Abstract
Study Objectives
To investigate the pre-sleep psychophysiological state and the arousal deactivation process across the sleep onset (SO) transition in adolescents.
Methods
Data were collected from a laboratory overnight recording in 102 healthy adolescents (48 girls, 12–20 years old). Measures included pre-sleep self-reported cognitive/somatic arousal, and cortical electroencephalographic (EEG) and electrocardiographic activity across the SO transition.
Results
Adolescent girls, compared with boys, reported higher pre-sleep cognitive activation (p = 0.025) and took longer to fall asleep (p < 0.05), as defined with polysomnography. Girls also showed a less smooth progression from wake-to-sleep compared with boys (p = 0.022). In both sexes, heart rate (HR) dropped at a rate of ~0.52 beats per minute in the 5 minutes preceding SO, and continued to drop, at a slower rate, during the 5 minutes following SO (p < 0.05). Older girls had a higher HR overall in the pre-sleep period and across SO, compared to younger girls and boys (p < 0.05). The EEG showed a progressive cortical synchronization, with increases in Delta relative power and reductions in Alpha, Sigma, Beta1, and Beta2 relative powers (p < 0.05) in the approach to sleep, in both sexes. Delta relative power was lower and Theta, Alpha, and Sigma relative powers were higher in older compared to younger adolescents at bedtime and across SO (p < 0.05).
Conclusions
Our findings show the dynamics of the cortical-cardiac de-arousing process across the SO transition in a non-clinical sample of healthy adolescents. Findings suggest a female-specific vulnerability to inefficient sleep initiation, which may contribute to their greater risk for developing insomnia.
Keywords: falling asleep, adolescence, arousal, heart rate, EEG, sex differences
Statement of Significance.
Sleep difficulties frequently emerge in adolescence on the backdrop of profound sleep maturation and biopsychosocial change, with older girls being particularly vulnerable to the development of insomnia symptoms. It is unknown whether the process of falling asleep, which involves the reorganization of multiple biological domains across the wake-to-sleep transition, differs across adolescence and in girls compared with boys. Our findings highlight the dynamics of the falling asleep process in healthy adolescents and suggest a female-specific vulnerability to inefficient sleep initiation, which may contribute to a higher risk for developing sleep disturbances in girls during adolescence as well as later in life.
Introduction
Falling asleep is a complex and frequently overlooked process in which a cascade of psychophysiological changes characterize the transition from wakefulness to sleep [1]. It is characterized by a progressive reduction in responsiveness to external stimuli and an increase in behavioral quiescence. The processing of sensory inputs changes together with the level and content of consciousness. When falling asleep, slow eye movements initially occur (reflecting drowsiness) and usually disappear at the first manifestation of specific cortical electroencephalographic (EEG) markers of consolidated sleep (e.g. spindles and K-complexes) [1]. Cortical activity shows a progressive spatiotemporal re-organization toward an overall decrease in complexity and increase in high-voltage synchronized EEG activity [1, 2]. Heart rate (HR) progressively drops, and the cardiac autonomic nervous system (ANS) modulation moves towards vagal dominance [3]. A cascade of other physiological changes like a drop in core body temperature, an increase in distal temperature, and a reduction in respiration rate also occur across the sleep onset (SO) transition, each with their own specific temporal dynamics [1].
Limited empirical evidence exists in explaining the fundamental process of SO, and its role in health and pathology. Many studies have focused on insomnia, in which difficulty falling asleep is a major diagnostic criterion and frequently a cardinal symptom of the disorder. Within this framework, abnormally up-regulated levels of cortical, somatic and cognitive activity (hyperarousal) play a key role in the pathophysiology of the disorder [4]. Hyperarousal is magnified under insomnia-specific circumstances such as when an individual is trying to sleep and interferes with the falling asleep and sleep maintenance processes. Insomnia sufferers exhibit high levels of pre-sleep anxiety, worry, intrusive thoughts [5], accompanied by evidence of central and autonomic nervous system hyperarousal around SO. Hyperarousal may manifest as elevated EEG Beta activity [6] and elevated HR and cardiac sympathetic ANS activity [7]. There may also be specific abnormalities in the normal falling asleep de-arousing processes. These include smaller declines in Alpha and Beta powers in the approach to SO [8], a slower increase in cortical synchronization [9] and the absence of a drop in cardiac ANS sympathetic activity [7] across the SO transition, in insomnia sufferers compared to controls.
There is a paucity of data exploring the falling asleep process in adolescents despite evidence that falling asleep difficulties are common during adolescence [10], and with historical trends showing that the prevalence of SO difficulties in adolescents has increased over time, being about 8% more common in 2005 than in 1983 [11]. Longer sleep onset latency (SOL) contributes to adolescents’ perceived sleep problems [12] and clinically, difficulty falling asleep is a cardinal symptom of insomnia disorder in adolescents [13]. Difficulty falling asleep is also evident in several other common conditions during adolescence, such as major depressive disorder [14]. Altered pre-sleep somatic arousal and cognitive processes such as catastrophizing, elevated worry, anxiety, and rumination are critical, potentially interfering with the processes underlying falling asleep and maintaining sleep in adolescence [15–17]. In the context of insomnia development, Fernandez-Mendoza and colleagues [18] found that adolescents complaining of insomnia symptoms such as self-reported difficulty in falling and staying asleep, had greater EEG beta power than controls before and during the first 5 minutes of NREM sleep after SO (first epoch of N2 sleep after lights-out). This greater cortical activation at SO was then maintained during NREM sleep across the night. In a second study [19], they found that greater cortical hyperarousal (high-NREM EEG beta power) in childhood (6–11 years) was associated with greater incidence of insomnia symptoms 8 years later (13–20 years). These results suggest that hyperarousal may be present even years before the manifestation of insomnia.
Findings in adolescents need to be contextualized within the profound normal developmental changes that occur during adolescence. Adolescence is accompanied by a dramatic age-related drop in slow-wave sleep and its activity (SWA; 0.3–4 Hz), reflecting changes in brain reorganization (e.g. synaptic pruning) [20, 21]. Changes in circadian and homeostatic biological maturation processes that delay SO times, coupled with social constraints such as early school start times, lead to changes in adolescents’ sleep timing and duration. Also, adolescent-specific stressors (e.g. academic pressure, peer-stress) and maladaptive behaviors (e.g. bedtime technology use, excessive caffeine consumption) may also interfere with the adolescents’ sleep pattern, and particularly with the falling asleep process, delaying the onset of sleep and contributing to poor sleep [13].
