Abstract
Most of our knowledge about the regulation and function of sleep is based on studies in a restricted number of mammalian species, particularly nocturnal rodents. Hence, there is still much to learn from comparative studies in other species. Birds are interesting because they appear to share key aspects of sleep with mammals, including the presence of two different forms of sleep, i.e. non-rapid eye movement (NREM) and rapid eye movement (REM) sleep. We examined sleep architecture and sleep homeostasis in the European starling, using miniature dataloggers for electroencephalogram (EEG) recordings. Under controlled laboratory conditions with a 12:12 h light–dark cycle, the birds displayed a pronounced daily rhythm in sleep and wakefulness with most sleep occurring during the dark phase. Sleep mainly consisted of NREM sleep. In fact, the amount of REM sleep added up to only 1~2% of total sleep time. Animals were subjected to 4 or 8 h sleep deprivation to assess sleep homeostatic responses. Sleep deprivation induced changes in subsequent NREM sleep EEG spectral qualities for several hours, with increased spectral power from 1.17 Hz up to at least 25 Hz. In contrast, power below 1.17 Hz was decreased after sleep deprivation. Sleep deprivation also resulted in a small compensatory increase in NREM sleep time the next day. Changes in EEG spectral power and sleep time were largely similar after 4 and 8 h sleep deprivation. REM sleep was not noticeably compensated after sleep deprivation. In conclusion, starlings display signs of NREM sleep homeostasis but the results do not support the notion of important REM sleep functions.
Keywords: birds, sleep phylogeny, sleep homeostasis, sleep deprivation, REM sleep, spectral analysis
Statement of Significance.
We studied sleep architecture and sleep homeostasis in a songbird, the European starling. The birds displayed both non-rapid eye movement (NREM) and rapid eye movement (REM) sleep but, surprisingly, REM sleep is only made up to 1~2% of total sleep time. In response to sleep deprivation, there was an increase in NREM sleep electroencephalogram (EEG) spectral power perhaps indicative of a sleep homeostatic response. Interestingly, power below 1.17 HZ showed an opposite response indicating that the mammalian delta power is not a universal indicator of sleep homeostasis. The low amount of baseline REM sleep and a lack of compensation of REM sleep loss after sleep deprivation suggest that starlings under laboratory conditions can almost do without REM sleep, which seems at odds with most theories on REM sleep function.
Sleep is a state of inactivity and diminished awareness of the surrounding that seems to be widespread in the animal kingdom. In fact, even though only a fraction of all animal species have been studied in detail, there is a general consensus that most species spend a large part of their lives asleep [1–3]. Sleep is thought to serve physiological functions that are of critical importance for the individuals’ performance and health, but what exactly these functions are, remains uncertain [4–6]. It is often assumed that the functions of sleep entail some form of recovery from preceding wakefulness, based on the finding that a need for sleep seems to build up during wakefulness. This notion is supported by the finding that extended wakefulness, or sleep deprivation, is associated with an increased drive for sleep and is followed by a compensatory rebound sleep [7, 8]. In other words, sleep appears to be homeostatically regulated in relation to how long animals have been awake [7, 8].
The questions regarding the regulatory principles and functions of sleep are complicated by the fact that sleep can come in two different forms, that is, non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep [8]. In mammals, particularly, the homeostatic regulation of NREM sleep is well established [8]. Extended wakefulness is often followed by a compensatory increase in both time and intensity of subsequent NREM sleep. The intensity of NREM sleep is reflected in the number of slow waves in the electroencephalogram (EEG) [9–12]. In several mammalian species, slow-wave activity in the range of 1–4 Hz was found to be an increasing function of the duration of prior wakefulness [13–16]. This slow-wave activity is highest at the beginning of sleep and then gradually declines in the course of the sleep phase suggesting that the need for NREM sleep is dissipating [13–16].
In mammals, rebounds of REM sleep have also been reported after sleep deprivation [10, 11, 17, 18] but these rebounds in REM sleep appear to be less predictable compared with NREM sleep [19–21]. In fact, it is still debated whether REM sleep is homeostatically regulated at all, and, if so, whether that is in relation to prior wakefulness or perhaps preceding NREM sleep [22–25]. Other factors that influence REM sleep are, for example, environmental temperature [26–28] and stress [29–31].
The vast majority of studies on the regulatory mechanisms and functional aspects of sleep were done in a handful of mammalian species, particularly nocturnal rodents such as mice and rats (see references above). Few other species have been studied in detail, often because they are not easily available or difficult to maintain under laboratory conditions [21]. Hence, there is still much to learn about sleep in other species groups [2, 32]. Birds are an interesting group in this respect because they share key features of sleep with mammals, including the presence of both NREM and REM sleep [33, 34]. Moreover, there are a number of reports suggesting that NREM sleep in birds may be homeostatically regulated in relation to wakefulness, suggesting it may serve functions similar to what has been proposed for mammals [35–38]. There are, however, also interesting differences in sleep between birds and mammals. For example, in mammals REM sleep on average makes up 18% of total sleep time [39], while in the few bird species for which this is known the amount of REM sleep is on average less than 10% of total sleep time [40]. Moreover, it was shown that some bird species under natural conditions are sometimes capable of persisting and apparently sustaining normal behavior with very little to no sleep at all for many days [41, 42]. Such findings challenge the common view based on studies in mammals that decreased performance and health is an inescapable outcome of sleep loss and beg for follow-up studies.
Studying sleep entails a special challenge in most bird species because of their ability to fly, but this constraint has been alleviated by the miniaturization of datalogger technology [32, 43, 44]. In the current study, we applied such miniature dataloggers to assess sleep architecture and sleep homeostasis in the European starling (Sturnus vulgaris). This species is an interesting model for sleep research because they can easily be maintained in captivity and are large enough to carry a datalogger without being hampered in their movements. Moreover, the starling is a common and widespread species that can be found living under a wide variety of environmental conditions, which makes it a suitable species for future studies aimed at ecological questions. In the present study, we measured baseline sleep in captive starlings under controlled conditions and addressed the question of sleep homeostasis by subjecting the birds to manual sleep deprivation of different durations (4 and 8 h).
