Abstract
Aim:
The present study aimed to evaluate the combined value of locoregional extension patterns (LEPs) and circulating cell-free Epstein–Barr virus (cf EBV) DNA for risk stratification of locoregionally advanced nasopharyngeal carcinoma (LA-NPC) to better guide therapeutic strategies.
Methods:
A total of 7227 cases of LA-NPC were reviewed retrospectively and classified into six groups according to their LEP (ascending, descending, or mixed type) and pre-treatment cf EBV-DNA load (⩾ versus <4000 copy/ml). Using a supervised statistical clustering approach, patients in the six groups were clustered into low, intermediate, and high-risk clusters. Progression-free survival (PFS), overall survival (OS), distant metastasis-free survival (DMFS), and locoregional relapse-free survival (LRRFS) were calculated using the Kaplan–Meier method and differences were compared using the log-rank test.
Results:
Survival curves for the low, intermediate, and high-risk clusters were significantly different for all endpoints. The 5-year survival rate for the low, intermediate, and high-risk clusters, respectively, were: PFS (83.5%, 73.2%, 62.6%, p < 0.001), OS (91.0%, 82.7%, 73.2%, p < 0.001), DMFS (92.3%, 83.0%, 73.4%, p < 0.001), and LRRFS (91.0%, 88.0%, 83.3%, p < 0.001). The risk clusters acted as independent prognostic factors for all endpoints. Among the patients in the high-risk cluster, neoadjuvant chemotherapy combined with concurrent chemoradiotherapy (CCRT) significantly improved the patients 5-year PFS (66.4% versus 57.9%, p = 0.014), OS (77.6% versus 68.6%; p < 0.002), and DMFS (76.6% versus 70.6%; p = 0.028) compared with those treated with CCRT.
Conclusion:
Our results could facilitate the development of risk-stratification and risk-adapted therapeutic strategies for patients with LA-NPC.
Keywords: Epstein–Barr virus, locoregional extension patterns, nasopharyngeal carcinoma, prognosis, risk stratification
Introduction
Nasopharyngeal carcinoma (NPC) is a distinct type of head and neck cancer with a strong correlation with Epstein-Barr virus (EBV) in endemic regions, aggressive natural locoregional history, and high risk of distant metastases. The highest incidence of NPC is observed in East and Southeast Asia.1,2 In the endemic areas, up to 80% of patients with newly diagnosed NPC present with locoregionally advanced (LA) disease.3,4 For such cases, platinum-based concurrent chemoradiotherapy (CCRT) is the main treatment modality.5–8 The 5-year overall survival (OS) rate for early-stage diseases is about 92.8–98.1%; however, the survival outcome of LA-NPC is still unsatisfactory, with a 5-year OS of 68.9–78.6%.9,10 Salvage treatments are challenging because of poor disease control.11,12 Currently, the standardized risk assessment for NPC is based primarily on the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) tumor-node-metastasis (TNM) staging system. However, given the intrinsic biological heterogeneity of tumors, patients with the same TNM staging have been observed to have markedly different survival outcomes. Therefore, the identification of other stratifying factors to accurately enable pre-treatment risk stratification and to facilitate therapeutic selection in LA-NPC is urgently required.
According to the locoregional extension patterns (LEPs) of NPC, LA-NPC can be divided into three subtypes with markedly different clinical biological behaviors, which emphasizes the natural characteristics of tumor progression in NPC, namely the ascending type, characterized as predominantly advanced local disease (T3–4) but with early-stage cervical lymph node disease (N0–1), and prone to suffer local failure; the descending type, characterized as predominantly advanced cervical lymph node disease (N2–3) but early-stage local disease (T1–2), and prone to suffer distant failure; and the mixed type, characterized as predominantly advanced local disease (T3–4) and advanced cervical lymph node disease (N2–3), and prone to suffer both local and distant failure.13–15 This suggests that the LEP-defined subtypes have the potential to provide additional biological information to the TNM staging system and could be used for prognosis prediction in LA-NPC.
In endemic regions, NPC is invariably associated with EBV infection. Theoretically, circulating cell-free (cf) EBV DNA could be released upon cancer cell apoptosis or necrosis.16–19 Thus, cf EBV DNA measurement is now used widely for its proven ability for population screening,20 prognostication,21–24 and tumor surveillance in patients with NPC.25–27 In particular, it was well demonstrated that the pre-treatment cf EBV DNA load correlates with the NPC tumor stage and survival.28–30 The complementary role of pre-treatment cf EBV DNA load in TNM staging for clinical prognostication has been determined in recent years,21,22 which suggests that this biomarker can capture crucial additional biological information regarding tumor intrinsic aggressiveness that is not presented by TNM staging.
Based on the above premise, both LEP-defined subtypes and pre-treatment cf EBV DNA load might have good value in reflecting the biological characteristics of tumor heterogeneity and might supplement the anatomical TNM staging system. In this context, the combination of those two factors might improve prognostication of NPC, which has not been well investigated. In the current study, we performed a real-world big-data intelligence platform-based study to determine the combined value of LEPs and pre-treatment cf EBV DNA load in risk stratification, with the aim of better guiding the individualized treatment of patients with LA-NPC.