The incidence of insomnia disorder changes across adolescence, being higher in post-pubertal girls compared to pre-pubertal girls and compared with boys [13, 22]. This female-prevalence for insomnia is then maintained at all ages [23], although PSG measures tend to show that females have more N3 (or slow-wave sleep) and less light N1 sleep than males [24]. One possible reason for the sex difference in insomnia that has not received much attention is that the falling asleep process could differ in girls, making them vulnerable to insomnia. Longer self-reported SOL in girls than boys has been reported [25]; however, a detailed investigation of sex differences in the falling asleep process in adolescents is lacking.
The purpose of the current study is to investigate the pre-sleep psychophysiological state and the physiology of the falling asleep de-arousing process in a non-clinical population of healthy male and female adolescents. Data were analyzed as a function of age and sex, using multidimensional data reflecting cognitive, cortical EEG, and cardiac ANS functioning. We hypothesized that girls would show greater difficulties in the arousal deactivation process across the SO transition than boys, potentially reflecting a female-specific “vulnerability” to sleep initiation problems and that the sex difference would be particularly evident in older adolescents.
Method
Participants
Healthy adolescents (n = 102, 48 girls, age range: 12–20 years old) who were in the SRI International baseline cohort of the longitudinal National Consortium on Alcohol and Neurodevelopment in Adolescence (NCANDA) study constituted the final sample. The study was approved by the SRI International Institutional Review Board. All adult participants consented to participate, with minors providing written assent along with consent from a parent/legal guardian. Details about the NCANDA methodology and sample characteristics [26], nocturnal PSG sleep [27], and ANS functioning during sleep [28] are described elsewhere. Sample characteristics are from data release version NCANDA_DATA_00010_V2.
A phone interview and a subsequent in-lab screening session were used to determine eligibility. Participants were free from severe current/past major DSM-IV disorders and medical conditions and were not currently using medications known to affect the central nervous system and/or the cardiovascular system. None of the participants had major sleep disorders as confirmed by in-lab clinical polysomnography (PSG).
Sample demographics are provided in Table 1. Ethnicity was self-identified. Pubertal status was determined using the Pubertal Development Scale (PDS) [29]. Self-reported sleep quality and depression symptoms were assessed with the Pittsburgh Sleep Quality Index (PSQI) [30], and the Center for Epidemiologic Studies Depression Scale (CES-D) [31], respectively. Height and weight were objectively measured and body mass index (BMI; kg × m-2) calculated. The number of adolescents drinking at levels above age-matched national norms is reported (see [26], for details).
Table 1.
Sample demographics
| Boys Mean (SD) | Girls Mean (SD) | |
|---|---|---|
| Sample, No. | 54 | 48 |
| Ethnicity | ||
| Caucasian, No. | 42 | 36 |
| Asian, No. | 9 | 9 |
| African-American, No. | 1 | 3 |
| Other/undeclared, No. | 2 | 0 |
| Age, years | 15.2 (2.0) | 15.4 (2.1) |
| PDS, scorea | 2.8 (0.7) | 3.3 (0.7) |
| BMI, kg × m−2 | 21.5 (4.2) | 21.1 (4.3) |
| PSQI, total scoreb | 3.8 (2.3) | 4.1 (1.9) |
| CES-D, total scorec | 5.2 (3.9) | 6.8 (6.8) |
| Exceeding drinking criteria, No. | 8 | 10 |
Ethnicity was self-identified. The number of adolescents drinking at levels above age-matched national norms is reported (see [26], for details). Data are provided as numbers (No.), percentage, or mean and SD when appropriate.
aData unavailable for three girls and one boy.
bData unavailable for two girls and one boy.
cData unavailable for eight girls and seven boys. Pubertal Development Scale (PDS); Pittsburgh Sleep Quality Index (PSQI); Center for Epidemiologic Studies Depression Scale (CES-D); Body Mass Index (BMI).
Procedures
Participants had a clinical/adaptation PSG night followed by a nonconsecutive standard PSG overnight recording (except for three participants who had consecutive nights due to schedule constraints). The standard PSG from which data were analyzed was, on average, 23 ± 19 days after the adaptation night, with four participants scheduled more than 2 months later (maximum, 118 days). All recordings were made in sound-attenuated and temperature-controlled bedrooms at the Human Sleep Research Program at SRI International.
Participants were instructed to avoid consuming beverages containing alcohol and/or caffeine after 3:00 p.m. on the day of the recording night. In the evening, once they arrived in the lab, participants had a breath alcohol test (S75 Pro, BACtrack Breathalyzers) and urine drug test (10 Panel iCup drug test, Instant Technologies, Inc.) to confirm the absence of recent alcohol or drug use. After the recording sensors were attached, they were allowed to engage in quiet activities (e.g. watching TV, reading) within the laboratory main areas. When ready to sleep, they went to their bedrooms and room lights were dimmed for the resting state recording. Right before bedtime (i.e. before lights-out) they had a 5-minute resting-state recording of EEG and electrocardiographic (ECG) activity while lying in bed in a supine position, awake and with their eyes closed (resting state), followed by a brief self-assessment of sleepiness (evaluated using a 0–100 mm visual analog scale [VAS], with higher scores indicating higher sleepiness), and cognitive and somatic arousal with the Pre-sleep Arousal Scale (PSAS) [32]. The PSAS evaluates the intensity of an individual’s symptoms (e.g. worry, depressive or anxious thoughts, muscle tension) when attempting to fall asleep. Greater scores indicate greater perceived arousal. The scale has 16 items with a 5-point Likert scale scoring ranging from “not at all” to “extremely.” A total score was calculated for the cognitive and somatic sub-scales (eight items each).
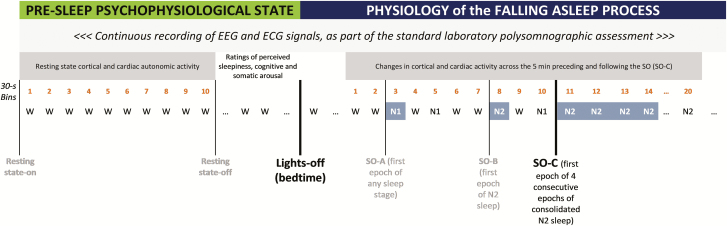
Lights were then turned out and participants were allowed to sleep. EEG and ECG signals were continuously recorded as part of the standard PSG assessment across the whole night (Figure 1).
Figure 1.
