Abstract
Study Objectives
To compare sleep–wake rhythms, melatonin, and cancer-related fatigue in pediatric patients with acute lymphoblastic leukemia (ALL) to healthy children and to assess the association between sleep–wake outcomes and cancer-related fatigue.
Methods
A national cohort of ALL patients (2–18 years) was included. Sleep–wake rhythms were measured using actigraphy and generated the following variables: Interdaily stability (IS): higher IS reflects higher stability; intradaily variability (IV): lower IV indicates less fragmentation; L5 and M10 counts: activity counts during the five least and 10 most active hours, respectively; and relative amplitude (RA): the ratio of L5 and M10 counts (higher RA reflects a more robust rhythm). The melatonin metabolite, 6-sulfatoxymelatonin (aMT6s), was assessed in urine. Cancer-related fatigue was assessed with the PedsQL Multidimensional Fatigue Scale. Using regression models sleep–wake rhythms, aMT6s, and cancer-related fatigue were compared to healthy children and associations between sleep–wake outcomes and cancer-related fatigue were assessed in ALL patients.
Results
In total, 126 patients participated (response rate: 67%). IS, RA, and M10 counts were lower in patients compared to healthy children (p < 0.001). aMT6s levels were comparable to healthy children (p = 0.425). Patients with ALL were more fatigued compared to healthy children (p < 0.001). Lower IS, RA and M10 counts and higher IV were significantly associated with more parent-reported cancer-related fatigue. Associations between sleep–wake rhythms and self-reported cancer-related fatigue were not statistically significant.
Conclusions
Sleep–wake rhythm impairment is associated with more cancer-related fatigue in pediatric ALL patients. Interventions aimed to improve sleep hygiene and encourage physical activity may reduce cancer-related fatigue.
Keywords: actigraphy, acute lymphoblastic leukemia, cancer-related fatigue, physical activity, children sleep–wake rhythms
Statement of Significance.
Sleep–wake cycle disruptions are associated with fatigue, amongst many other adverse health outcomes. Cancer-related fatigue is a common and distressing side-effect of anti-cancer treatments with few effective interventions available. The exact role of the sleep–wake rhythm in the pathophysiology of cancer-related fatigue in pediatric patients is unknown but could provide clues for improvement of cancer-related fatigue. Evidence on sleep–wake rhythms in pediatric cancer patients is limited. This study, therefore, aims to compare sleep–wake rhythms and cancer-related fatigue in pediatric patients with acute lymphoblastic leukemia (ALL) to healthy children and to evaluate the association between sleep–wake rhythm variables and cancer-related fatigue. The findings of this study suggest opportunities for interventions to improve cancer-related fatigue in pediatric patients with ALL.
Introduction
Sleep and wakefulness show a circadian rhythm, which is driven by the suprachiasmatic nuclei located in the anterior hypothalamus [1]. To align the circadian sleep–wake cycle to the environmental 24-hour light-dark cycle it needs to be synchronized by external cues, such as scheduled sleep, physical activity, meals and, most importantly, light [1–4]. The ability to maintain a robust sleep–wake rhythm can be seen as an independent indicator of health [5]. In addition, disruption and misalignment of sleep–wake rhythms have been associated with several adverse health outcomes, such as, chronic fatigue, impaired cognitive functioning, an increased risk of cardiovascular diseases, psychiatric diseases, diabetes and cancer [3, 6–8].
Disturbed sleep–wake rhythms have been reported in adults with cancer [9–11]. The severity of these disturbances is associated with worse cancer-specific outcomes (including impaired response to treatment and a decreased survival rate). Also, in pediatric patients with acute lymphoblastic leukemia (ALL) and with central nervous system (CNS) tumors, impaired sleep-rest activity patterns are described [12, 13]. Both in adult cancer patients and in pediatric cancer patients less robust sleep–wake rhythms were linked to more severe cancer-related fatigue [9, 12, 13].
Cancer-related fatigue is one of the most common side-effects of cancer treatments that persists after the end of treatment [14–17]. It is a distressing and disabling symptom that impairs school functioning and reduces the ability to participate in social roles and activities [18, 19]. Cancer-related fatigue is a multidimensional problem and underlying causal mechanisms are not yet well understood. A biopsychosocial model including demographic, biological, medical, functional and behavioral factors contributing to cancer-related fatigue seems likely [15]. The sleep–wake rhythm, including multiple factors of this biopsychosocial model (such as biological and behavioral factors), could play a role in the causality of cancer-related fatigue. Therefore, the sleep–wake rhythm could potentially provide clues to improve cancer-related fatigue in childhood cancer patients.
There are several methods of evaluating circadian rhythms. Sleep–wake rhythms can unobtrusively be assessed with actigraphy. Actigraphy is much less invasive compared to the gold standard polysomnography, and in addition, it can record sleep–wake patterns continuously over longer periods in an ambulant setting [20]. The recorded time-series of activity can be analyzed to obtain variables that quantify the fragmentation of the sleep–wake cycle, its ability to synchronize to the 24-hour light-dark cycle, and levels of daytime and nocturnal physical activity.
In addition, the assessment of melatonin has been used as an endogenous marker of circadian dysregulations. Melatonin production is not influenced by external influences other than light, unlike other circadian rhythm markers, and is, therefore, a powerful biomarker in the assessment of circadian dysregulation [21]. Endogenous disruption of melatonin production is not expected in pediatric patients with ALL, as structures involved in melatonin production and release (such as the suprachiasmatic nucleus and the pineal gland), are generally not affected. However, several factors during ALL therapy (such as treatment-related toxicities and hospitalization) could influence light exposure and subsequently melatonin production [21].
There is limited evidence on sleep–wake rhythms and the relation with cancer-related fatigue in pediatric cancer patients [12, 13]. Such a relationship could advance our knowledge regarding the etiology of cancer-related fatigue and guide the development of interventions to improve well-being of pediatric cancer patients. Furthermore, melatonin is used in clinical practice, although it has not been established yet whether melatonin levels are actually disturbed in pediatric cancer patients.