Materials and methods
Data extraction and study population
Using an NPC-specific database of the big-data intelligence platform at the Sun Yat-sen University Cancer Center (SYSUCC), we reviewed a total of 10,126 consecutive patients with newly diagnosed, histologically proven, non-disseminated NPC who were diagnosed between April 2009 and December 2015. To facilitate real-world research, an automatically and dynamically updated big data intelligence platform incorporating detailed electronic health records data extracted from routine healthcare was established in 2015.31 This allows oncologists to select eligible patients accurately, collect clinicopathological and therapeutic information of the patient, and track follow up.
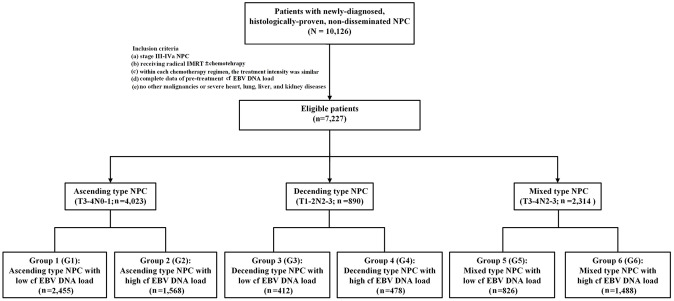
The inclusion criteria were as follows: (a) Stage III–IVa NPC (staged according to the 8th AJCC/UICC TNM staging system); (b) received radical intensity-modulated radiation therapy (IMRT) ± chemotherapy; (c) within each chemotherapy regimen, the treatment intensity was similar; (d) complete data of pre-treatment cf EBV DNA load; (e) no other malignancies or severe heart, lung, liver, and kidney diseases. Finally, a total of 7227 patients were found to be eligible. This study was reviewed and approved by the Institutional Review Board and the Research Ethics Committee of SYSUCC (Approval number: B2019-230-01). As our study was based on an analysis of patients’ routine clinical and treatment data, the research ethics committees of SYSUCC waived the requirement for individual informed consent.
Existing TNM staging cannot reasonably guide stratified treatment for patients with LA-NPC. Although CCRT is the standard treatment protocol for LA-NPC, controversy remains regarding the additional benefit of adding neoadjuvant chemotherapy (NACT) or adjuvant chemotherapy (ACT) to CCRT. Therefore, during the study period, our institutional guidelines recommended CCRT with or without NACT/ACT for LA-NPC. Within each chemotherapy regimen, the treatment intensity was similar. The CCRT regimen consisted of cisplatin administered during days 1, 22, and 43 of IMRT (weekly regimen, 80–100 mg/m2) for three cycles or weekly (triweekly regimen, 30–40 mg/m2) for six cycles, initiated on the first day of IMRT. A previous study demonstrated that the weekly regimen has comparable efficacy compared with a triweekly regimen for LA-NPC.32 NACT or ACT comprised cisplatin (80 mg/m2) with 5-fluorouracil (1000 mg/m2), cisplatin (75 mg/m2) with docetaxel (75 mg/m2), or cisplatin (60 mg/m2) with 5-fluorouracil (600 mg/m2) and docetaxel (60 mg/m2) every 3 weeks for two (8%) or three (92%) cycles. More details about the IMRT are provided in the supplemental methods. Reasons for not receiving recommended treatment regimens included age, organ dysfunction suggestive of intolerance to treatment, and an individual patient’s refusal.
Clinical staging and pre-treatment plasma EBV DNA assay
Before diagnosis and treatment, all patients underwent baseline evaluation, including physical examination, medical history, biochemistry and hematology profiles, quantitative analysis of pre-treatment cf EBV DNA, magnetic resonance imaging (MRI) with contrast of the suprasellar cistern to the collarbone, fiberoptic nasopharyngoscopy, chest radiography, chest radiography, abdominal sonography, and bone scintigraphy.
The quantitative cf EBV DNA assay was conducted in the laboratory of the Department of Molecular Diagnosis at SYSUCC.33 Our laboratory is experienced in measuring cf EBV DNA load, and was credentialed by Stanford University to offer qualitative cf EBV DNA assay as part of the EBV DNA-guided international NRG-HN001 trial for NPC [ClinicalTrials.gov identifier: NCT02135042]. In the present study, pre-treatment cf EBV DNA load was classified into a high- and low-risk group based on a cutoff value of 4000 copies/ml, which was identified and validated to have a powerful prognostic value in previously published studies.27,34 More details concerning the EBV DNA assay are described in the supplemental methods.