Methods
Animals and housing
A total of 12 adult starlings were used for this study (7 males and 5 females). Five of them were wild-caught animals obtained from the Max Planck Institute for Ornithology (Seewiesen, Germany) and the other seven were caught in the wild in the Netherlands (Oudehaske, 52°58′19.2″N 5°51′38.0″E). The birds were kept in groups in large outdoor aviaries until 2 weeks before the start of EEG recordings, for which the animals were individually housed indoors in a wooden cage (length = 79 cm, width = 60 cm, height = 60 cm). The cage floor was covered with bedding and a wooden branch in the center served as a perch. Water and food were provided ad libitum (food item number 6659; Kasper Faunafood, Woerden, The Netherlands). Each cage contained two light bulbs, and the light–dark cycle was set at 12:12 with lights-on from 8:00 am to 8:00 pm. In order to mimic twilight, a dim light was on for 10 minutes before lights-on and also for 10 minutes after lights-off. The temperature in the room was controlled at 21 ± 1 °C. All procedures were approved by the national Central Authority for Scientific Procedures on Animals (CCD) and the Institutional Animal Welfare Body (IvD, University of Groningen, The Netherlands).
Surgery
Surgeries for implantation of electrodes to record EEG were performed under isoflurane anesthesia (1.5–2%). The skull was carefully exposed and seven 0.5 mm holes were drilled for insertion of electrodes. Four EEG electrodes were placed in a left-to-right line over the rostral part of the telencephalon (two per hemisphere, 2 and 6 mm lateral from the midline). The location of the electrodes was based on previous research in birds [41–43]. The medial electrodes were over the hyperpallium and the lateral electrodes were over the mesopallium. Two reference electrodes were placed caudally near the cerebellum (one per hemisphere, 4 mm lateral of the midline) and one ground electrode was implanted over the right hemisphere (6 mm from the midline). All electrodes consisted of gold-plated pins with rounded tips (0.5 mm diameter, BKL Electronic 10120538, Lüdenscheid, Germany). They were inserted to the level of the dura mater and glued to the cranium with cyano-acrylic adhesive. All electrodes were wired to at 7-channel connector (BKL Electronic 10120302, Lüdenscheid, Germany) and then secured and isolated with Paladur dental acrylic (Heraeus Kulzer, Hanau, Germany). A light-weight protective plug was then attached to the connector (BKL Electronic 10120602, Lüdenscheid, Germany). After 2 weeks of recovery from surgery, dummy loggers were used to habituating the starlings to wearing the recording loggers. The dummy logger weight was gradually increased in 3 steps (1.5, 2.5, and 3.5 g) each one lasting 3 days. The final dummy logger weight was similar to the real datalogger weight and represented less than 5% of the total body weight. Recovery from surgery and habituation to the (dummy) loggers took place in the outdoor aviaries.
Data collection
To record and store EEG data, a neurologger 2A was attached to the connector on the head of the starlings (Neurologger 2A; Evolocus, Tarrytown, NY, USA). EEG was recorded with a sampling rate of 200 Hz. During data acquisition, the logger used a build-in high band pass filter of 1 Hz and a low band pass filter of 70 Hz. The first order high pass filter provided a relatively slow signal attenuation of 20 dB per decade, i.e. the amplitude of data between 1 and 0.1 Hz was gradually attenuated until a maximum of 10 times at 0.1 Hz. Therefore, the absolute power below 1 Hz was attenuated but could still be used for analysis. The logger also contained a three-axis accelerometer (LIS302DLH; STMicro-electronics Geneva, Switzerland) to measure head acceleration as a proxy for activity. Two ZA13 1.45 V batteries were used, which enabled the loggers to record data for about three-and-a-half days. Dummy loggers were replaced with neurologgers at noon and the subsequent dark–onset at 8 pm was defined as the start of the baseline.
Starlings were subjected to three treatments: control (C), 4 h of sleep deprivation (4SD), or 8 h of sleep deprivation (8SD). The control treatment consisted of a 3-day recording without intervention. The 4SD and 8SD treatment consisted of a sleep deprivation starting at the onset of the second dark phase for the duration of 4 or 8 h, respectively. Birds undergoing the 4SD or 8SD treatment were kept awake by means of “mild stimulation” [36, 45, 46]. Whenever a starling showed signs of inactivity and eye-closure, the cage was gently tapped and the animal was stimulated to be awake. The birds were subjected to all three treatments in balanced order, separated by at least 1 week. Because of technical problems with the loggers and/or batteries, we did not have complete 3-day recordings for all birds and conditions. The analysis is based on complete recordings for 9 C, 6 4SD, and 12 8SD.
Data analyses
EEG and accelerometry data were processed with RemLogic (Natus Medical, Pleasanton, California). All recordings were coded and then scored manually by an observer blind to the identity and treatment of the animals. All recordings were scored based on the same EEG derivation by the same person. Every 4-s epoch of the 3-days recording was scored as wakefulness (W), NREM sleep, or REM sleep according to the criteria described in Figure 1. Wakefulness was characterized by relatively low-amplitude, high-frequency EEG activity, and often with movements in the accelerometer signal. NREM sleep was scored when more than half of an epoch showed low-frequency activity with an amplitude approximately twice that of alert wakefulness. The onset of NREM sleep typically corresponded with a cessation of movement as indicated by the accelerometer signal. REM sleep was characterized by periods of EEG activation (>2 s) without noticeable head movement in the accelerometer signal or sometimes with signs of head dropping that were visible in the accelerometer data indicative of reduced muscle tone. Based on the 4-s scoring, we subsequently calculated the amounts of NREM sleep and REM sleep per hour.