Recently, a position paper on behalf of the International Psycho-Oncology Society Pediatrics Special Interest Group was published with recommendations to expand sleep research in pediatric oncology [22]. They recommended to address the gaps in understanding the relationship between sleep–wake rhythms and health outcomes along the trajectory of pediatric cancer. Furthermore, the authors mentioned the need for further research to guide melatonin use in pediatric oncology.
Therefore, this study aims to: (1) Determine sleep–wake rhythms, melatonin levels, and cancer-related fatigue in patients with ALL and compare these outcomes to healthy children, and (2) Evaluate the association between sleep–wake rhythm variables and cancer-related fatigue.
Methods
Patients and procedures
The results described here are part of the SLAAP [SLEEP]-study (SLeep in children with Acute lymphoblastic leukemia And their Parents), a multicenter study on sleep(-wake rhythms), quality of life and cancer-related fatigue in pediatric patients with ALL and functioning of their parents.
The assessment consisted of patient- and parent-reported questionnaires (sleep, quality of life, cancer-related fatigue, and parental distress), a sleep log, and a 1-week actigraphy assessment. In addition, a first-morning urine void was collected for the assessment of 6-sulfatoxymelatonin (aMT6s). Results on sleep–wake rhythms, melatonin levels, and cancer-related fatigue are reported here.
Patients were identified through the Dutch Childhood Oncology Group (DCOG) registry that includes all pediatric patients with a diagnosis of cancer or low-grade malignancy in the Netherlands. Patients were eligible if they were: (1) treated according to the DCOG ALL-11 treatment protocol, and (2) ≥2 years of age at assessment. Furthermore, parents and patients needed to master Dutch sufficiently to complete the questionnaires. Patients were recruited between August 2013 and July 2017 in the following Dutch pediatric oncology centers: Emma Children’s Hospital/Academic Medical Center and VU University Medical Center Amsterdam, Wilhelmina’s Children’s Hospital/University Medical Center Utrecht, Princess Máxima Center for pediatric oncology Utrecht, Sophia Children’s Hospital/Erasmus Medical Center Rotterdam, Beatrix Children’s Hospital/University Medical Center Groningen, Amalia Children’s Hospital/Radboud University Medical Center Nijmegen. Parents and patients of 12 years or older provided informed consent for participation.
The assessment was planned after the first treatment phase (i.e. induction and re-induction therapy), which lasted about 3 months and is considered the most intensive phase of ALL therapy. During the study assessment, ALL treatment consisted of four 2-week courses. Each course started with an approximately 4-day hospital admission because of administration of high dose methotrexate. The assessment was performed in-between admissions, in a home setting. During these intervals, patients only received oral anti-cancer medication (mercaptopurine) and generally experienced little treatment-related toxicity. Glucocorticoid therapy, a hallmark of ALL treatment and known for its effects on sleep, was not used during this treatment phase [12, 23, 24]. According to the ALL-11 protocol patients are stratified to the following risk groups (with increasing treatment intensity) based on response to treatment and cytogenetics: standard risk, medium risk, and high risk. At the time of the study assessment, most patients are not yet informed about their risk group stratification. Treatment did not include cranial radiation for any of the patients at time of the study assessment. Given the intensity of treatment and the regular hospital admissions patients generally do not attend school in this period.
The Institutional Review Board of the Erasmus Medical Center approved this study.
Measures
A survey regarding socio-demographics was completed by parents. Cancer-related fatigue was assessed by the valid and reliable questionnaire mentioned below. Since young or very ill patients are not able to fill out questionnaires themselves, parent-proxy reports were collected for all participants. In addition, self-reports were filled out by participants aged 8 years and older. Questionnaires were completed paper-pencil or through a secured online web portal depending on parent/patient preference. Parents only reported on child functioning, parental functioning was not described here. Sleep–wake rhythm variables were calculated based on a 7-day actigraphy assessment (all ages).
Socio-demographic information
Information on the following patient variables was collected: age, sex, time since diagnosis, parent-reported pre-existent sleep problems (yes or no), comorbidity (yes or no), sleep medication use (yes or no), bedroom sharing (yes or no).
Parents provided information on the following parental socio-demographics: parental age, sex, and highest attained educational level. Educational level was defined according to Statistics Netherlands (low educational level = no education, primary school, lower secondary education; middle educational level = upper secondary education, pre-university education, intermediate vocational education; high educational level = higher vocational education, university) [25]. For analyses, educational level was dichotomized as lower (low and middle educational level) versus higher educational level (high educational level).
Actigraphy derived sleep–wake rhythm
Actigraphy assessments were used to calculate sleep–wake rhythm variables. An actigraph (ActiGraph wGT3X-BT, Pensacola, FL) is a nonintrusive device that measures the occurrence and intensity of limb movements. Actigraphy has been validated against polysomnography and has proven to be an adequate method to measure sleep–wake patterns in infants, children, and adolescents [20, 26, 27]. Patients were instructed to wear the actigraph on their wrist for 24-hours for 7 days and to record sleep–wake schedule information (bedtime, wake time, nap times, non-wear times) in a sleep log to facilitate correct interpretation of the actigraphy data. With visual inspection of the data and based on sleep logs, invalid data was identified and removed from further analysis. Data was considered invalid in case probable non-wear time exceeded 3 consecutive hours [5]. A 24-hour period starting at the onset of this non-wear time was then removed from further analysis [5]. Sleep–wake rhythm variables were calculated only if valid data was available for at least 72 hours [28]. There are different approaches to quantify sleep–wake actigraphic recordings. Two commonly used methods are the cosinor analysis and the nonparametric method. Cosinor analysis fits a 24-hour cosine wave to the data and provides the estimated phase and amplitude. The method is parametric and presumes that the activity level variation over the day is best described with a 12:12 hour symmetrical sinusoidal. However, the sleep–wake rhythm is far from symmetrical and sinusoidal. In adults and adolescents, for example, there is an asymmetrical distribution of about 8 hours of sleep and 16 hours of wakefulness and in infants periods of sleep and wakefulness are more alternating over 24-hours. To better accommodate the non-sinusoidal, asymmetric activity pattern of everyday life, nonparametric methods have been proposed that do not make assumptions about the distribution of the rhythm. Accordingly, the nonparametric method seems to describe the sleep–wake pattern more accurately than cosinor analysis [28]. The following sleep–wake rhythm variables were obtained using nonparametric methods (definitions are provided in Table 1) [29]: Interdaily stability (IS), Intradaily variability (IV), M5 counts, M10 counts, and relative amplitude (RA).