Timeline for the assessment and evaluation of (1) the resting state before lights-out (bin 1-to-10 of the 5-minute wake eyes-closed period) and pre-sleep perceived sleepiness, cognitive and somatic arousal, and of (2) the changes in EEG and ECG activity across SO. The physiology of the falling asleep process (EEG power spectral analysis and ECG heart rate) is analyzed across the 5 minutes preceding (bin 1-to-10) and following (bin 11-to-20) the first epoch of four consecutive epochs of stable sleep (N2/N3) (SO-C) after bedtime, which we consider reflecting “how” an individual first approaches consolidated sleep. Each bin represents 30 seconds of data.
Participants were instructed not to use their phones or any electronic devices in bed; they were specifically told to turn devices off or to activate the silent mode, and to remove any alarms. All participants complied with the instructions. Before turning out the lights, participants did not receive any specific instructions. Participants self-selected their bedtimes and wake-up times. Girls who were post-menarche were studied irrespective of their menstrual cycle phase. However, as previously reported in the whole NCANDA sleep project baseline cohort, only five girls had detectable progesterone levels in saliva consistent with the luteal phase of their menstrual cycle [28].
Assessment of cortical and cardiac ANS activity before and across the falling asleep period
PSG was performed according to the American Academy of Sleep Medicine (AASM) guidelines [33], and included the recommended EEG derivations (F3, F4, C3, C4, O1, O2 referenced to the contralateral mastoid; 256 Hz sampled), ECG (512 Hz sampled), bipolar electrooculography, and submental electromyography (see ref. [27], for details about the NCANDA PSG data collection and outcomes). PSG signals were recorded during the resting state period and across the whole night. All signals were recorded using Compumedics Grael HD-PSG systems (Compumedics, Abbotsford, Victoria, Australia). PSG records were scored (wake, N1, N2, N3, rapid-eye-movement [REM] sleep) in 30-second epochs by experienced scorers following the AASM rules. Standard overnight PSG sleep parameters were calculated (see ref. [27], for details).
Spectral EEG analysis was performed on frontal EEG derivations (F3 and F4) based on evidence that anterior areas show earlier synchronization at SO [2], using EEGLAB toolbox [34] for MATLAB (MathWorks, Natick, MA), as previously described [27, 35]. EEG was re-referenced to the averaged mastoids and filtered at 0.3–36 Hz with half-amplitude cutoffs at 0.15 and 36.15 Hz. Fast Fourier Transform analysis was conducted on 2-second epochs, to facilitate artifact identification and removal (see below), using a sliding Hanning window to calculate power density values with 1 Hz resolution. Power density (µV2/Hz) values were then averaged across Delta (1 Hz to ≤4 Hz), Theta (>4 Hz to ≤8 Hz), Alpha (>8 Hz to ≤12 Hz), Sigma (>12 Hz to ≤15 Hz), Beta1 (>15 Hz to ≤23 Hz), and Beta2 (>23 Hz to ≤30 Hz) frequencies, and EEG relative power (expressed as ratios, ranging from 0 to 1) calculated for each of the frequency bands as a function of the EEG total power (1 Hz to ≤30 Hz), and then averaged between F3 and F4.
To minimize the potential impact of eye-movements and other artifacts (e.g. muscle artifacts), artifact rejection algorithms were implemented. For each 30-second window (equivalent to the 30-second PSG epochs), power density values (calculated at 2-second epochs) were assessed for artifact: in the time domain, if the maximum value of any epoch exceeded the 30-second window median by 10 times the median absolute deviation, that epoch was removed. In the frequency domain, for each band, if the power of any epoch exceeded the 30-second window median by 10 times the median absolute deviation, that epoch was also removed. Further, epochs containing scored arousals were removed from the analysis. All remaining non-artifact epochs were then averaged to create a 30-second power-density average that aligned with the PSG epochs. The proportion of rejected 2-second bins within each 30-second epoch was 37% ± 14% of the pre-SO period and 13% ± 11% of the post-SO period. This difference is attributed to greater variability in the individuals’ behavioral pattern in the pre-sleep phase compared to post-stable SO and has been shown elsewhere (in a similar analysis of the EEG changes across the SO transition [2]: the proportion of data rejected in the 5-minute preceding SO was 41% ± 16%, while 21% ± 16% of data were rejected post SO).
The ECG signal was digitally filtered with a fourth-order Butterworth bandpass filter (upper cutoff: 0.5 Hz; lower cutoff: 35 Hz). Customized algorithms were used to automatically detect R peaks, derive normal beat-to-beat HR, and compute standard heart rate variability (HRV) time-domain indices (SD of normal to normal R-R intervals [SDNN, ms], and root mean square of the successive differences in normal to normal R-R intervals [RMSSD, ms]), according to [36]. ECG signal samples with a value more than 10 SDs away from the mean were identified as outliers and were replaced by interpolating the remainder of ECG samples. Average R‐R interval was then estimated using power spectral analysis. An automatic peak detection algorithm available in MATLAB R2018a software (MathWorks, Inc., Natick, MA) was adopted to detect the ECG R peaks using half of the average R‐R interval as the minimum distance criterion between two successive peaks. To intensify the R peaks and minimize the effect of T waves with amplitudes higher than normal, the automatic peak detection algorithm was applied on the ECG signal derivative. R‐R intervals with a value more than 10 SDs away from their mean were identified as outliers [37]. Those cardiac cycles that happened to have an out‐of‐range ECG signal level or R‐R value were identified as invalid beats (corrupted by noise and artifacts or ectopic beats) and were replaced by interpolating the remainder of HR data.
Beat-to-beat HR was averaged in 30-second epochs, to match the PSG epochs, and was analyzed across the resting state window and SO, while HRV analysis was performed on the 5-minute resting state window only (Figure 1). All the algorithms were developed in MATLAB R2018a (MathWorks, Inc., Natick, MA).
PSG SO characterization
There is no scientific consensus on the precise moment when we fall asleep, which is considered more a process rather than a moment in time, and multiple definitions of the process have been previously used [1]. We characterized SO using multiple definitions based on 30-second PSG staging across the wake-to-sleep transition (Figure 1): SO-A: SO defined as the occurrence of the first PSG epoch of any sleep stage (usually N1 sleep) after bedtime (standard AASM definition [33]); SO-B: SO defined as the occurrence of the first epoch of N2 sleep after bedtime, which has been previously used in analyses focused on physiological changes occurring across the SO transition [2, 38]; SO-C: SO defined as the occurrence of the first epoch of four consecutive epochs of stable sleep (N2/N3) after bedtime, which we consider a more conservative definition reflecting “how an individual approaches consolidated sleep.”