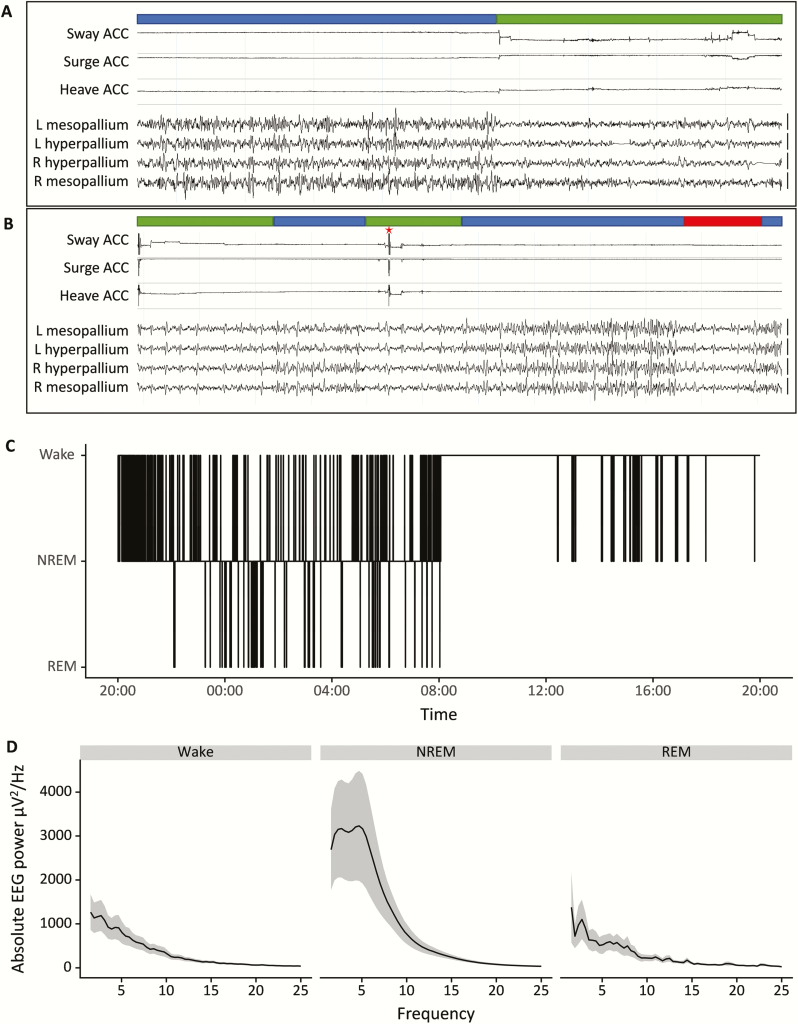
Figure 1.
(A and B) Representative EEG traces and accelerometer signals of a starling. The channels shown represent a three-axis accelerometery (Sway, Surge, and Heave) and four EEG signals (L + R Hyperpallium and L + R Mesopallium). Based on these signals, each 4-s epoch is scored as Wakefulness (green bar), NREM sleep (blue bar), or REM sleep (red bar). Epochs with artifacts (red asterisk) were omitted prior to spectral analysis. Vertical bars on the right of the EEG traces denote 100 μV. (C) A hypnogram of an individual starling of the control group during the baseline day, scored for Wake, NREM sleep, and REM sleep. (D) Mean absolute power spectra of the baseline day in the control group for Wake, NREM, and REM sleep, the shaded areas indicate the standard error of the mean (SEM).
EEG data of all 4-s epochs were further subjected to fast Fourier transformation (FFT) to calculate spectral power density for different frequency bins. This yielded 256 frequency bins with a bin-width of 0.39 Hz. EEG artifacts were visually detected and the corresponding FFT values were omitted from the spectral analysis of NREM sleep EEG. Epochs were labeled as artifacts when movements seen in the accelerometer channels caused peaks in the EEG at least twice the normal amplitude (e,g., stage changes in epochs that largely consisted of NREM sleep). This was the case for 20.4% ± 2.0 of the NREM sleep epochs. To correct for interindividual differences in NREM sleep EEG signal strength, for each 3-day recording the spectral power values of each frequency bin of each NREM sleep epoch were normalized by expressing them relative to the power in the same frequency bin averaged for all 12-h baseline dark phase NREM sleep epochs.
Statistics
Data were analyzed in R with linear mixed models lme4 [47, 48], including bird ID as a random effect. The package lsmeans was used for posthoc Tukey HSD tests [49]. Data in text and figures are expressed as mean ± SEM.
Results
Figure 1 shows representative EEG and accelerometer signals, hypnogram, and absolute spectral power from an individual starling and illustrates the distinct vigilance states known from other studies in both birds and mammals. The starlings spent much of the 12 h baseline dark phase sleeping and were awake most of the light phase, except for some sleep in the middle of the light phase (Figures 1, C and 2, also Table S1). Most of the sleep consisted of NREM sleep (on average 82.8 ± 1.7% of the 12 h dark phase and 98.4 ± 0.5% of total sleep time in the dark phase; on average 6.7 ± 1.9% of the 12 h light phase and 99.9 ± 0.02% of total sleep time in the light phase). Strikingly, only a marginal amount of the baseline sleep consisted of REM sleep (on average 1.3 ± 0.4% of the 12 h dark phase and 1.6 ± 0.5% of total sleep time in the dark phase; practically no REM sleep in the light phase). For unknown reasons, the birds in the control condition had a slightly lower amount of REM sleep on the second recording day as compared with the first day (4.1 ± 1.1 min and 6.8 ± 1.7 min, respectively; see Table S1).
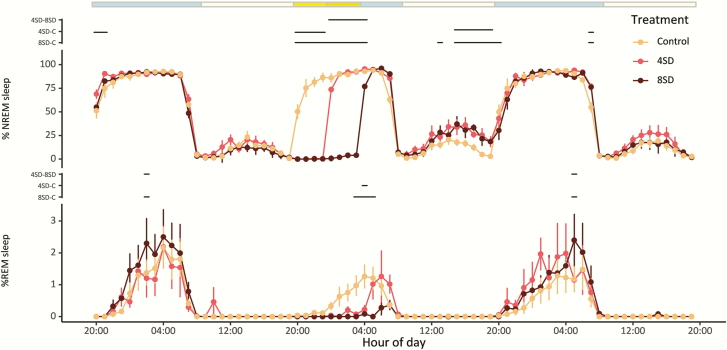
Figure 2.
The sleep architecture of starlings during a 3-day recording for the control (n = 9), 4SD (n = 6), and 8SD (n = 12) treatments. The upper panel shows the percentage of NREM sleep per hour, the lower panel shows the percentage of REM sleep per hour. The colored bar on the top indicates the light–dark cycle (blue: dark phase; light yellow: light phase) and the timing of the sleep deprivation (bright yellow: 4 h SD during the dark phase; bright+dark yellow: 8 h SD during the dark phase). Significant differences between treatments are indicated by the dashed lines (lmer model and Tukey HSD posthoc test, p < 0.05).