Table 1.
Definitions of the sleep–wake rhythm variables
| Variable | Range | Definition |
|---|---|---|
| Intradaily variability | 0–2 | An estimate of the 24-hour rest-activity rhythm and reflects the fragmentation of the rhythm, a higher intradaily variability indicates a more fragmented rhythm |
| Interdaily stability | 0–1 | An estimate of the stability of the rhythm, and describes the synchronization of the rhythm, wherein 1 signifies a perfect synchronization to the light-dark cycle |
| L5 counts | 0–∞ | Activity counts of the least active 5 hours of the day. |
| M10 counts | 0–∞ | Activity counts of the most active 10 hours of the day. |
| Relative amplitude | 0–1 | Ratio of the difference and the sum of M10 and L5 counts. A higher relative amplitude indicates a bigger difference between the least and most active period during the day, hence a better sleep–wake rhythm. |
These variables were also obtained in healthy children aged 2–18 years with the same type of actigraph: ActiGraph wGT3X-BT, Pensacola, FL. Children were not eligible if they visited a health care provider for sleep disturbances in the preceding 3 months, used any type of sleep medication (including melatonin), or had a medical condition that could potentially affect their sleep(–wake rhythm). These healthy children were not matched to the study population described here, but analyses were controlled (as described below) for factors that could potentially affect the outcomes of the current study. Valid actigraphy data was available for 85 healthy children (median age: 8.5 years [interquartile range 5.5–15.3], 50.6% boys, highest parental educational level: 34.1% lower educational level and 65.9% higher educational level). Additional information on the recruitment, in- and exclusion criteria, and socio-demographics of the sample of healthy children is provided as Supplementary Material.
Urinary aMT6s
Metabolites of melatonin can be easily assessed in blood, saliva, and urinary samples. Melatonin levels show intra-individual stability but inter-individual variability [30]. In-home salivary sampling has shown to be a feasible method for the assessment of dim-light melatonin onset (DLMO) in pediatric cancer patients [31]. However, considering the young age of most ALL patients, the multiple study assessments (questionnaires, actigraphy, melatonin), and the timing of assessment during treatment, the burden of salivary DLMO assessment was considered too high. To reduce the study burden, a single morning urine sample was therefore used for the assessment of aMT6s, as this highly correlates with total nocturnal plasma melatonin levels [21, 32, 33]. Patients and parents were instructed to collect the first-morning void urine in the second part of the measurement week. Since urinary aMT6s levels are significantly correlated when compared on consecutive days, urine collection was not restricted to a specific day during the measurement week [21]. Patients and parents were asked to store the urine in the refrigerator until the sample was returned to the research team. Bojkowski et al. [34] showed stable aMT6s levels even at room temperature for 5 days. Urine samples were stored at −80°C until analysis. Stability of aMT6s levels over time has been proven and it is, therefore, suitable for delayed laboratory processing [21]. The aMT6s levels were analyzed by isotope dilution mass spectrometry using online solid-phase extraction in combination with liquid chromatography and tandem mass spectrometry (LC–MS/MS), at the Department of Laboratory Medicine, University Medical Center Groningen. Intra-assay imprecision was below 2.5% and inter-assay imprecision was below 5.4%. The quantification limit for aMT6s was determined at 0.2 nmol/L. Concentrations of aMT6s were adjusted for urinary creatinine levels. In the sample of healthy children mentioned above (Supplemental Material), morning void urine samples were collected to provide age-appropriate urinary aMT6s levels in healthy children. Valid aMT6s values were available for 90 healthy children (median age: 8.9 years [5.6–15.7], 52.2% boys).
Cancer-related fatigue
The Dutch version of the PedsQL Multidimensional Fatigue Scale (PedsQL MFS) was used to assess cancer-related fatigue [35, 36]. The PedsQL MFS is designed to measure child and parent perceptions of fatigue in pediatric patients. The questionnaire consists of 18-items and assesses the occurrence of problems during the past week. A 5-point Likert scale (almost always, frequent, sometimes, almost never, never) is used. The items allow for an overall fatigue score and three subscales scores (each six items): general fatigue (e.g.: “I feel tired” or “I feel too tired to spent time with my friends”), sleep-rest fatigue (e.g.: “I spent a lot of time in bed” or “I feel tired when I wake up in the morning”), and cognitive fatigue (e.g.: “It is hard for me to keep my attention on things” or “It is hard for me to remember more than one thing at a time”). Items are rescored to a 0–100 score. A higher score is indicative of better functioning, that is, less cancer-related fatigue. The subscale scores were used in this study. Scores in healthy Dutch children have previously been collected [35]. The original dataset of this population was used for analyses. The internal consistency of the subscales was adequate in Dutch healthy children as well as in current study population (Cronbach’s alpha: parent-reports > 0.70 and self-reports > 0.60).
Statistical analysis
Mann Whitney U tests and chi-square tests were used to assess differences in patient age and sex between participants and non-participants and patients that were not invited for the study.