To analyze the cortical and cardiac changes occurring across the SO transition, we considered SO-C as the reference point to align the data. Differences between SO-B and SO-C were minimal, with SO-B and SO-C being coincident in 82% of the sample, with a difference of 0.88 ± 2.7 minutes (mean ± SD).
Statistical analyses
PSAS-cognitive, PSAS-somatic, SOL-A, SOL-B, SOL-C, SDNN, and RMSSD were log-transformed to improve normality. Sex was originally coded as 0 for boys and 1 for girls, and then centered at the proportion of girls in the sample (0.471). Age was measured in years and centered at the average age (15.26).
Linear regression models were used for the analysis of the resting state period. Dependent variables were: resting-state EEG relative power in Delta, Theta, Alpha, Sigma, Beta1 and Beta2 frequency bands, HR and HRV SDNN and RMSSD measures (calculated across the 5-minute resting-state period), self-reported measures of sleepiness, cognitive (PSAS-cognitive) and somatic (PSAS-somatic) arousal, and SO latencies from lights-out to SO-A, SO-B, and SO-C. Independent variables included sex, age, and the interaction term, sex × age. The formula for the regression was Yi = c + β 1 Age + β 2 Sex + β 3 Age × Sex + ei where Sex and Age are mean-centered, c is a constant, and i indexes participants.
To analyze the cortical and cardiac measures across the falling asleep process (after lights-out), separate linear regressions were performed to analyze changes across the 5-minute preceding (observations in bins 1-to-10) and following (observations in bins 11-to-21) SO-C (Figure 1). The dependent variables in these regressions were EEG relative power in the Delta, Theta, Alpha, Sigma, Beta1 and Beta2 frequency bands, and HR. The independent variables were bin (centered at the midpoint of the bin range and considered as an interval-level variable), sex (as described above), age (as described above), and all two- and three-factor interactions. Variances for the regression coefficients were estimated using the Huber and White robust method as implemented in Stata [39, 40], which adjusts for within-cluster correlation and heteroskedasticity and provides accurate assessments of the sample-to-sample variability of the parameter estimates even when the model is misspecified. The model was given by Yij = c + β 1 Sex + β 2 Bin + β 3 Age + β 4 Sex × Bin + β 5 Sex x Age + β 6 Bin × Age +β 7 Bin × Sex × Age + eij, where i indexes participant and j indexes bin, and the eij are not assumed independent nor homoskedastic. The amount of PSG wake was only analyzed in the 5 minutes preceding SO-C (there were no wake epochs in the post SO-C period) using a multi-level mixed-effects logistic model. The model was logit(pij) = c + β 1 Sex + β 2 Bin + β 3 Age + β 4 Sex × Bin + β 5 Sex × Age + β 6 Bin × Age +β 7 Bin × Sex × Age + ei, ei is the variance component attributable to person i, pij is the logit of the probability of the j-th observation on person i being 1, the probability of Wake being equal to 1 is given by exp(r)/(1+exp(r)) where r is the right-hand side of the equation for logit(pij) and the individual observations are from a Bernoulli distribution with probability pij.
Effects were considered significant at p < 0.05. Regression coefficients (β), standard errors (SE) and degree of freedom are reported for the significant models. All analyses were performed using Stata/SE 14.1 for Windows.
Results
Pre-sleep psychophysiological state: bedtime cortical, cardiac ANS, and cognitive activity
Means and SDs for the pre-sleep psychophysiological measures are provided in Table 2. Before bedtime, girls, as compared to boys, reported greater cognitive arousal (β = 0.065, SE = 0.029, t87 = 2.28, p = 0.025), regardless of age. No sex differences or sex × age interactions were found in pre-sleep somatic arousal or sleepiness.
Table 2.
Physiological (cardiac autonomic and cortical activity) and self-reported (sleepiness, cognitive, and somatic arousal) pre-sleep measures (mean (SD)), in boys and girls
| Boys Mean (SD) | Girls Mean (SD) | ||
|---|---|---|---|
| Self-reported | Cognitive arousal, PSAS cognitive scoresa | 12.0 (4.8) | 14.0 (4.2)* |
| Somatic arousal, PSAS somatic scoresa | 8.8 (1.8) | 9.2 (2.0) | |
| Sleepiness, 0–100 mm VASa | 68.9 (18.9) | 74.7 (17.1) | |
| Autonomic | ECG Heart rate, bpm | 66.9 (9.2) | 68.5 (9.2)* |
| HRV RMSSD, ms | 76.4 (46.5) | 76.3(45.2) | |
| HRV SDNN, ms | 76.8 (46.5) | 72.7 (32.1)* | |
| Cortical | EEG Delta, relative power | 0.43 (0.13) | 0.42 (0.12) |
| EEG Theta, relative power | 0.17 (0.05) | 0.18 (0.05) | |
| EEG Alpha, relative power | 0.22 (0.10) | 0.22 (0.10) | |
| EEG Sigma, relative power | 0.05 (0.02) | 0.06 (0.02) | |
| EEG Beta1, relative power | 0.03(0.01) | 0.03 (0.02) | |
| EEG Beta2, relative power | 0.01 (0.01) | 0.01 (0.01) |
aData unavailable for eight males and three females. RMSSD = Root mean square of the successive differences in normal to normal R-R intervals; SDNN = SD of normal to normal R-R intervals; VAS = Visual analogic scale.
*Significant sex or sex × age interactions.
There was a sex × age interaction for resting-state HR (β = 2.773, SE = 0.864, t98 = 3.21, p = 0.002) and SDNN (total HRV; β = −0.037, SE = 0.018, t98 = −2.04, p = 0.044) indicating higher HR and lower SDNN (total HRV) during the resting state in older girls compared to boys. Resting-state HR was lower in older than younger boys, while it was similar or slightly higher in older than younger girls. In contrast, SDNN during resting state showed a greater age-related reduction in girls, compared to boys.
Quantitative EEG analyses showed that resting-state EEG Delta relative power (β = −0.020, SE = 0.006, t98 = −3.48, p = 0.001) was lower and EEG Alpha relative power (β = 0.019, SE = 0.005, t98 = 4.11, p < 0.001) was higher in older versus younger adolescents.
PSG SO latency and sleep/wake stage shifting in the approach to SO
Table 3 shows PSG-defined SO parameters in boys and girls. Girls took longer than boys to fall asleep, evident across different SO definitions: SOL-A (β = 0.159, SE = 0.076, t98 = 2.09, p = 0.039), SOL-B (β = 0.139, SE = 0.065, t98 = 2.12, p = 0.036), SOL-C (β = 0.168, SE = 0.066, t98 = 2.55, p = 0.012) (Table 3). 13% of girls and 6% of boys spent ≥ 30 minutes to fall asleep (SOL-A, AASM definition [33]), with 67% of girls and 78% of boys falling asleep within 15 minutes. Bedtimes did not significantly differ between boys and girls.