The mild stimulation procedure during the 4SD and 8SD treatment was highly effective in keeping the animals awake (Figure 2). Upon cessation of the sleep deprivation treatment, the birds quickly went to sleep and for the remainder of the dark phase displayed a similarly high proportion of time in NREM sleep as in the undisturbed control condition. In the light phase following the sleep deprivation, both the 4SD and 8SD group displayed slightly but significantly more NREM sleep compared with the control condition (lmer model with Tukey HSD posthoc test, p < 0.05), indicating some compensatory day-time napping to make up for the sleep that was lost (Figure 2, top panel). In contrast, REM sleep was not only suppressed during the sleep deprivation but was still suppressed during the remainder of the dark phase, particularly in the 8SD group (lmer model with Tukey posthoc test, p < 0.05, Figure 2, lower panel). The REM sleep that was lost during and immediately following the sleep deprivation was not compensated during the subsequent light phase (Figure 2, lower panel). During the third recording day, there were no major differences in sleep between the three treatment groups, except for small increases in NREM and REM sleep toward the end of the night. The patterns in relative NREM sleep EEG spectral power between 0 and 25 Hz for the three recording days are shown in heat maps in Figure 3, with a brighter color indicating a higher spectral power. To better visualize the effect of sleep deprivation, the heatmaps in Figure 4 depict the deviations in NREM sleep EEG spectral power between the experimental sleep deprivation conditions and the non-sleep-deprived control condition, either for the same clock time or for the time since sleep onset.
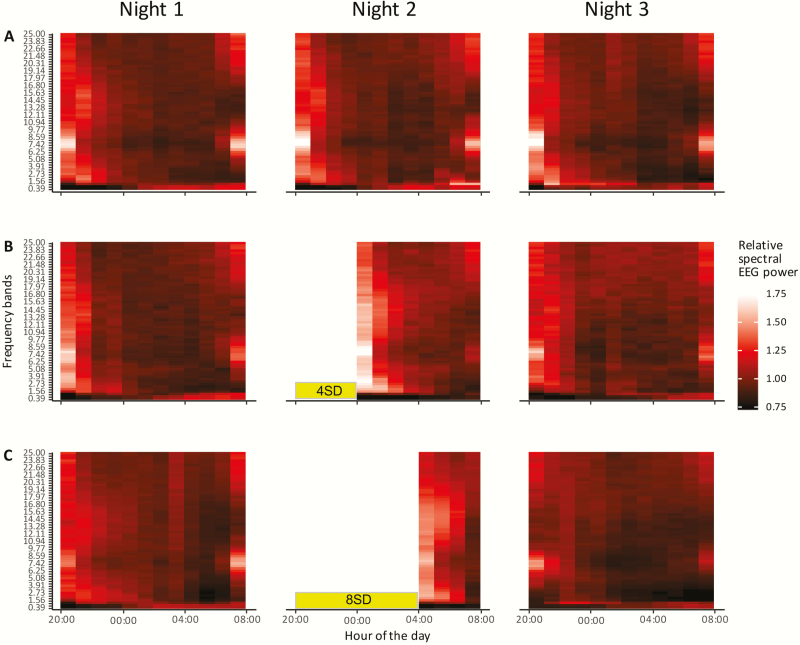
Figure 3.
Heatmap of normalized NREM sleep EEG spectral power during three consecutive nights. Y-axis shows EEG frequency from 0 to 25 Hz with a bandwidth of 0.39 Hz: X-axis shows the time of day. (A) Normalized spectral EEG heatmaps of the control treatment; (B) 4SD treatment; and (C) 8SD treatment. The sleep deprivation periods are indicated by the yellow bars. A brighter color with a value above 1 indicates a higher spectral power in a frequency bin compared with the average baseline dark phase power in that same frequency bin. A darker color with a value below 1 indicates a lower power in that frequency bin as compared with the average baseline dark phase power in that frequency bin.
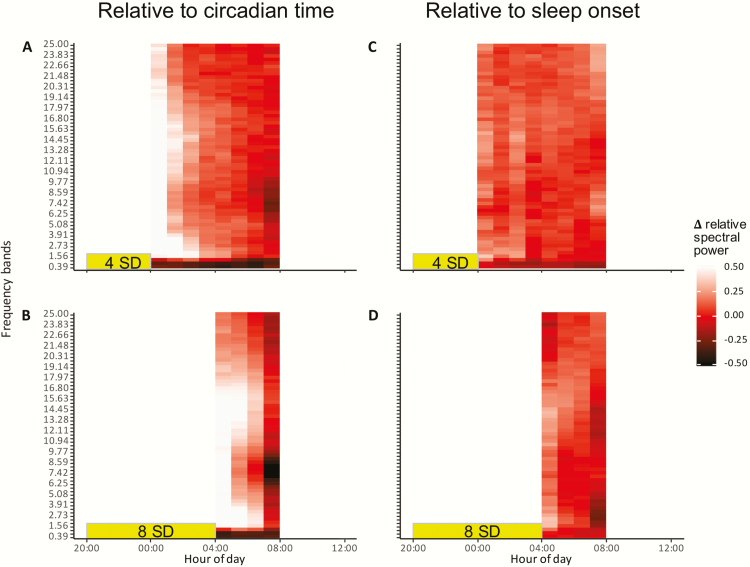
Figure 4.
Differences in normalized NREM sleep EEG spectral power between the experimental treatments and the control treatment on either the same clock time (panel A and C) or relative to sleep onset (panel B and D).
During baseline, the relative NREM sleep EEG power in a wide range of frequency bins between 1 and 25 Hz was highest at the beginning of the dark phase and then declined in the course of the night (Figures 3 and 5). In some frequency bins, spectral power slightly increased toward the end of the dark phase. In contrast, spectral power in the lower three frequency bins (0–1.17 Hz) showed an opposite pattern, with low power at the beginning of the dark phase and a gradual increase in the course of the night (Figures 3 and 5).