Descriptive statistics of sleep–wake rhythm variables, urinary aMT6s values corrected for creatinine levels (µmol/mol creatinine), and cancer-related fatigue scores were presented. Patients’ outcomes were compared to outcomes of healthy children using linear regression models. Regression models were adjusted for patient age, sex, and sleep medication use as differences in these outcomes between patients with ALL and healthy children could affect sleep–wake rhythm outcomes, melatonin levels, and cancer-related fatigue. The regression models for sleep–wake rhythm outcomes and cancer-related fatigue were also adjusted for highest attained parental educational level. Patients with ALL that used melatonin were excluded from the urinary aMT6s analysis. Differences between patients and healthy children were expressed in a regression coefficient (B) with a 95% confidence interval (CI).
Additionally, concordance between parent- and self-reported cancer-related fatigue scores was determined with Pearson or Spearman correlation coefficients as appropriate. Correlations between 0.2 and 0.5 were considered small, 0.5–0.8 moderate, and ≥0.8 strong.
Associations between sleep–wake rhythm variables and cancer-related fatigue were evaluated with linear regression models. Models were adjusted for age, sex, and sleep medication use. The adjusted regression coefficient (B) with a 95% CI were presented to express the magnitude of the association.
IBM SPSS statistics version 22.0 was used for all analysis. A two-sided p-value of <0.05 was considered statistically significant.
Results
Study population
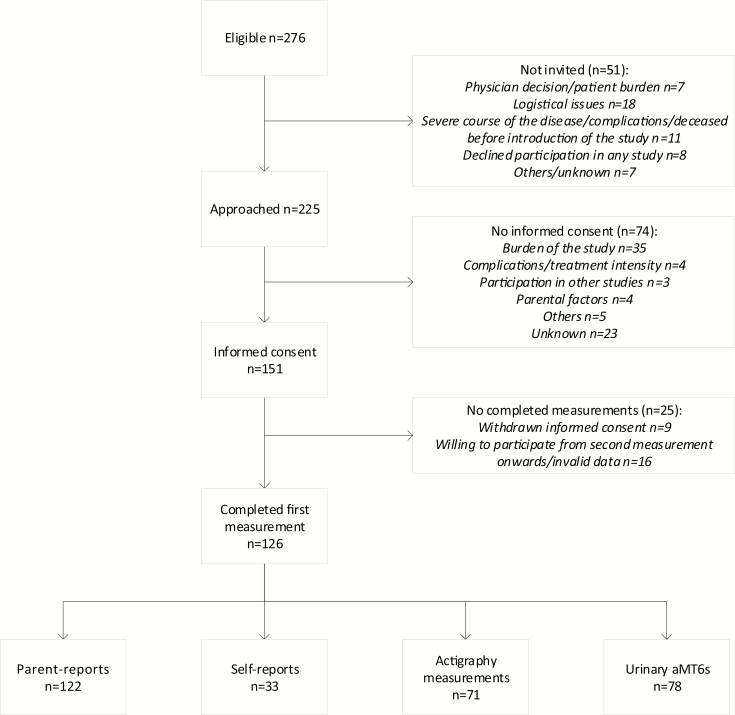
Of the 276 patients that were eligible for the study according to the DCOG registry, 225 were invited to participate (Figure 1). Fifty-one patients were not invited for the study, mainly because of a severe course of the disease or logistical reasons. Informed consent was provided for 151 patients (response rate 67%). The perceived burden of the study was the main reason for non-participation. At least one of the study assessments was completed by 126 children (parent-reports n = 122, self-reports n = 33, actigraphy n = 71, urine samples n = 78). Parent-reports on cancer-related fatigue, actigraphy assessment, and melatonin assessment were completed by 55 participants, of whom 20 participants also completed self-reports.
Figure 1.
Patient enrollment.
Baseline characteristics
Age and sex were not statistically different between participants and non-participants and those not invited for the study (Table 2).
Table 2.
Baseline characteristics
| Study participants N = 126 | Non-participants N = 99 | Not invited N = 51 | |
|---|---|---|---|
| Child- and treatment-related factors | |||
| Child age at diagnosis—Median [IQR] | 5.1 [3.1; 9.3] | 5.5 [3.5; 11.9] | 5.8 [3.8; 11.5] |
| Female child sex—N (%) | 51 (40.5) | 42 (42.1) | 23 (45.1) |
| Time since diagnosis in months—Median [IQR] | 4.5 [4.1; 5.1] | ||
| Preexisting sleep problems—N (%) | 19 (15.1) | ||
| Use of sleep medication—N (%) | 8 (6.3) | ||
| Comorbid chronic illness—N (%)a | 8 (6.3) | ||
| Sharing a bedroom—N (%) | 26 (20.6) | ||
| Parental- and family factors | |||
| Parental age | 37.0 [34.0; 43.0] | ||
| Female parental sex—N (%) | 98 (77.8) | ||
| Educational level—N (%) | |||
| Low | 5 (4.0) | ||
| Middle | 36 (28.6) | ||
| High | 82 (65.1) | ||
| Unknown | 3 (2.4) | ||
| Country of birth—N (%) | |||
| Netherlands | 116 (92.1) | ||
| Otherb | 7 (5.6) | ||
| Unknown | 3 (2.4) |
aDown syndrome (n = 3), autism (n = 1), hypermobility (n = 1), coeliac disease (n = 1), anorectal malformation (n = 1), and cavernomas in the brain (n = 1).
bBrazil (n = 1), Kenya (n = 1), Romania (n = 1), South Africa (n = 1), Surinam (n = 2), and Turkey (n = 1).
Sleep medication was used by 8 participants at time of the study: melatonin (n = 5), lorazepam (n = 1), unknown (n = 2). In 19 patients, pre-existent sleep problems were reported by parents and these consisted of problems with initiating and maintaining sleep (n = 15), somnambulating (n = 1), need of sibling in the room (n = 1), and two parents reported less need for sleep than other children.
The median age at diagnosis of patients that completed self-reports was 12.2 years (age range [min–max]: 7.5–17.9), 5.9 years (age range: 1.9–17.9) for those with valid sleep–wake rhythm data (actigraphy derived) and 6.1 years (age range: 2.3–17.9) for patients with valid urinary aMT6s levels. Somewhat more girls participated in the actigraphy assessments (46.5%) compared to the total population. Time since diagnosis was comparable to the total population in those who participated in self-reports, actigraphy, and urinary assessments.