LEP-defined subtypes combined with pre-treatment cf EBV DNA load defined six groups
All patients with LA-NPC were classified into six groups based on their LEP-defined subtypes [ascending type (T3–4N0–1) versus descending type (T1–2N2–3) versus mixed type NPC (T3–4N2–3) and pre-treatment cf EBV DNA load (low, <4000 versus high, ⩾4000 copies/ml)] as follows: Group 1 (G1), ascending type NPC with a low cf EBV DNA load; group 2 (G2), ascending type NPC with a high cf EBV DNA load; group 3 (G3), descending type NPC with a low cf EBV DNA load; group 4 (G4), descending type NPC with a high cf EBV DNA load; group 5 (G5), mixed type NPC with a low cf EBV DNA load; and group 6 (G6): Mixed type NPC with a high cf EBV DNA load.
Population follow-up strategy and endpoints
After completing treatment, the patients attended regular follow-up appointments at least every 3 months during the first 3 years and every 6 months thereafter until their death. They were also followed via phone if their recent examinations were not recorded. Follow-up duration was calculated from the first day of therapy to last contact or death. The study primary endpoint was progression-free survival (PFS, defined as the time from treatment initiation to the first event or death from any cause). The secondary endpoints were overall survival (OS, defined as the time from the date of treatment initiation to death from any cause), distant metastasis-free survival (DMFS, defined as the time from the date of treatment initiation to first distant failure), and locoregional relapse-free survival (LRRFS, defined as from the date of treatment initiation to the first local or regional recurrence or both), respectively.
Statistical analysis
All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) v23.0 (IBM, Armonk, NY, USA) and R version 3.4.4 (http://www.r-project.org). All reported p values were two-sided, and a p value <0.05 was considered to demonstrate statistical significance. The patients’ clinicopathological and treatment characteristics were compared using the χ2 or Fisher’s exact test. Survival results were calculated using the Kaplan–Meier method and survival comparisons were made using the log-rank method. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using the Cox proportional hazards models. We performed supervised clustering according to relative intergroup HRPFS to reduce the number of six subgroups to three distinct risk clusters. The association between the redefined risk clusters, clinicopathological and treatment characteristics, and survival were also evaluated using univariable and multivariable Cox proportional hazards model. The discriminatory performance of the TNM staging system and the risk clusters was measured using the concordance index (C-index).
Results
Patient and treatment characteristics
A total of 7227 eligible patients with LA-NPC were identified (Figure 1), comprising 4023 (55.7%) patients with ascending type NPC, 890 (12.3%) patients with descending type NPC, and 2314 (32.0%) patients with mixed type NPC. Detailed clinical and treatment characteristics of the patients at baseline are summarized in Table 1. The median follow-up of the entire study was 50.8 months (interquartile range 39.4–65.4 months).
Figure 1.
Flowchart of patients included in the study.
Ascending type NPC: Patients with stage T3–4N0–1 NPC; descending type NPC: patients with stage T1–2N2–3 NPC; mixed type NPC: Patients with stage T3–4N2–3 NPC; low cf EBV DNA load, cf EBV DNA load <4000 copies/ml; high cf EBV DNA load, cf EBV DNA load ⩾4000 copies/ml.
Table 1.
Clinical and treatment characteristics of patients with ascending type, descending type, and mixed type nasopharyngeal carcinoma at baseline.
| Characteristic | Ascending type NPC (T3–4N0–1, n = 4023, %) | Descending type NPC (T1–2N2–3, n = 890, %) | Mixed type NPC (T3–4N2–3, n = 2314, %) | p value† |
|---|---|---|---|---|
| Age (years) | 0.188 | |||
| <45 | 1929 (47.9) | 449 (50.4) | 1156 (50.0) | |
| ⩾45 | 2094 (52.1) | 441 (49.6) | 1158 (50.0) | |
| Sex | 0.025 | |||
| Male | 2927 (72.8) | 675 (75.8) | 1746 (75.5) | |
| Female | 1096 (27.2) | 215 (24.2) | 568 (24.5) | |
| WHO histological type | 0.778 | |||
| Differentiated | 102 (2.5) | 19 (2.1) | 56 (2.4) | |