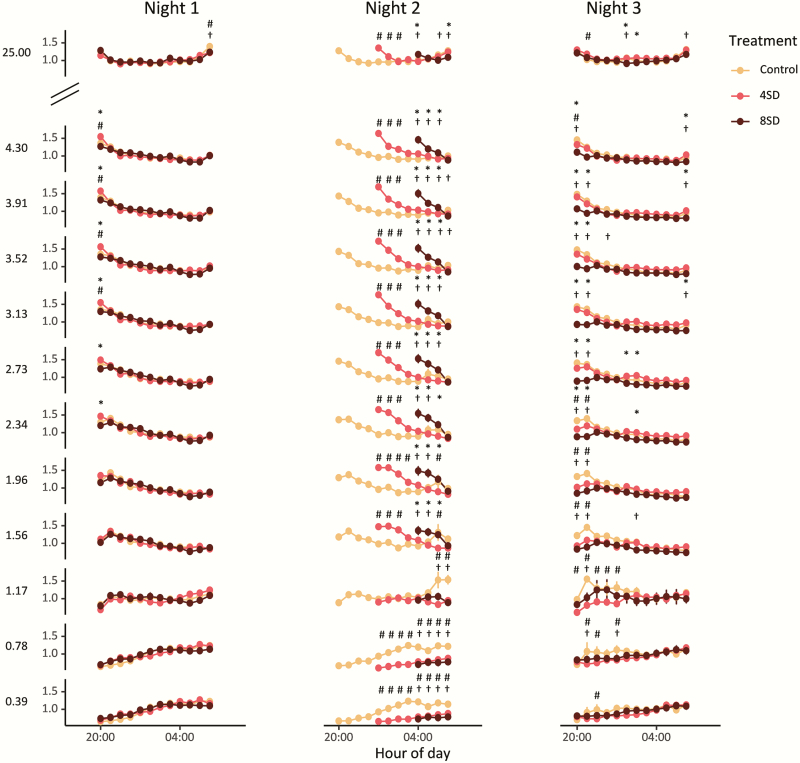
Figure 5.
Normalized EEG spectral power over the course of the three nights for the three different treatments (yellow = control, red = 4SD, and black = 8SD). The first 10 Hz bands are plotted (0.39–4.30 Hz) and the 25 Hz bands. After sleep deprivation an increase in spectral power over a broad range was visible (1.56–25 Hz) and a decrease occurs in the range of 0–0.78 Hz). All significant differences are indicated by the symbols * indicates 4SD–8SD, # indicates control–4SD, and † indicates control–8SD (lmer model and Tukey HSD posthoc test, symbols indicate p < 0.05).
After sleep deprivation of both 4 and 8 h, an increase in EEG spectral power occurred over a broad range of frequencies as compared with the power at the same time of the night under the control condition (Figures 4, A and C and 5). This increase occurred in a frequency range from 1.17 up to 25 Hz, but particularly in the ranges of 1.17–3 Hz and 11–18 Hz, the increase seemed to last longer (Figures 4, A and C and 5).
In contrast, EEG spectral power in the lowest frequency bins (0–0.78 Hz) showed an opposite pattern with decreased power after sleep deprivation as compared with the control condition at the same clock time and this decrease persisted for a large part of the night (Figures 4, A and C and 5). In the 0.78–1.17 Hz bin, no clear effect of sleep deprivation was visible (Figure 5).
When the relative NREM sleep EEG spectral power following sleep deprivation was compared with the spectral power following sleep–onset at the start of the night in the control condition, there were no significant differences (lmer model: treatment, F2,24=1.76, p = 0.194, Figure 4, B and D). In other words, spectral power after sleep deprivation did not increase beyond the levels seen at the beginning of the baseline night and the decrease in power in the course of sleep followed a similar pattern.
Importantly, contrary to the expectation that longer sleep deprivation would result in larger changes in EEG power, the changes in power that occurred after 4 and 8 h sleep deprivation were largely similar (Figure 5).
Discussion
Under controlled laboratory conditions with a 12 h light–12 h dark cycle, starlings displayed a pronounced daily rhythm in sleep and wakefulness with most of the sleep occurring during the dark phase. Sleep mainly consisted of NREM sleep. In fact, the amount of REM sleep displayed in the birds under these conditions was very low and amounted to no more than 1~2% of total sleep time. We successfully sleep deprived the starlings for 4 or 8 h by manual stimulation. Sleep deprivation resulted in a small compensatory increase in NREM sleep the day after and also induced clear changes in subsequent NREM sleep EEG spectral qualities, with increased spectral power over a broad frequency range above 1.17 Hz and a decrease in spectral power in the frequency range below 1.17 Hz when compared with the same time of the baseline night. There was no evidence that REM sleep that was lost during sleep deprivation was compensated.
We aimed to test homeostatic regulation of sleep in starlings by subjecting the birds to different durations of sleep deprivation during their normal night-time sleep phase. There was no immediate increase in sleep time during the remainder of the night immediately after sleep deprivation, presumably because levels of sleep already approached the maximum possible under baseline conditions, but the birds seemed to partly compensate for the loss of sleep by a delayed increase in NREM sleep time the next day. However, this increase in day-time napping was not nearly enough to compensate for the lost NREM sleep and, also, it was quantitatively similar after 4 and 8 h sleep deprivation. We continued the recordings for another 24 h but there was very little additional compensation for the loss of sleep during the second recovery night and day.
Part of the NREM sleep that was lost during sleep deprivation may have been compensated by an increase in sleep intensity, reflected in spectral changes in the EEG. In mammals, the intensity of NREM sleep is thought to be reflected in the amount of EEG slow waves and EEG spectral power in the slow 1–4 Hz delta range and was found to be an increasing function of the duration of prior wakefulness [13–16]. In the mammalian species studied, EEG slow-wave activity was increased after sleep deprivation and then gradually declined in the course of the sleep phase, suggesting a dissipating need for NREM sleep [8]. In our birds, sleep deprivation also caused changes in EEG spectral composition during subsequent sleep that lasted for several hours, which may suggest a sleep homeostatic response. However, these changes were not completely similar to what has been reported for mammals. First, whereas mammals most often show a predominant increase in power in the lower frequencies, the starlings showed a consistent increase in spectral power across a wide frequency range up to at least 25 Hz. While different from mammals, this finding is in line with previous EEG findings in other birds such as pigeons [36]. Strikingly, we found an unexpected drop in EEG spectral power for the slow frequencies below 1.17 Hz. Such complex changes in EEG spectral power after sleep deprivation clearly indicated that the mammalian delta power or slow-wave activity is not a universal indicator of sleep intensity that can be extended to all birds.