Actigraphy derived sleep–wake outcomes
Patients with ALL had a significantly lower IS and RA compared to healthy children, indicating a less stable and less robust sleep–wake rhythm (Table 3). M10 counts were significantly lower in ALL patients. IV and M5 counts were not significantly different from healthy children.
Table 3.
Sleep–wake rhythm variables (actigraphy derived) in patients with ALL compared to healthy children.
| Patients with ALL (N = 71) mean ± SD or median [IQR] | Healthy children (N = 85) mean ± SD or median [IQR] | B(95% CI)a | p-value | |
|---|---|---|---|---|
| Interdaily stability | 0.58 ± 0.13 | 0.62 ± 0.13 | −0.07 (−0.10; −0.04) | <0.001 |
| Intradaily variability | 0.74 ± 0.24 | 0.72 ± 0.20 | 0.06 (−0.01; 0.12) | 0.070 |
| Relative amplitude | 0.92 ± 0.05 | 0.95 ± 0.03 | −0.03 (−0.04; −0.02) | <0.001 |
| L5 counts | 48.6 [36.4–72.8] | 46.3 [36.2–63.5] | 3.0 (−5.5; 11.5) | 0.485 |
| M10 counts | 1752.5 ± 758.3 | 2187.1 ± 709.3 | −599.5 (−784.2; −414.7) | <0.001 |
aAdjusted for patient age, sex, sleep medication use and highest attained parental educational level.
Urinary aMT6s values
In patients with ALL, the mean aMT6s value was 26.70 (SD: 20.64) µmol/mol creatinine, compared to 24.15 (SD: 19.69) µmol/mol creatinine in healthy children. Adjusted for age and sex, urinary aMT6s levels were not statistically different between patients and healthy children (B −2.12 (95% CI): (−7.35; 3.11) p-value: 0.425).
Cancer-related fatigue
Parent-reported general and sleep-rest fatigue scores were significantly lower (indicating more cancer-related fatigue) in patients with ALL compared to scores in healthy children, while scores on the cognitive fatigue scale were comparable (Table 4). Self-reported general fatigue scores were significantly lower in ALL patients, whereas sleep-rest and cognitive fatigue scores were not significantly different from scores in healthy children. In families where parent-proxy and child dyad reports were available, parent-proxies reported somewhat lower general fatigue scores (58.3 interquartile range [IQR]: 39.6–75.0) but similar sleep-rest (72.0 ± 15.5) and cognitive fatigue scores (72.9 [IQR: 62.5–95.8]) compared to child self-reports. Concordance between parent-proxy and child dyads was moderate (correlation coefficients 0.56–0.65, p < 0.01).
Table 4.
Cancer-related fatigue (PedsQL MFS scores) in patients with ALL compared to healthy children
| PedsQL MFS scale | Patients with ALL mean ± SD or median [IQR] | Healthy children [33] mean ± SD or median [IQR] | B (95% CI)a | p-value |
|---|---|---|---|---|
| Parent reports | N = 122 | N = 497 | ||
| General fatigue | 56.61 ± 25.51 | 83.33 [70.83–91.67] | −25.60 (−29.11; −22.10) | <0.001 |
| Sleep-rest fatigue | 70.83 [56.25–83.33] | 87.50 [75.00–95.83] | −16.11 (−19.40; −12.82) | <0.001 |
| Cognitive fatigue | 75.00 [66.67–95.83] | 79.17 [66.67–95.83] | −1.29 (−5.04; 2.47) | 0.502 |
| Self-reports | N = 33 | N = 298 | ||
| General fatigue | 70.83 [50.00–83.33] | 79.17 [70.83–91.67] | −12.86 (−18.47; −7.25) | <0.001 |
| Sleep-rest fatigue | 70.38 ± 15.99 | 74.60 ± 14.84 | −4.59 (−10.30; 1.28) | 0.115 |
| Cognitive fatigue | 75.00 [56.25–91.67] | 79.17 [66.67–91.67] | −5.64 (−12.52; 1.24) | 0.108 |
aAdjusted for patient age, sex, sleep medication use, and highest attained parental educational level.
Association between sleep–wake rhythms and cancer-related fatigue
A higher IS (i.e. more stable rhythm), a higher RA (i.e. more robust rhythm), and higher M10 counts (i.e. more physical activity during the day) were all significantly associated with higher parent-reported general and sleep-rest fatigue scores (indicating less fatigue) (Table 5). Higher IV (i.e. more fragmented rhythm) was significantly associated with lower parent-reported sleep-rest fatigue scores (indicating more fatigue). L5 counts were not significantly associated with parent-reported cancer-related fatigue. None of the sleep–wake rhythm variables were significantly associated with self-reported cancer-related fatigue.
Table 5.
Association between sleep–wake rhythm variables (actigraphy derived) and cancer-related fatigue (PedsQL MFS scores)
| B (95% CI)a | B (95% CI)a | B (95% CI)a | |
|---|---|---|---|
| Parent reports (n = 69) | General fatigue | Sleep-rest fatigue | Cognitive fatigue |
| Interdaily stability (0.1 change) | 5.53 (0.06; 10.99)* | 4.87 (0.90; 8.84)* | −1.21 (−5.44; 3.02) |
| Intradaily variability (0.1 change) | −2.02 (−4.92; 0.89) | −2.59 (−4.66; −0.52)* | −0.004 (−3.17; 3.16) |
| Relative amplitude (0.1 change) | 16.12 (2.97; 29.26)* | 14.06 (4.56; 23.55)* | −0.77 (−11.12; 9.59) |
| L5 counts (10 counts change) | 0.00 (−2.18; 2.19) | 0.10 (−1.51; 1.71) | −0.22 (−1.87; 1.42) |
| M10 counts (10 counts change) | 0.18 (0.08; 0.28)** | 0.16 (0.09; 0.23)** | 0.02 (−0.06; 0.10) |
| Self-reports (n = 24) | General fatigue | Sleep-rest fatigue | Cognitive fatigue |
| Interdaily stability (0.1 change) | −0.94 (−8.90; 7.03) | 3.46 (−3.94; 10.87) | −5.76 (−14.58; 3.06) |
| Intradaily variability (0.1 change) | −1.60 (−4.63; 1.43) | −2.42 (−5.15; 0.31) | 0.81 (−2.81; 4.42) |
| Relative amplitude (0.1 change) | 8.32 (−10.76; 27.40) | 9.38 (−8.62; 27.37) | 0.34 (−22.32; 23.01) |
| L5 counts (10 counts change) | 0.82 (−3.03; 4.68) | 2.25 (−1.27; 5.77) | −0.57 (−5.06; 3.93) |
| M10 counts (10 counts change) | 0.07 (−0.10; 0.24) | 0.13 (−0.02; 0.29) | −0.12 (−0.32; 0.07) |
aAdjusted for patient age, sex, sleep medication use and highest attained parental educational level.