| Undifferentiated | 3921 (97.5) | 871 (97.9) | 2258 (97.6) | |
| T classification* | <0.001 | |||
| T1 | 0 (0.0) | 365 (40.9) | 0 (0.0) | |
| T2 | 0 (0.0) | 526 (59.1) | 0 (0.0) | |
| T3 | 2776 (69.0) | 0 (0.0) | 1603 (69.3) | |
| T4 | 1247 (31.0) | 0 (0.0) | 711 (30.7) | |
| N classification* | <0.001 | |||
| N0 | 705 (17.5) | 0 (0.0) | 0 (0.0) | |
| N1 | 3318 (82.5) | 0 (0.0) | 0 (0.0) | |
| N2 | 0 (0.0) | 530 (59.6) | 1505 (65.1) | |
| N3 | 0 (0.0) | 360 (40.4) | 807 (34.9) | |
| Overall Stage* | <0.001 | |||
| III | 2777 (69.0) | 530 (59.6) | 1024 (44.3) | |
| IV | 1246 (31.0) | 360 (40.4) | 1290 (55.7) | |
| Cigarette consumption | 0.001 | |||
| No | 2626 (65.3) | 548 (61.6) | 1403 (60.6) | |
| Yes | 1397 (34.7) | 342 (38.4) | 911 (39.4) | |
| Alcohol consumption | <0.001 | |||
| No | 3504 (87.1) | 727 (81.7) | 1970 (85.1) | |
| Yes | 519 (12.9) | 163 (18.3) | 344 (14.9) | |
| Family of cancer history | 0.068 | |||
| No | 2951 (73.4) | 682 (76.6) | 1740 (75.2) | |
| Yes | 1072 (26.6) | 208 (23.4) | 574 (24.8) | |
| Pre-treatment cf EBV DNA load, copy/ml | <0.001 | |||
| <4000 | 2455 (61.0) | 412 (46.3) | 826 (35.7) | |
| ⩾4000 | 1568 (39.0) | 478 (53.7) | 1488 (64.3) | |
| HGB (g/l) | 0.058 | |||
| <120 | 271 (6.7) | 60 (6.7) | 192 (8.3) | |
| ⩾120 | 3752 (93.3) | 830 (93.3) | 2122 (91.7) | |
| LDH (U/l) | <0.001 | |||
| <245 | 3805 (94.6) | 812 (91.2) | 2048 (88.5) | |
| ⩾245 | 218 (5.4) | 78 (8.8) | 266 (11.5) | |
| ALB (g/l) | 0.002 | |||
| <40 | 358 (8.9) | 80 (9.0) | 267 (11.5) | |
| ⩾40 | 3665 (91.1) | 810 (91.0) | 2047 (88.5) | |
| CRP (mg/l) | <0.001 | |||
| <1.0 | 1222 (30.4) | 296 (33.3) | 557 (24.1) | |
| 1.0–3.0 | 1544 (38.4) | 333 (37.4) | 840 (36.3) | |
| ⩾3.0 | 1257 (31.2) | 261 (29.3) | 917 (39.6) | |
| Treatment modality | <0.001 | |||
| IMRT alone | 202 (5.0) | 21 (2.4) | 62 (2.7) | |
| NACT+IMRT alone | 339 (8.4) | 89 (10.0) | 219 (9.5) | |
| CCRT | 1670 (41.5) | 315 (35.4) | 583 (25.2) | |
| NACT+CCRT | 1628 (40.5) | 417 (46.9) | 1313 (56.7) | |
| CCRT+ACT | 184 (4.6) | 48 (5.4) | 137 (5.9) |
Ascending type NPC: patients with stage T3–4N0–1 NPC; Descending type NPC: patients with stage T1–2N2–3 NPC; Mixed type NPC: patients with stage T3–4N2–3 NPC; Statistical comparisons between three types NPC were computed using the Chi-square test.
According to the 8th edition of the AJCC/UICC staging system.
Clinicopathologic and treatment characteristics were compared using the χ2 test, or Fisher’s exact test, a p-value of 0.05 indicates a significant difference.
ACT, adjuvant chemotherapy; ALB, albumin; CCRT, concurrent chemoradiotherapy; cf EBV DNA, circulating cell-free Epstein-Barr virus deoxyribonucleic acid; CRP, C-reactive protein; HGB, hemoglobin; IMRT, intensity modulated radiation therapy; LDH, lactate dehydrogenase; NACT, neoadjuvant chemotherapy; WHO, World Health Organization.
In terms of treatment characteristics, all patients received radical IMRT and 96.1% of patients received platinum-based chemotherapy. A majority of the patients received NACT followed by CCRT (n = 3358, 46.5%) or CCRT alone (n = 2568, 35.5%). During the follow-up period, 10.7% (775/7227) of patients experienced local-regional recurrence, 14.6% (1054/7227) experienced distant metastasis, and 15.3% (1109/7227) of patients died. The 5-year PFS, OS, DMFS, and LRRFS for the entire cohort were 75.1%, 84.0%, 84.7%, and 88.3%, respectively.
Subgroup survival analysis stratified by LEP-defined subtypes and pre-treatment cf EBV DNA load
A total of 2455 (34.0%), 1568 (21.7%), 412 (5.7%), 478 (6.6%), 826 (11.4%), and 1488 (20.6%) patients were categorized as G1–G6, respectively. Their detailed clinical characteristics are summarized in the Supplemental Table S1. The number of events for each group is listed in Supplemental Table S2. For G1–G6, the 5-year PFS values were 83.5%, 74.3%, 83.7%, 69.8%, 72.8%, and 62.6%, respectively (p < 0.001); the 5-year OS values were 90.9%, 84.1%, 91.4%, 80.3% 80.9%, and 74.2%, respectively (p < 0.001); the 5-year DMFS values were 92.5%, 84.6%, 91.5%, 77.9%, 82.7%, and 73.4%, respectively (p < 0.001); and the 5-year LRRFS values were 90.9%, 88.8%, 91.7%, 87.7%, 86.4%, and 83.3%, respectively (p < 0.001, Supplemental Table S3).