In our starlings, the 4 and 8 h sleep deprivation did not only induce similar increases in sleep time during recovery, but the changes in EEG spectral power were also largely similar for the two different durations of sleep deprivation. Thus, the spectral changes in the NREM sleep EEG did not clearly reflect the duration of prior wakefulness as reported for some mammalian species [8]. There are several possible explanations for this lack of a dose-dependent effect. One potential explanation is that the maximum sleep debt and maximum homeostatic sleep pressure was already reached after 4 h of sleep deprivation. A second potential explanation is that the build-up of sleep debt in relation to prior wakefulness was there but it was not proportionally reflected in the EEG during subsequent recovery sleep. This could be due to the fact that birds have a rather different organization of their neuronal networks than mammals [50, 51]. Hence, the build of sleep debt at the molecular and cellular level may translate differently to EEG changes in birds and mammals [52]. Both of these hypotheses could potentially be addressed using read-outs other than EEG to assess if sleep deprivation has dose-dependent effects on, for example, molecular markers, single cell-activity, arousal threshold, or behavioral performance.
A third explanation is that with longer sleep deprivation some of the sleep pressure that builds up starts “leaking” into the waking state, with scattered and perhaps local slow-waves appearing in the waking EEG such that there is no additional increase in SWA at the onset of true sleep. This phenomenon of sleep deprivation-induced slow-waves intruding the waking state has indeed been reported in mammals [53]. It would be hard to quantify this in the birds because of the frequent movement artifacts in the waking EEG but, also, because these waking-state slow-waves could go undetected with a restricted number of EEG electrodes when they occur locally on the background of global wakefulness.
A fourth explanation for the lack of a clear wake-duration dependent sleep response in our starlings is that sleep is not homeostatically regulated in this species. This explanation may not seem very likely because it is at odds with some of the most influential theories on sleep homeostasis and sleep function that proposes that sleep is a recovery process from prior wakefulness, for example, to replenish brain energy stores that were depleted in the course of wakefulness [54], or to downscale synapses that were potentiated during waking neuronal activity [55]. However, the view that sleep is homeostatically regulated in relation to the duration of prior wakefulness is largely based on studies in only a handful of mammalian species and no single theory is undisputed or unequivocally proven. Moreover, other major theories imply sleep may not necessarily depend on the quantity and duration of prior wakefulness but, instead, may be related to the quality of wakefulness, i.e. to process and store very specific waking experiences and to support learning and memory processes [6, 56]. Indeed, there are numerous studies showing that sleep may support the formation of specific memories, not only in mammals but in birds as well [57–59], particularly in relation to song learning [60, 61].
Moreover, while it is often assumed that sleep in mammals and other animals such as birds represent similar states that have a common evolutionary origin, it is not excluded that a primitive common sleep state evolved into more complex states with different functions in different taxonomic groups. Thus, homeostatic regulation of sleep in relation to the duration of wakefulness as it is found in mammals may not be present in exactly the same way in birds. In fact, this notion is supported by recent findings showing that birds under natural conditions may go with little to no sleep for many days or even weeks in a row, apparently sustaining normal behavior and performance [41, 42]. For example, an EEG study in wild frigate birds showed that these animals can spend up to 10 days on the wing foraging over sea with on average only 42 min sleep per day and it is unclear whether they compensate for any of the sleep lost in flight [42]. In another EEG study under natural conditions, it was shown that male pectoral sandpipers in the reproductive season get very little sleep during a 3-week period of intense competition for access to fertile females [41]. Interestingly, the males that slept the least ultimately produced the most offspring suggesting that decreased performance is not an inescapable outcome of sleep loss. These findings clearly challenge the generality of the common view of wake-dependent sleep homeostasis emerging from studies in mammals.
Indeed, another intriguing finding is that the starlings had very little REM sleep under baseline conditions and when that little bit was prevented by sleep deprivation it did not seem to be recovered. While the amount of REM sleep was low in all birds, there was some variation in between individuals, which may have been caused in part by variation in age, sex, and origin of the birds. However, the current study was not designed to address these specific variables.
Also, REM sleep was slightly lower during the second night compared with the first night in the control group, however, this did not reach statistical significance and may have reflected spontaneous day to day variation, especially since the overall amount of REM sleep is very low and a few epochs of REM sleep already make a difference. Another reason might be that the experimental manipulation of the sleep-deprived birds in the same room caused a mild suppression of REM sleep in the control animals.
The less than 2% REM sleep in our starlings agrees with an earlier study in this species reporting a similar minimal amount of REM sleep [62]. We initially anticipated that the low amount of REM sleep reported in this earlier study could have been an artifact, due to the measurement conditions. The birds were connected to a head cable for EEG recordings, possibly interfering with the expression of their natural sleep behavior. Such interference was less likely in the present study, given that our starlings were equipped with miniature dataloggers that posed no restrictions on their normal body posture and behavior.
Although the amount of REM sleep we found in starlings is among the lowest reported for birds, it is certainly not exceptional. Low amounts of REM sleep were reported for several other bird species from different orders, for example, the rook (less than 2% of total sleep time) [63], budgerigar (less than 4% of total sleep time) [64], turtle dove (less than 5% of total sleep time) [65], and quail (less than 6% of total sleep time) [66]. Overall, the amount of REM sleep in birds varies a great deal between species, ranging from the minimal amount in starlings and rooks to higher mammalian-like numbers in, for example, white-crowned sparrows (about 16% of total sleep-time) [67] and zebra finches (about 25% of total sleep time) [68]. It is yet unknown what is causing this variation in the amount of REM sleep among bird species but there does not appear to be a simple taxonomic explanation as illustrated by substantial differences even within orders, for example, between songbirds such as starlings and white-crowned sparrows or zebra finches.
The low amount of REM sleep in the starlings and the fact that sleep deprivation-induced loss of REM sleep was not compensated adds to ongoing discussions on how REM sleep is regulated and what its functions may be. The current data clearly do not support the view that REM sleep is homeostatically regulated and serves an important recovery function that relates to the duration of prior wakefulness or prior NREM sleep [22]. In fact, it appears that starlings housed under the controlled laboratory conditions can almost do without REM sleep and are therefore at odds with any theory on REM sleep function.