*p-value < 0.05; **p-value < 0.001.
Discussion
This study provides unique information on sleep–wake rhythms, melatonin levels, cancer-related fatigue and the association between sleep–wake rhythm variables and cancer-related fatigue in a large sample of pediatric patients with ALL, after the first, most intensive phase of therapy, when a first recovery of the disease and treatment can be expected.
The stability and robustness of the sleep–wake cycle were reduced in pediatric patients with ALL compared to healthy children. Furthermore, they were less active during the day. There may be several reasons for the reduced stability and robustness of the rhythm. First, the activity levels may be low overall after the intensive first part of therapy, when most patients are not yet able to participate in social activities, sports activities, and to attend school. Second, as mentioned previously, external stimuli are needed to synchronize the biological clock to the light-dark cycle and in pediatric patients treated for ALL some of these stimuli could be disrupted [3]. For example, parents of ALL patients may be more lenient with limit setting and the reinforcement of rules with regards to sleep hygiene [37, 38].
Daytime napping fragments the sleep–wake rhythm and has been described in pediatric patients with ALL [23, 24]. However, fragmentation was not found in our study. The low physical activity levels in our sample could have led to less fragmentation of the rhythm. Furthermore, assessments were planned in home setting in a phase with less treatment-related toxicity, enabling a first recovery, which might have reduced the fragmentation of the rhythm. Moreover, patients in the previous studies that reported fragmentation were on maintenance treatment and received dexamethasone, whereas assessments in the current study took place in a period without glucocorticoids [23, 24].
Melatonin levels were comparable to healthy children. Shifted light–dark exposure affects melatonin production [21]. Disturbed melatonin production and release as a result of the reduced sleep–wake rhythm stability in our study population could have been expected, but was not found. However, although morning aMT6s highly correlate with nocturnal peak melatonin levels, DLMO cannot be assessed with a single morning urine collection. The DLMO may therefore still be different in pediatric patients with ALL compared to healthy children. Melatonin was used in 4% (5/125) of the patients in the current study. Melatonin is available over the counter in the Netherlands and clinicians are therefore not always aware of the use of melatonin. However, as melatonin is only indicated in patients with circadian rhythm disorders, it is not only important that clinicians are aware of melatonin supplementations but also that they should be cautious in prescribing melatonin. Although melatonin may seem relatively safe, possible late effects of long-term use during early development are insufficiently known. Moreover, incorrect dosing and timing of exogenous melatonin can affect the biological clock and cause adverse circadian phase shifting [39]. Prescription of melatonin by a healthcare provider that is familiar with melatonin and circadian rhythm disorders is, therefore, advised [39]. Furthermore, non-medicated options (such as timed bright light and dim light manipulations) should also be considered to support endogenous melatonin release in individual cases where the circadian rhythm is disturbed [39].
In accordance with literature, more cancer-related fatigue was reported in this ALL population, with the exception of cognitive cancer-related fatigue [14, 16]. The absence of cognitive cancer-related fatigue, which is in line with a previous study in adolescents with cancer, is possibly due to the lower cognitive demands and expectations in this early phase of therapy [16]. Similar as in pediatric patients with ALL during maintenance treatment, a more impaired sleep–wake rhythm was associated with higher levels of parent-reported cancer-related fatigue [12]. Furthermore, we confirmed a previously reported association between increased cancer-related fatigue and lower physical activity levels [40, 41].
The association with sleep–wake outcomes was not found for self-reported cancer-related fatigue. The small number of available self-reports could have limited the sensitivity to find a significant association. Moreover, differences in patient- and parent-reported outcomes are common [42–44]. Coping mechanisms differ for patients and parents. Patients may change their judgment of symptoms during the course of a cancer treatment, described as “response shift,” while parents may overreport symptoms due to concerns about their child’s health [37, 42, 45].
Given the many adverse health outcomes and even the worse cancer-related outcomes that have been associated with impaired sleep–wake rhythms in studies on adults, efforts to improve sleep–wake rhythms in pediatric cancer patients are important [3, 10, 11]. More stringent bedtime routines and participation in appropriate levels of physical activity as soon as possible might enhance the robustness of the sleep–wake rhythm. Clinicians should, therefore, be aware of healthy sleep behaviors and pay attention to sleep hygiene and physical activity in this population. Interventions combining sleep hygiene education and physical activity may enhance the sleep–wake rhythm and have already proven feasibility and acceptability in pediatric patients with ALL [46, 47]. Furthermore, the association between physical activity and cancer-related fatigue implicates that such interventions may provide opportunities to improve cancer-related fatigue in this population.