Table 3.
Polysomnographic-defined sleep onset measures (mean (SD)) derived from a single night recording in adolescent boys and girls
| Boys Mean (SD) | Girls Mean (SD) | ||
|---|---|---|---|
| Sleep onset | Bedtime, hour:minutes | 22:50 (00:46) | 22:52 (00:43) |
| SOL-A, minutes | 11.9 (13.6) | 16.1 (16.5)* | |
| SOL-B, minutes | 14.5 (14.5) | 19.8 (17.3)* | |
| SOL-C, minutes | 14.8 (14.5) | 21.3 (17.4)* |
Sleep onset latency (SOL; -A, first epoch of any sleep stage; -B, first epoch of N2 sleep; -C, first epoch of first four consecutive epochs of stable sleep [N2/N3]).
*Significant sex main effects on sleep onset outcomes.
In approaching stable sleep (SOL-C) there was a progressive non-linear reduction in the proportion of PSG wake epochs (β = −0.831, SE = 0.062, z = −13.50, p < 0.001). In addition, a sex × bin interaction effect (β = 0.117, SE = 0.051, z = 2.30, p = 0.022) indicated that the proportion of wake epochs declined more rapidly in boys compared to girls, reflecting a smoother progression from wake-to-sleep in boys. No sex × age interactions or age main effects were found in any of the models.
Changes in cardiac and cortical activities across the SO transition
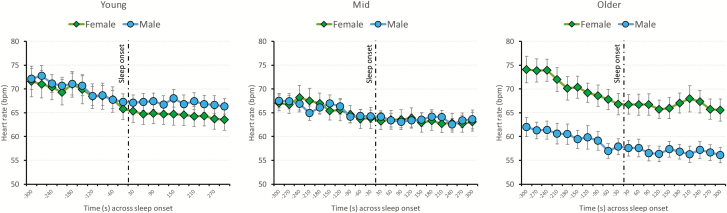
The rate of change in HR across SO was similar between sexes and across age. In the five minutes preceding SO-C, HR progressively slowed (β = −0.533, SE = 0.058, t101 = −9.14, p < 0.001) in both boys and girls (HR drop of ~0.53 bpm every 30 seconds in the 5 minutes preceding SO-C). In the 5 minutes after SO-C, HR continued to drop in both sexes (β = −0.088, SE = 0.025, t101 = −3.56, p = 0.001), at a lower rate (~0.09 bpm drop every 30 seconds in the 5 minutes after SO-C) (Figure 2).
Figure 2.
Mean (±SEs) heart rate plotted every 30 seconds across the 5 minutes preceding and 5 minutes following SO, defined as the first epoch of four consecutive epochs of stable sleep (N2/N3) (SO-C), separately for girls (green rhombuses) and boys (blue circles). For illustrative purpose only, the sample was split in thirds according to the age distribution: young (<14 years; N = 34), mid (≥14 years, <17 years; N = 34), and older (≥17 years; N = 34) age categories. However, age was included as a continuous factor in the statistical models.
As for the resting state data, there was a sex × age interaction effect for HR for both pre- (β = 1.307, SE = 0.046, t101 = 2.84, p = 0.005) and post-SO-C (β = 1.289, SE = 0.428, t101 = 3.01, p = 0.003), showing faster HR in older girls than older boys. HR was slower in older than younger boys, while it remained constant or was slightly faster in older than younger girls.
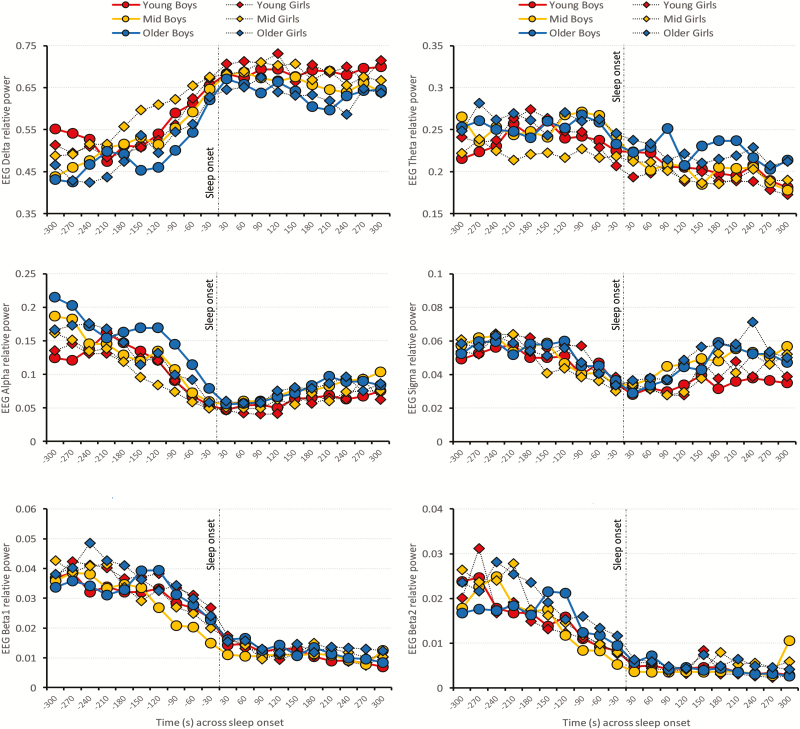
EEG Delta relative power progressively increased (β = 0.0176, SE = 0.0017, t101 = 10.19, p < 0.001) at a rate of ~1.8% every 30 seconds in the 5 minutes preceding SO-C (an overall cumulative increase of ~17.6%), in both boys and girls. In contrast, EEG Alpha (β = −0.0114, SE = 0.0012, t101 = −9.54, p < 0.001), Sigma (β = −0.0024, SE = 0.0028, t101 = −8.50, p < 0.001), Beta1 (β = −0.0012, SE = 0.0002, t101 = −7.29, p < 0.001) and Beta2 (β = −0.0017, SE = 0.0003, t101 = −6.04, p < 0.001) relative powers progressively decreased in the approach to SO-C, in boys and girls. No changes were found in EEG Theta relative power in the approach to SO-C.