Supplementary Material
Acknowledgments
This study was supported by an Adaptive Life Program scholarship from the Groningen Institute for Evolutionary Life Sciences and an Ubbo Emmius scholarship provided by the Faculty of Science and Engineering at the University of Groningen.
Funding
NCR was supported by the Max Planck Society.
Conflict of interest statement. None declared.
References
- 1. Campbell SS, et al.. Animal sleep: a review of sleep duration across phylogeny. Neurosci Biobehav Rev. 1984;8(3):269–300. [DOI] [PubMed] [Google Scholar]
- 2. Lesku JA, et al.. Phylogenetics and the correlates of mammalian sleep: a reappraisal. Sleep Med Rev. 2008;12(3):229–244. [DOI] [PubMed] [Google Scholar]
- 3. Nath RD, et al.. The jellyfish cassiopea exhibits a sleep-like state. Curr Biol. 2017;27(19):2984–2990.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benington JH, et al.. Cellular and molecular connections between sleep and synaptic plasticity. Prog Neurobiol. 2003;69(2):71–101. [DOI] [PubMed] [Google Scholar]
- 5. Siegel JM. Clues to the functions of mammalian sleep. Nature. 2005;437(7063):1264–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Raven F, et al.. The role of sleep in regulating structural plasticity and synaptic strength: implications for memory and cognitive function. Sleep Med Rev. 2018;39:3–11. [DOI] [PubMed] [Google Scholar]
- 7. Benington JH. Sleep homeostasis and the function of sleep. Sleep. 2000;23(7):959–966. [PubMed] [Google Scholar]
- 8. Deboer T. Behavioral and electrophysiological correlates of sleep and sleep homeostasis. Curr Top Behav Neurosci. 2015;25:1–24. [DOI] [PubMed] [Google Scholar]
- 9. Blake H, Gerard RW. Brain potentials during sleep. Am J Physiol. 1937;119:692–703. [Google Scholar]
- 10. Friedman L, et al.. Effects of sleep deprivation on sleepiness, sleep intensity, and subsequent sleep in the rat. Sleep. 1979;1(4):369–391. [DOI] [PubMed] [Google Scholar]
- 11. Borbély AA, et al. . Sleep deprivation: effects on sleep and EEG in the rat. J Comp Physiol A 1979;133:71–87. [Google Scholar]
- 12. Borbély AA, et al.. Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981;51(5):483–495. [DOI] [PubMed] [Google Scholar]
- 13. Tobler I, et al.. Sleep EEG in the rat as a function of prior waking. Electroencephalogr Clin Neurophysiol. 1986;64(1):74–76. [DOI] [PubMed] [Google Scholar]
- 14. Dijk DJ, et al.. EEG power density during nap sleep: reflection of an hourglass measuring the duration of prior wakefulness. J Biol Rhythms. 1987;2(3):207–219. [DOI] [PubMed] [Google Scholar]
- 15. Franken P, et al.. Sleep homeostasis in the rat: simulation of the time course of EEG slow-wave activity. Neurosci Lett. 1991;130(2):141–144. [DOI] [PubMed] [Google Scholar]
- 16. Huber R, et al.. Effects of sleep deprivation on sleep and sleep EEG in three mouse strains: empirical data and simulations. Brain Res. 2000;857(1–2):8–19. [DOI] [PubMed] [Google Scholar]
- 17. Dement W. The effect of dream deprivation. Science. 1960;131(3415):1705–1707. [DOI] [PubMed] [Google Scholar]
- 18. Borbély AA, et al.. Effect of sleep deprivation on sleep and EEG power spectra in the rat. Behav Brain Res. 1984;14(3):171–182. [DOI] [PubMed] [Google Scholar]
- 19. Cartwright RD, et al.. Individual differences in response to REM deprivation. Arch Gen Psychiatry. 1967;16(3):297–303. [DOI] [PubMed] [Google Scholar]
- 20. Endo T, et al.. Selective REM sleep deprivation in humans: effects on sleep and sleep EEG. Am J Physiol. 1998;274(4):R1186–R1194. [DOI] [PubMed] [Google Scholar]
- 21. Coolen A, et al.. Telemetric study of sleep architecture and sleep homeostasis in the day-active tree shrew Tupaia belangeri. Sleep. 2012;35(6):879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Benington JH, et al.. Does the function of REM sleep concern non-REM sleep or waking? Prog Neurobiol. 1994;44(5):433–449. [DOI] [PubMed] [Google Scholar]
- 23. Benington JH. Debating how REM sleep is regulated (and by what). J Sleep Res. 2002;11(1):29–31; discussion 31. [DOI] [PubMed] [Google Scholar]
- 24. Franken P. Long-term vs. short-term processes regulating REM sleep. J Sleep Res. 2002;11(1):17–28. [DOI] [PubMed] [Google Scholar]
- 25. Ocampo-Garcés A, et al.. Homeostasis of REM sleep after total and selective sleep deprivation in the rat. J Neurophysiol. 2000;84(5):2699–2702. [DOI] [PubMed] [Google Scholar]
- 26. Roussel B, et al.. Effect of ambient temperature on the sleep-waking cycle in two strains of mice. Brain Res. 1984;294(1):67–73. [DOI] [PubMed] [Google Scholar]
- 27. Amici R, et al.. The influence of a heavy thermal load on REM sleep in the rat. Brain Res. 1998;781(1–2):252–258. [DOI] [PubMed] [Google Scholar]
- 28. Amici R, et al.. Cold exposure and sleep in the rat: REM sleep homeostasis and body size. Sleep. 2008;31(5):708–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rampin C, et al.. Immobilisation stress induces a paradoxical sleep rebound in rat. Neurosci Lett. 1991;126(2):113–118. [DOI] [PubMed] [Google Scholar]
- 30. Meerlo P, et al.. Restraint increases prolactin and REM sleep in C57BL/6J mice but not in BALB/cJ mice. Am J Physiol Regul Integr Comp Physiol. 2001;281(3):R846–R854. [DOI] [PubMed] [Google Scholar]
- 31. Sanford LD, et al.. Differential effects of controllable and uncontrollable footshock stress on sleep in mice. Sleep. 2010;33(5):621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rattenborg NC, et al. . Sleep research goes wild: new methods and approaches to investigate the ecology, evolution and functions of sleep. Philos Trans R Soc B. 2017;372:20160251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lesku JA, et al.. Avian sleep. Curr Biol. 2014;24(1):R12–R14. [DOI] [PubMed] [Google Scholar]