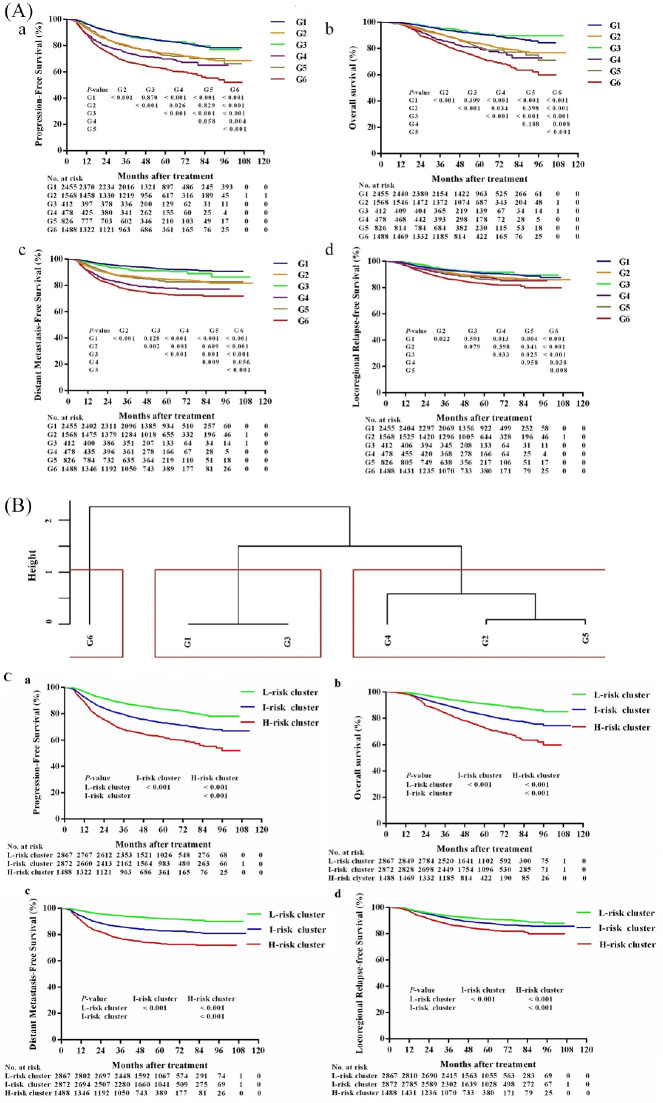
However, not all inter-group prognoses were significantly different, and the corresponding survival curves for G1–G6 were not completely separated (Figure 2A). Patients from G6 had the poorest PFS, OS, and LRRFS among the six groups. Patients from G1 and G3 had significantly better PFS, OS, DMFS, and LRRFS values compared with those for patients from G2, G4, G5, and G6. Patients in G1 had comparable PFS, OS, DMFS, and LRRFS values to those from patients in G3. In addition, patients in G2 had comparable PFS, OS, and LRRFS values to those from patients in G4 and G5.
Figure 2.
(A) Kaplan–Meier plots of survival outcomes for patients with locoregionally advanced nasopharyngeal carcinoma in six subgroups stratified by locoregional extension patterns combined with pre-treatment cf EBV DNA load. a PFS; b OS; cDMFS; d LRFS. G1 (group 1): The ascending type NPC (T3–4N0–1) with low cf EBV DNA load (<4000 copies/ml); G2 (group 2): The ascending type NPC with high cf EBV DNA load (⩾4000 copies/ml); G3 (group 3): The descending type NPC (T1) 2N2–3)with low cf EBV DNA load; G4 (group 4): The descending type NPC with high cf EBV DNA load; G5 (group 5): The mixed type NPC (T3–4N2–3) with low cf EBV DNA load; G6 (group 6): The mixed type NPC with high cf EBV DNA load. (B) G1–G6 were clustered into three risk clusters using a supervised clustering approach. Clustering analysis was performed according to the inter-group HRPFS to reduce the number of groups. We finally identified three distinct risk clusters: G1 and G3 were assigned into risk cluster 1 (low-risk cluster); G2, G4, and G5 were assigned into risk cluster 2 (intermediate-risk cluster); and G6 was assigned into risk cluster 3 (high-risk cluster). (C) Kaplan–Meier plots of survival outcomes for patients with locoregionally advanced nasopharyngeal carcinoma in three distinct risk clusters. a PFS; b OS; c DMFS; d LRFS.
DMFS, distant metastasis-free survival; H-risk, high-risk; HR, hazard ratio; I-risk, intermediate-risk; L-risk, low-risk; LRFS, locoregional relapse-free survival; OS, overall survival; PFS, progression-free survival
Construction of three distinct risk clusters to predict differential prognosis
Considering the overlap in survival curves between G1–G6 (Figure 2A), clustering analyses were performed according to the relative HRPFS between each group to reduce the number of groups. We finally identified three distinct risk clusters (Figure 2B): G1 and G3 were assigned into risk cluster 1 (n = 2867); G2, G4, and G5 were assigned into risk cluster 2 (n = 2872); and G6 was assigned into risk cluster 3 (n = 1488).
