24 (K24AG055602).
Conflict of interest statement. None declared.
References
- 1. Morin CM, et al. Epidemiology of insomnia. Sleep Med Clin. 2013;8(3):281–297. [Google Scholar]
- 2. Morin CM, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123–130. [DOI] [PubMed] [Google Scholar]
- 3. Mai E, et al. Insomnia: prevalence, impact, pathogenesis, differential diagnosis, and evaluation. Sleep Med Clin. 2008;3(2):167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. [DOI] [PubMed] [Google Scholar]
- 5. Ellis JG, et al. The natural history of insomnia: focus on prevalence and incidence of acute insomnia. J Psychiatr Res. 2012;46(10):1278–1285. [DOI] [PubMed] [Google Scholar]
- 6. Ford DE, et al. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262(11):1479–1484. [DOI] [PubMed] [Google Scholar]
- 7. Foley DJ, et al. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep. 1999;22(Suppl 2):S366–72. [PubMed] [Google Scholar]
- 8. Leblanc M, et al. Incidence and risk factors of insomnia in a population-based sample. Sleep. 2009;32(8):1027–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abiona T, et al. The natural history of insomnia in the ibadan study of ageing. Sleep. 2011;34(7):965–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Analytics Z. The Zogby Poll; 2019. https://zogbyanalytics.com/. Accessed May 17, 2019.
- 11. National Institute of Health. ResearchMatch; 2019. https://www.researchmatch.org/. Accessed May 17, 2019.
- 12. Morin CM, et al. Dysfunctional beliefs and attitudes about sleep (DBAS): validation of a brief version (DBAS-16). Sleep. 2007;30(11):1547–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drake CL, et al. Vulnerability to stress-related sleep disturbance and hyperarousal. Sleep. 2004;27(2):285–291. [DOI] [PubMed] [Google Scholar]
- 14. Holm JE, et al. The Daily Hassles Scale (Revised): does it measure stress or symptoms? Behav Assess. 1992;14:465–482. [Google Scholar]
- 15. Hewitt PL, et al. The perceived stress scale: factor structure and relation to depression symptoms in a psychiatric sample. J Psychopathol Behav Assess. 1992;14(3):247–257. [Google Scholar]
- 16. Holmes TH, et al. The social readjustment rating scale. J Psychosom Res. 1967;11(2):213–218. [DOI] [PubMed] [Google Scholar]
- 17. Mendoza TR, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85(5):1186–1196. [DOI] [PubMed] [Google Scholar]
- 18. Johns M. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. [DOI] [PubMed] [Google Scholar]
- 19. Morin CM. Insomnia: Psychological Assessment and Management. New York, NY: Guilford Press; 1993. [Google Scholar]
- 20. Bastien CH, et al. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. [DOI] [PubMed] [Google Scholar]
- 21. Broomfield NM, et al. Towards a valid, reliable measure of sleep effort. J Sleep Res. 2005;14(4):401–407. [DOI] [PubMed] [Google Scholar]
- 22. Ellis J, et al. Sleep preoccupation in poor sleepers: psychometric properties of the Sleep Preoccupation Scale. J Psychosom Res. 2007;63(6):579–585. [DOI] [PubMed] [Google Scholar]
- 23. Mastin DF, et al. Assessment of sleep hygiene using the Sleep Hygiene Index. J Behav Med. 2006;29(3):223–227. [DOI] [PubMed] [Google Scholar]
- 24. Kroenke K, et al. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saunders JB, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption‐II. Addiction. 1993;88(6):791–804. [DOI] [PubMed] [Google Scholar]
- 26. Skinner H. Drug Use Questionnaire (DAST-20). Addict Res Found. 1982;9–10. doi:10.1037/t31524-000 [Google Scholar]
- 27. Semler CN, et al. Monitoring for sleep-related threat: a pilot study of the Sleep Associated Monitoring Index (SAMI). Psychosom Med. 2004;66(2):242–250. [DOI] [PubMed] [Google Scholar]
- 28. Carney CE, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35(2):287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taylor DJ, et al. Epidemiology of insomnia, depression, and anxiety. Sleep. 2005;28(11):1457–1464. [DOI] [PubMed] [Google Scholar]
- 30. Ellis JG, et al. Acute insomnia: current conceptualizations and future directions. Sleep Med Rev. 2012;16(1):5–14. [DOI] [PubMed] [Google Scholar]
- 31. Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1(3):195–204. [PubMed] [Google Scholar]
- 32. Perlis ML, et al. Models of insomnia. In: Kryger M, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 5th ed. St. Louis, MO: Elsevier; 2011:850–865. [Google Scholar]
- 33. Perlis ML, et al. Etiology and pathophysiology of insomnia. In: Kryger M, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. St. Louis, MO: Elsevier; 2005:714–725. [Google Scholar]
- 34. Spielman AJ, et al. The varied nature of insomnia. In: Hauri, PJ, ed. Case Studies in Insomnia. New York: Plenum Publishing Corporation; 1991:1–18. [Google Scholar]
- 35. Bonnet MH, et al. Hyperarousal and insomnia. Sleep Med Rev. 1997;1(2):97–108. [DOI] [PubMed] [Google Scholar]
- 36. Grandner MA. Social-ecological model of sleep health. In: Grandner MA, ed. Sleep and Health. San Diego, CA: Elsevier; 2019: 45–53. [Google Scholar]
- 37. Spielman AJ, et al. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am. 1987;10(4): 541–553. [PubMed] [Google Scholar]
- 38. Semler CN, et al. An experimental investigation of daytime monitoring for sleep-related threat in primary insomnia sleep-related threat in primary insomnia. Elsevier. 2016;9931(October):146–161. doi:10.1080/02699930600639462 [Google Scholar]
- 39. Ellis J, et al. The role of preoccupation in attributions for poor sleep. Sleep Med. 2007;8(3):277–280. [DOI] [PubMed] [Google Scholar]
- 40. Perlis ML, et al. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res. 1997;6(3):179–188. [DOI] [PubMed] [Google Scholar]
- 41. Buysse DJD, et al. A neurobiological model of insomnia. Drug Discov Today. 2012:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Plante DT, et al. The role of GABA in primary insomnia. Sleep. 2012;35(6):741–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Plante DT, et al. Reduced γ-aminobutyric acid in occipital and anterior cingulate cortices in primary insomnia: a link to major depressive disorder? Neuropsychopharmacology. 2012;37(6):1548–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]