Figure 3 illustrates the patterns of change for cortical frontal EEG relative powers in Delta, Theta, Alpha, Sigma, Beta1, and Beta2 frequency bands across the SO period. Once reaching SO-C, EEG Delta relative power (β = −0.0023, SE = 0.0008, t101 = −2.78, p = 0.007) started declining slightly across the 5 minutes post SO. Similarly, once reaching SO-C, EEG Theta relative power (β = −0.0024, SE = 0.0005, t101 = −4.93, p < 0.001) started declining across the 5 minutes post SO. An inverted trend is evident in EEG Sigma (β = 0.0018, SE = 0.0003, t101 = 6.38, p < 0.001) and Alpha (β = 0.0035, SE = 0.0004, t101 = 9.53, p < 0.001) relative powers, which started rising across the 5-minute period after SO-C. Beta1 (β = −0.0005, SE = 0.0001, t101 = −4.81, p < 0.001) continued progressively dropping across the 5 minutes post SO-C.
Figure 3.
Patterns of change (mean values) in EEG relative power (averaged for F3 and F4) in Delta, Theta, Alpha, Sigma, Beta1, and Beta2 frequencies bands across the 5 minutes preceding and 5 minutes periods following SO, defined as the first epoch of four consecutive epochs of stable sleep (N2/N3) (SO-C). Data are plotted for boys (circles) and girls (rhombuses). For illustrative purpose only, the sample was split in thirds according to the age distribution: young (<14 years, red; N = 34), mid (≥14 years, <17 years, yellow; N = 34) and older (≥17 years, blue; N = 34) age categories. However, age was included as a continuous factor in the statistical models.
There was a significant main effect of age for EEG Delta power in the period before SO-C (β = −0.0099, SE = 0.0039, t101 = −2.53, p = 0.013), as well as for EEG Delta (β = −0.0119, SE = 0.0008, t101 = −4.78, p < 0.001), Theta (β = 0.0057, SE = 0.0016, t101 = 3.48, p < 0.001), Alpha (β = 0.0032, SE = 0.0013, t101 = 2.49, p = 0.015) and Sigma (β = 0.0026, SE = 0.0012, t101 = 2.23, p = 0.028) relative powers post SO-C, indicating lower EEG Delta relative power (drop-rate of ~1% every year), and higher EEG Theta, Alpha and Sigma relative powers in older compared with younger boys and girls. No sex × age, bins × age or bins × sex interactions were significant for any of the EEG frequency bands. Average EEG absolute power in Delta, Theta, Alpha, Sigma, Beta1, and Beta2 frequency bands across SO are provided in Supplementary Table 1, separately for boys and girls, and the time-course of single Hz EEG absolute power across the SO transition is provided in Supplementary Figure 1. Changes in absolute EEG power bands across SO were similar to the patterns seen for relative power.
Discussion
To our knowledge, this is the first study showing the dynamics of the pre-sleep psychophysiological state and cortical and cardiac de-arousing processes across the wake-to-sleep transition in a non-clinical sample of healthy adolescent boys and girls. In our study, girls approached sleep in a greater state of pre-sleep negative cognition (elevated worry, rumination, depressing, or anxious thoughts) than boys, and had a delayed onset to consolidated sleep, with a less smooth progression from wake-to-sleep than boys. These findings support the existence of a female-specific vulnerability to sleep initiation, which may potentially set girls at high risk to develop sleep disturbances in adolescence as well as later in life.
The finding of elevated pre-sleep cognitive arousal in girls supports existing population-based evidence of sex differences in sleep-related cognitive processing. Catastrophic worry (e.g. worry about school, relation with others) was common in a community sample of young (11–12 years) adolescent females [41], higher in older (16–18 years) adolescent girls compared to boys [42], and associated with poor self-reported sleep quality [41]. Also, catastrophic worry partially mediated the relationship between adolescents’ sleep disturbances and the occurrence of depressive symptoms [42]. Interestingly, a meta-analytic review highlighted the fact that adolescent girls have a greater tendency for ruminating than adolescent boys, which does not seem to be evident in children [43], suggesting potential sex differences in developmental changes in pre-sleep-related cognition. Further studies are needed to comprehensively evaluate bedtime cognition in adolescent boys and girls, its relationship with mood and anxiety, and its interference with the physiological de-arousing process.
Our results of prolonged PSG-defined SOL in girls confirmed our previous laboratory finding of longer latencies to N2 sleep in girls compared to boys based on an extended NCANDA sample [27], and results from large population-based studies based on self-reported SOL (see ref. [25], for example). In addition, in our study, 13% of girls versus 6% of boys spent ≥ 30 minutes to fall asleep (using the AASM standard definition of SOL, SOL-A), that is considered a clinical cutoff for difficulties falling asleep in the insomnia literature, further supporting the evidence of greater perceived SO difficulties in girls compared to boys [11].
Of interest, girls displayed a less clear wake-to-sleep transition than boys, which was reflected in a blunted reduction in the proportion of PSG wake time in approaching stable sleep (SO-C). While boys seemed to have less trouble in proceeding from wake-to-sleep, girls had more switches from “wake–brief period of sleep (N1/N2)–wake,” before ultimately reaching consolidated sleep, possibly reflecting less efficiency in reaching stable sleep. The significant sex effects that we found for pre-sleep cognitive arousal, SOL, and the wake-to-sleep transition were equally apparent in younger and older age groups, which does not support our hypothesis that effects would be more evident in older girls. Our results, therefore, show that adolescent girls, regardless of age, differ from boys in pre-sleep arousal and reaching consolidated sleep. However, girls and boys showed a similar pattern of change in cortical EEG and cardiac activities around the onset of stable sleep. In both sexes, HR dropped rapidly in the 5 minutes preceding stable sleep at a rate of ~0.53 bpm every 30 seconds and continued to drop at a slower rate once stable sleep was reached.
A progressive cortical synchronization (increases in EEG Delta relative power) and a reduction in EEG high-frequency activity characterized the approach to sleep in both sexes. As shown in Supplementary Figure 1, our findings are similar to those reported by De Gennaro et al. [38] in healthy young male adults. We found no evidence of age- or sex-specific differences in the time-course of EEG changes across SO in this adolescent group. However, Spiess and colleagues [44] by employing high-density EEG recordings and specific analyses of EEG oscillatory rhythms, highlighted several differences in the falling asleep process between children (8–11 years) and young adults (20–25 years) (the sample consisted of 67% males, and sex differences or sex × age interactions were not examined). For example, changes in slow-wave density and amplitude showed a similar temporal course across the SO transition in children, while they were dissociated in adults. Also, adults and children showed different EEG patterns across different cortical regions (see ref. [44] for details), which we did not examine. Future work should compare the spatiotemporal cortical reorganization that occurs across the SO transition in male and female adolescents and adults.