This study provides valuable additional information to the existing literature, as it is the first study in pediatric patients with ALL to examine sleep–wake rhythm variables using nonparametric methods and to determine melatonin levels. However, some limitations of the study needs to be mentioned. First, not all patients participated in all study assessments (questionnaires, actigraphy, and melatonin assessment). Hence, participation bias, for example, based on treatment toxicity, cannot completely be ruled out. The study may, therefore, have underestimated sleep–wake rhythm disturbances and cancer-related fatigue. Second, because of the small sample of available self-reports these results should be interpreted with caution. Third, families with a higher level of education were overrepresented in our sample compared to the general Dutch population [25]. Since a lower socio-economic status has been associated with less healthy sleep behaviors, our study may have underestimated the prevalence and severity of disturbed sleep–wake rhythms [48]. Finally, nocturnal enuresis was not registered. Obtaining first-morning aMT6s levels, we possibly have not captured the total melatonin secretion in children with nocturnal enuresis. With this method, we might, therefore, have underestimated aMT6s levels. However, Wada et al. [49] reported similar associations between aMT6s levels and socio-demographics in their total sample as compared to the subgroup of children that probably did not urinate during the night.
In conclusion, pediatric patients with ALL have a less stable and less robust sleep–wake rhythm, are less physically active and experience more cancer-related fatigue after the first, most intensive phase of therapy. Sleep–wake rhythm impairment is associated with increased levels of cancer-related fatigue in these patients. Clinicians should, therefore, pay attention to sleep hygiene and stimulate physical activity guided by the physical condition of patients. Longitudinal studies are important to unravel the development of the association between sleep–wake rhythms and cancer-related fatigue during and after treatment. Furthermore, interventions aiming at improving sleep hygiene and encouraging physical activity as early as possible are needed, to eventually reduce cancer-related fatigue, a common and distressing symptom in pediatric oncology patients.
Funding
The research described in this article is supported by the Dutch Cancer Society (VU 2014-6703).
Supplementary Material
Acknowledgments
We thank the research nurses of the participating medical centers for the inclusion and follow-up of patients. We also thank the Department of Laboratory Medicine of the University Medical Center Groningen for performing the aMT6s analyses.
Conflict of interest statement. None declared.
References
- 1. Hofstra WA, et al. . How to assess circadian rhythm in humans: a review of literature. Epilepsy Behav. 2008;13(3):438–444. [DOI] [PubMed] [Google Scholar]
- 2. Duffy JF, et al. . Entrainment of the human circadian system by light. J Biol Rhythms. 2005;20(4):326–338. [DOI] [PubMed] [Google Scholar]
- 3. Baron KG, et al. . Circadian misalignment and health. Int Rev Psychiatry. 2014;26(2):139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scheer FA, et al. . Environmental light and suprachiasmatic nucleus interact in the regulation of body temperature. Neuroscience. 2005;132(2):465–477. [DOI] [PubMed] [Google Scholar]
- 5. Luik AI, et al. . Stability and fragmentation of the activity rhythm across the sleep-wake cycle: the importance of age, lifestyle, and mental health. Chronobiol Int. 2013;30(10):1223–1230. [DOI] [PubMed] [Google Scholar]
- 6. Luik AI, et al. . Associations of the 24-h activity rhythm and sleep with cognition: a population-based study of middle-aged and elderly persons. Sleep Med. 2015;16(7):850–855. [DOI] [PubMed] [Google Scholar]
- 7. Eismann EA, et al. . Circadian effects in cancer-relevant psychoneuroendocrine and immune pathways. Psychoneuroendocrinology. 2010;35(7):963–976. [DOI] [PubMed] [Google Scholar]
- 8. Pedersen M, et al. . Sleep-wake rhythm disturbances and perceived sleep in adolescent chronic fatigue syndrome. J Sleep Res. 2017;26(5):595–601. [DOI] [PubMed] [Google Scholar]
- 9. Berger AM, et al. . Patterns of circadian activity rhythms and their relationships with fatigue and anxiety/depression in women treated with breast cancer adjuvant chemotherapy. Support Care Cancer. 2010;18(1):105–114. [DOI] [PubMed] [Google Scholar]
- 10. Mormont MC, et al. . Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clin Cancer Res. 2000;6(8):3038–3045. [PubMed] [Google Scholar]
- 11. Savard J, et al. . Breast cancer patients have progressively impaired sleep-wake activity rhythms during chemotherapy. Sleep. 2009;32(9):1155–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rogers VE, et al. . Impairment in circadian activity rhythms occurs during dexamethasone therapy in children with leukemia. Pediatr Blood Cancer. 2014;61(11):1986–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rogers VE, et al. . Relationship between circadian activity rhythms and fatigue in hospitalized children with CNS cancers receiving high-dose chemotherapy. Support Care Cancer. 2019. doi:10.1007/s00520-019-04960-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Darezzo Rodrigues Nunes M, et al. . Fatigue and sleep experiences at home in children and adolescents with cancer. Oncol Nurs Forum. 2015;42(5):498–506. [DOI] [PubMed] [Google Scholar]
- 15. Barsevick AM, et al. ; National Cancer Institute Clinical Trials Planning Meeting. Recommendations for high-priority research on cancer-related fatigue in children and adults. J Natl Cancer Inst. 2013;105(19):1432–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Daniel LC, et al. . Fatigue in adolescents with cancer compared to healthy adolescents. Pediatr Blood Cancer. 2013;60(11):1902–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nap-van der Vlist MM, et al. . Fatigue in childhood chronic disease. Arch Dis Child. 2019. doi:10.1136/archdischild-2019-316782. [DOI] [PubMed] [Google Scholar]