According to the survival rates, risk clusters 1, 2, and 3 were termed as low, intermediate, and high-risk clusters, respectively. At the last follow up, for the low, intermediate, and high-risk clusters, 238 (8.3%), 315 (11.0%), and 222 (14.9%) patients had experienced local-regional recurrence, respectively (p < 0.001); and 208 (7.3%), 467 (16.3%), and 379 (25.5%) had experienced distant metastasis, respectively (p < 0.001, Supplemental Table S4). Their respective 5-year PFS values were 83.5%, 73.2%, and 62.6% (p < 0.001); their 5-year OS values were 91.0%, 82.7%, and 73.2% (p < 0.001); their 5-year DMFS values were 92.3%, 83.0%, and 73.4% (p < 0.001); and their 5-year LRRFS values were 91.0%, 88.0%, and 83.3% (p < 0.001, Supplemental Table S5). Survival curves for all endpoints were significantly segregated among patients in the three risk clusters (Figure 2C).
The risk clusters (low versus intermediate versus high-risk cluster) developed in our study showed better discrimination performance than the 8th UICC/AJCC TNM staging system (stage III versus stage IVa): The C-index scores of 8th UICC/AJCC TNM staging system for PFS, OS, DMFS, and LRRFS prediction were 0.582 (95% CI, 0.558–0.606), 0.598 (95% CI, 0.568–0.629), 0.591 (95% CI, 0.561–0.622), and 0.572 (95% CI, 0.533–0.612), respectively; and the C-index scores of the risk clusters developed in our study for PFS, OS, DMFS, and LRRFS prediction were 0.607 (95% CI, 0.583–0.632), 0.622 (95% CI, 0.59–0.653), 0.643 (95% CI, 0.613–0.673), and 0.573 (95% CI, 0.537–0.609), respectively.
The univariate analysis results are illustrated in Supplemental Table S6, which showed a significant association between the established risk clusters and PFS, OS, DMFS, and LRRFS The results of multivariate analyses (Table 2) indicated that the risk clusters were independent risk factors for PFS, OS, DMFS, and LRRFS. Compared with those in the low-risk cluster, patients in the intermediate-risk cluster (HRPFS = 1.506, 95% CI: 1.328–1.709, p < 0.001; HROS = 1.816, 95% CI: 1.551–2.126, p < 0.001; HRDMFS = 1.919, 95% CI: 1.609–2.288, p < 0.001; HRLRRFS = 1.362, 95% CI: 1.148–1.616, p < 0.001) and high-risk cluster (HRPFS = 2.072, 95% CI: 1.749–2.454, p < 0.001; HROS = 2.579, 95% CI: 2.170–3.066, p < 0.001; HRDMFS = 2.642, 95% CI: 2.112–3.307, p < 0.001; HRLRRFS = 1.984, 95% CI: 1.644–2.394, p < 0.001) had significantly inferior PFS, OS, DMFS, and LRRFS.
Table 2.
Summary of multivariate Cox proportional hazard regression analysis of independent prognostic factors for 7227 locoregionally advanced nasopharyngeal carcinomas.
| Endpoint | Characteristic | HR (95% CI) | p |
|---|---|---|---|
| PFS | |||
| Age (⩾45 versus <45, years) | 1.191 (1.082–1.311) | <0.001 | |
| Gender (Female versus Male) | 0.793 (0.699–0.901) | <0.001 | |
| WHO histological type (Undifferentiated versus Differentiated) | 0.639 (0.502–0.812) | <0.001 | |
| N classification | 0.002 | ||
| (N1 versus N0) | 1.466 (1.183–1.817) | <0.001 | |
| (N2 versus N0) | 1.555 (1.225–1.973) | <0.001 | |
| (N3 versus N0) | 1.415 (1.104–1.813) | 0.006 | |
| Overall Stage (IV versus III) | 1.702 (1.518–1.908) | <0.001 | |
| Risk clusters | <0.001 | ||
| (Intermediate-risk versus Low-risk cluster) | 1.506 (1.328–1.709) | <0.001 | |
| (High-risk versus Low-risk cluster) | 2.072 (1.749–2.454) | <0.001 | |
| Cigarette consumption (Yes versus No) | 1.128 (1.015–1.254) | 0.025 | |
| ALB (⩾40 versus <40 g/l) | 0.777 (0.674–0.895) | <0.001 | |
| LDH (⩾245 versus <245 U/l) | 1.331 (1.144–1.549) | <0.001 | |