Although we did not find sex or age differences in CNS or ANS variables underlying the falling asleep de-arousing processes, significant age and age × sex interactions in basal cortical and cardiac activities were evident. Overall, older girls exhibited higher HR before and across SO, compared to younger girls and boys. This effect was accompanied by an age-related reduction in total HRV, as measured during the resting state, in girls, which was not evident in boys. These findings extend those of our previous investigation [28] showing sex × age interactions in HR and cardiac ANS function during nighttime sleep, and appears to reflect normal sex differences in ANS maturation rather than SO-specific sex- and age-dependent shifting in ANS control. We only investigated a 5-minute resting period before lights-out and the 10-minute period across SO, and found no sex × age interaction effects in the falling asleep process. Possibly, a longer time period of analysis, such as across the hour before bedtime, might reveal sex-age divergence in the preparation to sleep, if it does exist. Also, similar to the converging findings of age-related changes in sleep EEG across adolescence (e.g. strong reduction in sleep EEG Delta power in older compared to younger adolescence) [21, 27, 45], our study showed that EEG Delta relative power was lower (~1% lower with every additional year of age), and EEG Theta, Alpha and Sigma relative powers were higher in older compared to younger adolescents, most likely reflecting a trait signature of the brain maturation processes occurring in adolescence, rather than age-related differences in SO-specific cortical activity. Indeed, we and others have shown dramatic changes in EEG delta power with age across adolescence, which is evident during wake, NREM sleep, and REM sleep [27, 46–48]. Also, we found that age-related changes in brain structure (reduction in gray matter volume and cortical thickness, likely suggesting changes in synaptic pruning and myelination [20]) explained between 3% and 9% of variance, and partially mediated the relationship between age and delta activity during NREM sleep [21].
Our study has some limitations, which need to be acknowledged. We did not account for next-day stressful situations (e.g. school exams) or current stress levels, which may have confounded the assessments of pre-sleep psychophysiological state and sleep processes. For example, Wang et al. [49] reported that 78.3% of adolescents preparing for their College Entrance Exam, reported spending more than 30 minutes to fall asleep in the month preceding the exam. Also, despite no differences in SOL being found, Dewald et al. [50] reported greater actigraphic sleep fragmentation in adolescents (12–17 years old; 71% girls) when sleeping during exam weeks (high stress) versus regular school weeks (low stress). Further investigations should account for potential effects of stress. On the other hand, we controlled for several other factors (e.g. caffeine, bedtime electronic media use, alcohol and drug use), which could potentially affect pre-sleep arousal and falling asleep processes ([51, 52]). Participants self-selected their bedtimes and there were no sex, age or sex × age interactions in the timing of lights-out or self-reported sleepiness levels. However, we cannot completely exclude potential sex and age differences in endogenous circadian timing, which was not assessed in the current study. Future studies should include experimental manipulations of the pre-sleep psychophysiological state (e.g. up-regulation) and longitudinal evaluations of adolescents’ falling asleep processes to provide new insight into potential age- and sex-related vulnerabilities for sleep initiation, as well as relationships between different pre-sleep psychophysiological states and the subsequent falling asleep process.
In conclusion, here we have described the cognitive, cortical, and cardiac autonomic dynamics of the falling asleep process in healthy adolescents. Our findings suggest a female-specific vulnerability to inefficient sleep initiation which may underly sex-specific risks (greater in girls) for the development of sleep disturbances in adolescence as well as later in life.
Supplementary material
Supplementary material is available at SLEEP online.
Supplementary Figure 1. Time-course of single Hz electroencephalographic (EEG) absolute power (mean values averaged between F3 and F4; log µV2/Hz) across the 5 min preceding and following SO, defined as the first epoch of four consecutive epochs of stable sleep (N2/N3) (SO-C), in boys (right) and girls (left).
Funding
This study was supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) grant AA021696 (I.M.C. + F.C.B.) and 3U01AA021696-07S1 supplement grant (FCB), and by the National Heart, Lung and Blood Institute (NHLBI) grant R01 HL139652 (M.d.Z.). The content is solely the responsibility of the authors and does not necessarily represent the official views the National Institutes of Health.
Conflict of interest statement. We declare no conflict of interest related to the current work. M.d.Z., F.C.B., and I.C. have received research funding unrelated to this work from Ebb Therapeutics Inc., Fitbit Inc., International Flavors & Fragrances Inc., Noctrix Health, Inc., and Verily Life Sciences, LCC.
References
- 1. Ogilvie RD. The process of falling asleep. Sleep Med Rev. 2001;5(3):247–270. [DOI] [PubMed] [Google Scholar]
- 2. Marzano C, et al. How we fall asleep: regional and temporal differences in electroencephalographic synchronization at sleep onset. Sleep Med. 2013;14(11):1112–1122. [DOI] [PubMed] [Google Scholar]
- 3. Okamoto-Mizuno K, et al. Heart rate variability and body temperature during the sleep onset period. Sleep Biol Rhythms. 2008;6(1):42–49. [Google Scholar]
- 4. Levenson JC, et al. The pathophysiology of insomnia. Chest. 2015;147(4):1179–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harvey AG. A cognitive model of insomnia. Behav Res Ther. 2002;40(8):869–893. [DOI] [PubMed] [Google Scholar]
- 6. Perlis ML, et al. Beta/Gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep. 2001;24(1):110–117. [DOI] [PubMed] [Google Scholar]
- 7. de Zambotti M, et al. Sleep onset and cardiovascular activity in primary insomnia. J Sleep Res. 2011;20(2):318–325. [DOI] [PubMed] [Google Scholar]
- 8. Staner L, et al. Sleep microstructure around sleep onset differentiates major depressive insomnia from primary insomnia. J Sleep Res. 2003;12(4):319–330. [DOI] [PubMed] [Google Scholar]
- 9. Merica H, et al. The EEG of the sleep onset period in insomnia: a discriminant analysis. Physiol Behav. 1992;52(2):199–204. [DOI] [PubMed] [Google Scholar]
- 10. Gradisar M, et al. Recent worldwide sleep patterns and problems during adolescence: a review and meta-analysis of age, region, and sleep. Sleep Med. 2011;12(2):110–118. [DOI] [PubMed] [Google Scholar]