- 18. Knight SJ, et al. . School functioning in adolescents with chronic fatigue syndrome. Front Pediatr. 2018;6:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salter A, et al. . The association of fatigue and social participation in multiple sclerosis as assessed using two different instruments. Mult Scler Relat Disord. 2019;31:165–172. [DOI] [PubMed] [Google Scholar]
- 20. Ancoli-Israel S, et al. . The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. [DOI] [PubMed] [Google Scholar]
- 21. Mirick DK, et al. . Melatonin as a biomarker of circadian dysregulation. Cancer Epidemiol Biomarkers Prev. 2008;17(12):3306–3313. [DOI] [PubMed] [Google Scholar]
- 22. Daniel LC, et al. . A call to action for expanded sleep research in pediatric oncology: a position paper on behalf of the International Psycho-Oncology Society Pediatrics Special Interest Group. Psychooncology. 2019 104:1090–1095. doi:10.1002/pon.5242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosen G, et al. . The effects of dexamethasone on sleep in young children with acute lymphoblastic leukemia. Sleep Med. 2015;16(4):503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hinds PS, et al. . Dexamethasone alters sleep and fatigue in pediatric patients with acute lymphoblastic leukemia. Cancer. 2007;110(10):2321–2330. [DOI] [PubMed] [Google Scholar]
- 25. Standaard Onderwijsindeling 2016 [Standard Educational Classification]. Den Haag/Heerlen, Netherlands: Centraal Bureau voor de Statistiek [Statistics Netherlands]; 2016. [Google Scholar]
- 26. Sadeh A, et al. . The role of actigraphy in sleep medicine. Sleep Med Rev. 2002;6(2):113–124. [DOI] [PubMed] [Google Scholar]
- 27. Sadeh A, et al. . The role of actigraphy in the evaluation of sleep disorders. Sleep. 1995;18(4):288–302. [DOI] [PubMed] [Google Scholar]
- 28. Mitchell JA, et al. . Variation in actigraphy-estimated rest-activity patterns by demographic factors. Chronobiol Int. 2017;34(8):1042–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Van Someren EJ, et al. . Bright light therapy: improved sensitivity to its effects on rest-activity rhythms in Alzheimer patients by application of nonparametric methods. Chronobiol Int. 1999;16(4):505–518. [DOI] [PubMed] [Google Scholar]
- 30. Mahlberg R, et al. . Normative data on the daily profile of urinary 6-sulfatoxymelatonin in healthy subjects between the ages of 20 and 84. Psychoneuroendocrinology. 2006;31(5):634–641. [DOI] [PubMed] [Google Scholar]
- 31. Mandrell BN, et al. . In-home salivary melatonin collection: methodology for children and adolescents. Dev Psychobiol. 2018;60(1):118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cook MR, et al. . Morning urinary assessment of nocturnal melatonin secretion in older women. J Pineal Res. 2000;28(1):41–47. [DOI] [PubMed] [Google Scholar]
- 33. Graham C, et al. . Prediction of nocturnal plasma melatonin from morning urinary measures. J Pineal Res. 1998;24(4):230–238. [DOI] [PubMed] [Google Scholar]
- 34. Bojkowski CJ, et al. . Melatonin secretion in humans assessed by measuring its metabolite, 6-sulfatoxymelatonin. Clin Chem. 1987;33(8):1343–1348. [PubMed] [Google Scholar]
- 35. Gordijn M, et al. . Fatigue in children: reliability and validity of the Dutch PedsQL™ Multidimensional Fatigue Scale. Qual Life Res. 2011;20(7):1103–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Varni JW, et al. . The PedsQL in pediatric cancer: reliability and validity of the pediatric quality of life inventory generic core scales, multidimensional fatigue scale, and cancer module. Cancer. 2002;94(7):2090–2106. [DOI] [PubMed] [Google Scholar]
- 37. Long KA, et al. . Child-rearing in the context of childhood cancer: perspectives of parents and professionals. Pediatr Blood Cancer. 2014;61(2):326–332. [DOI] [PubMed] [Google Scholar]
- 38. McCarthy MC, et al. . Are parenting behaviors associated with child sleep problems during treatment for acute lymphoblastic leukemia? Cancer Med. 2016;5(7):1473–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Keijzer H, et al. . Why the dim light melatonin onset (DLMO) should be measured before treatment of patients with circadian rhythm sleep disorders. Sleep Med Rev. 2014;18(4):333–339. [DOI] [PubMed] [Google Scholar]
- 40. Hooke MC, et al. . Use of a fitness tracker to promote physical activity in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2016; 63(4): 684–689. [DOI] [PubMed] [Google Scholar]
- 41. Van Dijk-Lokkart EM, et al. . Longitudinal development of cancer-related fatigue and physical activity in childhood cancer patients. Pediatr Blood Cancer. 2019;66(12):e27949. [DOI] [PubMed] [Google Scholar]
- 42. Gordijn MS, et al. . Sleep, fatigue, depression, and quality of life in survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60(3):479–485. [DOI] [PubMed] [Google Scholar]
- 43. Parsons SK, et al. . Comparing longitudinal assessments of quality of life by patient and parent in newly diagnosed children with cancer: the value of both raters’ perspectives. Qual Life Res. 2012;21(5):915–923. [DOI] [PubMed] [Google Scholar]
- 44. Upton P, et al. . Parent-child agreement across child health-related quality of life instruments: a review of the literature. Qual Life Res. 2008;17(6):895–913. [DOI] [PubMed] [Google Scholar]
- 45. Visser MR, et al. . How response shift may affect the measurement of change in fatigue. J Pain Symptom Manage. 2000;20(1):12–18. [DOI] [PubMed] [Google Scholar]
- 46. Zupanec S, et al. . A sleep hygiene and relaxation intervention for children with acute lymphoblastic leukemia: a pilot randomized controlled trial. Cancer Nurs. 2017;40(6):488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Moyer-Mileur LJ, et al. . Fitness of children with standard-risk acute lymphoblastic leukemia during maintenance therapy: response to a home-based exercise and nutrition program. J Pediatr Hematol Oncol. 2009;31(4):259–266. [DOI] [PubMed] [Google Scholar]
- 48. Acebo C, et al. . Sleep/wake patterns derived from activity monitoring and maternal report for healthy 1- to 5-year-old children. Sleep. 2005;28(12):1568–1577. [DOI] [PubMed] [Google Scholar]
- 49. Wada K, et al. . Associations of urinary 6-sulfatoxymelatonin with demographics, body mass, sex steroids, and lifestyle factors in preschool Japanese children. Ann Epidemiol. 2013;23(2):60–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.