| Treatment modality | <0.001 | ||
| (IMRT alone versus CCRT) | 1.481 (1.170–1.875) | 0.001 | |
| (NACT+IMRT alone versus CCRT) | 0.997 (0.842–1.182) | 0.976 | |
| (NACT+CCRT versus CCRT) | 0.821 (0.735–0.917) | <0.001 | |
| (CCRT+ACT versus CCRT) | 1.064 (0.862–1.314) | 0.563 | |
| OS | |||
| Age (⩾45 versus <45, years) | 1.468 (1.299–1.658) | <0.001 | |
| Gender (Female versus Male) | 0.737 (0.632–0.860) | <0.001 | |
| WHO histological type (Undifferentiated versus Differentiated) | 0.591 (0.447–0.781) | <0.001 | |
| Overall Stage (IV versus III) | 1.799 (1.583–2.046) | <0.001 | |
| Risk clusters | <0.001 | ||
| (Intermediate-risk versus Low-risk cluster) | 1.816 (1.551–2.126) | <0.001 | |
| (High-risk versus Low-risk cluster) | 2.579 (2.170–3.066) | <0.001 | |
| HGB (120 versus <120 g/l) | 0.747 (0.597–0.934) | 0.010 | |
| ALB (⩾40 versus <40 g/l) | 0.781 (0.655–0.932) | 0.006 | |
| LDH (⩾245 versus <245 U/l) | 1.475 (1.234–1.764) | <0.001 | |
| CRP | 0.021 | ||
| (1.0–3.0 versus <1.0 mg/l) | 1.075 (0.916–1.261) | 0.378 | |
| (⩾3.0 versus <1.0 mg/l) | 1.234 (1.053–1.446) | 0.009 | |
| Treatment modality | <0.001 | ||
| (IMRT alone versus CCRT) | 1.577 (1.195–2.080) | 0.001 | |
| (NACT+IMRT alone versus CCRT) | 0.992 (0.805–1.222) | 0.939 | |
| (NACT+CCRT versus CCRT) | 0.830 (0.723–0.954) | <0.001 | |
| (CCRT+ACT versus CCRT) | 1.019 (0.780–1.331) | 0.890 | |
| DMFS | |||
| Gender (Male versus Female) | 0.636 (0.541–0.748) | <0.001 | |
| WHO histological type (Undifferentiated versus Differentiated) | 0.660 (0.483–0.903) | 0.009 | |
| N classification | <0.001 | ||
| (N1 versus N0) | 1.874 (1.331–2.639) | <0.001 | |
| (N2 versus N0) | 2.073 (1.439–2.984) | <0.001 | |
| (N3 versus N0) | 2.231 (1.537–3.240) | <0.001 | |
| Overall Stage (IV versus III) | 1.565 (1.344–1.822) | <0.001 | |
| Risk clusters | <0.001 | ||
| (Intermediate-risk versus Low-risk cluster) | 1.919 (1.609–2.288) | <0.001 | |
| (High-risk versus Low-risk cluster) | 2.642 (2.112–3.307) | <0.001 | |
| HGB (⩾120 versus <120 g/l) | 0.758 (0.601–0.956) | 0.019 | |
| ALB (⩾40 versus <40 g/l) | 0.774 (0.646–0.928) | 0.006 | |
| LDH (⩾245 versus <245 U/l) | 1.547 (1.292–1.852) | <0.001 | |
| Treatment modality | <0.001 | ||
| (IMRT alone versus CCRT) | 1.464 (1.067–2.007) | 0.018 | |
| (NACT+IMRT alone versus CCRT) | 0.991 (0.794–1.236) | 0.933 | |
| (NACT+CCRT versus CCRT) | 0.814 (0.705–0.940) | 0.005 | |
| (CCRT+ACT versus CCRT) | 1.167 (0.899–1.515) | 0.246 | |
| LRRFS | |||
| Age (⩾45 versus <45, years) | 1.179 (1.022–1.359) | 0.024 | |
| WHO histological type (Undifferentiated versus Differentiated) | 0.629 (0.439–0.902) | 0.012 | |
| T classification | <0.001 | ||
| (T2 versus T1) | 1.407 (0.899–2.200) | 0.135 | |
| (T3 versus T1) | 1.006 (0.687–1.472) | 0.976 | |
| (T4 versus T1) | 1.707 (1.161–2.511) | 0.007 | |
| Cigarette consumption (Yes versus No) | 1.162 (1.006–1.343) | 0.041 | |
| Risk clusters | <0.001 | ||
| (Intermediate-risk versus Low-risk cluster) | 1.362 (1.148–1.616) | <0.001 | |
| (High-risk versus Low-risk cluster) | 1.984 (1.644–2.394) | <0.001 | |
ACT, adjuvant chemotherapy; ALB, albumin; HGB, hemoglobin; CCRT, concurrent chemoradiotherapy; CI, confidence intervals; CRP, C-reactive protein; DMFS, distant metastasis-free survival; HR, hazard ratio; IMRT, intensity modulated radiation therapy; LDH, lactate dehydrogenase; LRRFS, locoregional relapse-free survival; NACT, neoadjuvant chemotherapy; OS, overall survival; PFS, progression-free survival; WHO, World Health Organization.