- 11. Pallesen S, et al. Time trends in sleep-onset difficulties among Norwegian adolescents: 1983–2005. Scand J Public Health. 2008;36(8):889–895. [DOI] [PubMed] [Google Scholar]
- 12. Short MA, et al. Identifying adolescent sleep problems. PLoS One. 2013;8(9):e75301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Zambotti M, et al. Insomnia disorder in adolescence: diagnosis, impact, and treatment. Sleep Med Rev. 2018;39:12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dahl RE, et al. Sleep onset abnormalities in depressed adolescents. Biol Psychiatry. 1996;39(6):400–410. [DOI] [PubMed] [Google Scholar]
- 15. Alfano CA, et al. Pre-sleep arousal and sleep problems of anxiety-disordered youth. Child Psychiatry Hum Dev. 2010;41(2):156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hiller RM, et al. Trying to fall asleep while catastrophising: what sleep-disordered adolescents think and feel. Sleep Med. 2014;15(1):96–103. [DOI] [PubMed] [Google Scholar]
- 17. Heath M, et al. The role of pre-sleep cognitions in adolescent sleep-onset problems. Sleep Med. 2018;46:117–121. [DOI] [PubMed] [Google Scholar]
- 18. Fernandez-Mendoza J, et al. Insomnia is associated with cortical hyperarousal as early as adolescence. Sleep. 2016;39(5):1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fernandez-Mendoza J, et al. Childhood high-frequency EEG activity during sleep is associated with incident insomnia symptoms in adolescence. J Child Psychol Psychiatry. 2019;60(7):742–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Feinberg I, et al. Sleep EEG changes during adolescence: an index of a fundamental brain reorganization. Brain Cogn. 2010;72(1):56–65. [DOI] [PubMed] [Google Scholar]
- 21. Goldstone A, et al. The mediating role of cortical thickness and gray matter volume on sleep slow-wave activity during adolescence. Brain Struct Funct. 2018;223(2):669–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Paus T, et al. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9(12):947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suh S, et al. Sex Differences in insomnia: from epidemiology and etiology to intervention. Curr Psychiatry Rep. 2018;20(9):69. [DOI] [PubMed] [Google Scholar]
- 24. Ohayon MM, et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–1273. [DOI] [PubMed] [Google Scholar]
- 25. Hysing M, et al. Sleep patterns and insomnia among adolescents: a population-based study. J Sleep Res. 2013;22(5):549–556. [DOI] [PubMed] [Google Scholar]
- 26. Brown SA, et al. The National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA): a Multisite Study of Adolescent Development and Substance Use. J Stud Alcohol Drugs. 2015;76(6):895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baker FC, et al. Age-Related differences in sleep architecture and electroencephalogram in adolescents in the national consortium on alcohol and neurodevelopment in adolescence sample. Sleep. 2016;39(7):1429–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Zambotti M, et al. Sex- and age-dependent differences in autonomic nervous system functioning in adolescents. J Adolesc Health. 2018;62(2):184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Petersen AC, et al. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc. 1988;17(2):117–133. [DOI] [PubMed] [Google Scholar]
- 30. Buysse DJ, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 31. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 32. Nicassio PM, et al. The phenomenology of the pre-sleep state: the development of the pre-sleep arousal scale. Behav Res Ther. 1985;23(3):263–271. [DOI] [PubMed] [Google Scholar]
- 33. Iber C,. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specification. In. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 34. Delorme A, et al. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. [DOI] [PubMed] [Google Scholar]
- 35. Willoughby AR, et al. Partial K-Complex recovery following short-term abstinence in individuals with alcohol use disorder. Alcohol Clin Exp Res. 2015;39(8):1417–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 37. Forouzanfar M, et al. Automatic analysis of pre-ejection period during sleep using impedance cardiogram. Psychophysiology. 2019;56(7):e13355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. De Gennaro L, et al. The boundary between wakefulness and sleep: quantitative electroencephalographic changes during the sleep onset period. Neuroscience. 2001;107(1):1–11. [DOI] [PubMed] [Google Scholar]
- 39. Rogers WA, et al. Maxillary definitive obturators: rationale of design. J Dent Technol. 1996;13(9):19–26. [PubMed] [Google Scholar]
- 40. Froot KA. Consistent covariance matrix estimation with cross-sectional dependence and heteroskedasticity in financial data. J Finan Quant Anal. 1989;24(3):333–355. [Google Scholar]
- 41. Noone DM, et al. Catastrophizing and poor sleep quality in early adolescent females. Behav Sleep Med. 2014;12(1):41–52. [DOI] [PubMed] [Google Scholar]
- 42. Danielsson NS, et al. Sleep disturbance and depressive symptoms in adolescence: the role of catastrophic worry. J Youth Adolesc. 2013;42(8):1223–1233. [DOI] [PubMed] [Google Scholar]
- 43. Rood L, et al. The influence of emotion-focused rumination and distraction on depressive symptoms in non-clinical youth: a meta-analytic review. Clin Psychol Rev. 2009;29(7):607–616. [DOI] [PubMed] [Google Scholar]
- 44. Spiess M, et al. How do children fall asleep? A high-density EEG study of slow waves in the transition from wake to sleep. Neuroimage. 2018;178:23–35. [DOI] [PubMed] [Google Scholar]
- 45. Colrain IM, et al. Changes in sleep as a function of adolescent development. Neuropsychol Rev. 2011;21(1):5–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Feinberg I, et al. Longitudinal sleep EEG trajectories indicate complex patterns of adolescent brain maturation. Am J Physiol Regul Integr Comp Physiol. 2013;304(4):R296–R303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Baker FC, et al. Developmental changes in the sleep electroencephalogram of adolescent boys and girls. J Sleep Res. 2012;21(1):59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jenni OG, et al. Spectral analysis of the sleep electroencephalogram during adolescence. Sleep. 2004;27(4):774–783. [PubMed] [Google Scholar]
- 49. Wang G, et al. Sleep patterns and academic performance during preparation for college entrance exam in Chinese adolescents. J Sch Health. 2016;86(4):298–306. [DOI] [PubMed] [Google Scholar]
- 50. Dewald JF, et al. Adolescents’ sleep in low-stress and high-stress (exam) times: a prospective quasi-experiment. Behav Sleep Med. 2014;12(6):493–506. [DOI] [PubMed] [Google Scholar]
- 51. Cain N, et al. Electronic media use and sleep in school-aged children and adolescents: a review. Sleep Med. 2010;11(8):735–742. [DOI] [PubMed] [Google Scholar]
- 52. Calamaro CJ, et al. Adolescents living the 24/7 lifestyle: effects of caffeine and technology on sleep duration and daytime functioning. Pediatrics. 2009;123(6):e1005–e1010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.