- 34. Beckers GJ, et al.. An in depth view of avian sleep. Neurosci Biobehav Rev. 2015;50:120–127. [DOI] [PubMed] [Google Scholar]
- 35. Jones SG, et al.. Homeostatic regulation of sleep in the white-crowned sparrow (Zonotrichia leucophrys gambelii). BMC Neurosci. 2008;9:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Martinez-Gonzalez D, et al.. Increased EEG spectral power density during sleep following short-term sleep deprivation in pigeons (Columba livia): evidence for avian sleep homeostasis. J Sleep Res. 2008;17(2):140–153. [DOI] [PubMed] [Google Scholar]
- 37. Rattenborg NC, et al.. Avian sleep homeostasis: convergent evolution of complex brains, cognition and sleep functions in mammals and birds. Neurosci Biobehav Rev. 2009;33(3):253–270. [DOI] [PubMed] [Google Scholar]
- 38. Lesku JA, et al.. Ostriches sleep like platypuses. PLoS One. 2011;6(8):e23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lesku JA, et al.. A phylogenetic analysis of sleep architecture in mammals: the integration of anatomy, physiology, and ecology. Am Nat. 2006;168(4):441–453. [DOI] [PubMed] [Google Scholar]
- 40. Roth TC 2nd, et al.. A phylogenetic analysis of the correlates of sleep in birds. J Sleep Res. 2006;15(4):395–402. [DOI] [PubMed] [Google Scholar]
- 41. Lesku JA, et al.. Adaptive sleep loss in polygynous pectoral sandpipers. Science. 2012;337(6102):1654–1658. [DOI] [PubMed] [Google Scholar]
- 42. Rattenborg NC, et al.. Evidence that birds sleep in mid-flight. Nat Commun. 2016;7:12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vyssotski AL, et al.. Miniature neurologgers for flying pigeons: multichannel EEG and action and field potentials in combination with GPS recording. J Neurophysiol. 2006;95(2):1263–1273. [DOI] [PubMed] [Google Scholar]
- 44. Vyssotski AL, et al.. EEG responses to visual landmarks in flying pigeons. Curr Biol. 2009;19(14):1159–1166. [DOI] [PubMed] [Google Scholar]
- 45. van der Borght K, et al.. Hippocampal cell proliferation across the day: increase by running wheel activity, but no effect of sleep and wakefulness. Behav Brain Res. 2006;167(1):36–41. [DOI] [PubMed] [Google Scholar]
- 46. Hagewoud R, et al.. Sleep deprivation impairs spatial working memory and reduces hippocampal AMPA receptor phosphorylation. J Sleep Res. 2010;19(2):280–288. [DOI] [PubMed] [Google Scholar]
- 47. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: 2017. https://www.r-project.org/ [Google Scholar]
- 48. Bates D, et al. . Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 49. Lenth RV. Least-squares means: the R package lsmeans. J Stat Softw. 2016;69(1):1–33. [Google Scholar]
- 50. Medina L, et al.. Do birds possess homologues of mammalian primary visual, somatosensory and motor cortices? Trends Neurosci. 2000;23(1):1–12. [DOI] [PubMed] [Google Scholar]
- 51. Avian Brain Nomenclature Consortium. Avian brains and a new understanding of vertebrate brain evolution. Nat Rev Neurosci. 2005;6(2):151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Van der Meij J, et al. . Intra-“cortical” activity during avian non-REM and REM sleep: variant and invariant traits between birds and mammals. SleepJ. 2019;42(2):zsy230. [DOI] [PubMed] [Google Scholar]
- 53. Vyazovskiy VV, et al.. Local sleep in awake rats. Nature. 2011;472(7344):443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Benington JH, et al.. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45(4):347–360. [DOI] [PubMed] [Google Scholar]
- 55. Tononi G, et al.. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10(1):49–62. [DOI] [PubMed] [Google Scholar]
- 56. Diekelmann S, et al.. The memory function of sleep. Nat Rev Neurosci. 2010;11(2):114–126. [DOI] [PubMed] [Google Scholar]
- 57. Jackson C, et al.. Dynamics of a memory trace: effects of sleep on consolidation. Curr Biol. 2008;18(6):393–400. [DOI] [PubMed] [Google Scholar]
- 58. Brawn TP, et al.. Sleep-dependent consolidation of auditory discrimination learning in adult starlings. J Neurosci. 2010;30(2):609–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Brawn TP, et al.. Sleep-dependent reconsolidation after memory destabilization in starlings. Nat Commun. 2018;9(1):3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Derégnaucourt S, et al.. How sleep affects the developmental learning of bird song. Nature. 2005;433(7027):710–716. [DOI] [PubMed] [Google Scholar]
- 61. Shank SS, et al.. Sleep and sensorimotor integration during early vocal learning in a songbird. Nature. 2009;458(7234):73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Szymczak JT. Sleep pattern in the starling (Sturnus vulgaris). Acta Physiol Pol. 1985;36(5–6):323–331. [PubMed] [Google Scholar]
- 63. Szymczak JT. Daily distribution of sleep states in the rook Corvus frugilegus. J Comp Physiol A. 1987;161(2):321–327. [DOI] [PubMed] [Google Scholar]
- 64. Ayala-Guerrero F. Sleep patterns in the parakeet Melopsittacus undulatus. Physiol Behav. 1989;46(5):787–791. [DOI] [PubMed] [Google Scholar]
- 65. Walker LE, et al.. A continuum of sleep and shallow torpor in fasting doves. Science. 1983;221(4606):194–195. [DOI] [PubMed] [Google Scholar]
- 66. Mexicano G, et al.. Sleep characteristics in the quail Coturnix coturnix. Physiol Behav. 2014;129:167–172. [DOI] [PubMed] [Google Scholar]
- 67. Rattenborg NC, et al.. Migratory sleeplessness in the white-crowned sparrow (Zonotrichia leucophrys gambelii). PLoS Biol. 2004;2(7):E212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Low PS, et al.. Mammalian-like features of sleep structure in zebra finches. Proc Natl Acad Sci USA. 2008;105(26):9081–9086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.