Individualized therapeutic strategies based on three risk clusters
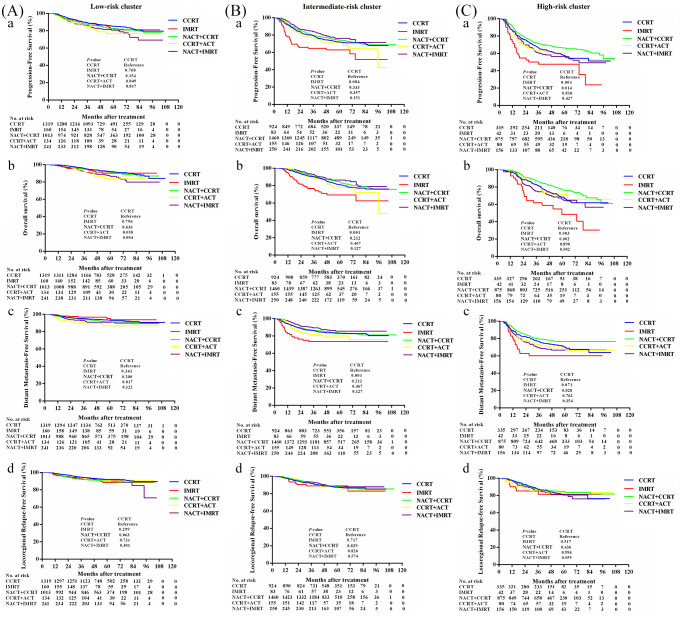
We further explored the best individualized treatment regimens for the three risk clusters by comparing the efficacy of other treatment regimens with the standard treatment regimen “CCRT” (Figure 3). Patients in the high-risk groups were likely to benefit from therapy intensification with NACT+CCRT over CCRT in terms of PFS, OS, DMFS; however, we did not observe a superiority for treatment modality over CCRT in the low and intermediate-risk clusters. Within the low-risk and intermediate-risk clusters, patients receiving IMRT alone, NACT+CCRT, CCRT+ACT, or NACT+IMRT did not show significantly better survival compared with those who received the standard treatment modality of CCRT. For the high-risk cluster, NACT+CCRT was superior to CCRT in terms of PFS (5-year PFS: 66.4% versus 57.9%; HR = 0.775, 95% CI: 0.632–0.949; p = 0.014), OS (5-year OS: 77.6% versus 68.6%; HR = 0.684, 95% CI: 0.535–0.873; p < 0.002), and DMFS (5-year DMFS: 76.6% versus 70.6%; HR = 0.759, 95% CI: 0.593–0.971; p = 0.028). However, patients treated with NACT+CCRT and CCRT had comparable LRRFS values (5-year LRRFS: 84.7% versus 80.6%; HR = 0.879, 95% CI: 0.636–1.215; p = 0.436). In the high-risk cluster, patients who received IMRT alone, CCRT+ACT, or NACT+IMRT did not show significantly better survival compared with those who received the standard treatment modality of CCRT.
Figure 3.
Efficacies of different treatment regimens for patients in low-risk (A), intermediate-risk (B), and high-risk (C) clusters. a PFS; b OS; c DMFS; d LRFS.
ACT, adjuvant chemotherapy; CCRT, concurrent chemoradiotherapy; DMFS, distant metastasis-free survival; IMRT, intensity modulated radiation therapy; LRFS, locoregional relapse-free survival; NACT, neoadjuvant chemotherapy; OS, overall survival; PFS, progression-free survival.
When adjusting for other factors at multivariate analysis, treatment modality (NACT+CCRT versus CCRT) was identified as an independent prognostic factor for PFS (HR = 0.690; 95% CI, 0.561–0.849; p < 0.001), OS (HR = 0.652; 95% CI, 0.509–0.833; p = 0.001), and DMFS (HR = 0.677; 95% CI, 0.528–0.870; p = 0.002; Table 3).
Table 3.
Summary of multivariate Cox proportional hazard regression analysis of independent prognostic factors for locoregionally advanced nasopharyngeal carcinoma in high-risk cluster.
| Endpoint | Characteristic | HR (95% CI) | p |
|---|---|---|---|
| PFS | |||
| T classification (T4 versus T3) | 1.375 (1.047–1.806) | 0.022 | |
| LDH (⩾245 versus <245 U/l) | 1.323 (1.064–1.645) | 0.012 | |
| ALB (⩾40 versus <40 g/l) | 0.664 (0.531–0.829) | <0.001 | |
| Treatment modality | <0.001 | ||
| (IMRT alone versus CCRT) | 1.672 (1.077–2.593) | 0.022 | |
| (NACT+IMRT alone versus CCRT) | 1.040 (0.778–1.390) | 0.792 | |
| (NACT+CCRT versus CCRT) | 0.690 (0.561–0.849) | <0.001 | |
| (CCRT+ACT versus CCRT) | 0.967 (0.660–1.417) | 0.862 | |
| OS | |||
| Age (⩾45 versus <45, years) | 1.257 (1.017–1.553) | 0.034 | |
| N classification (N3 versus N2) | 1.301 (1.060–1.597) | 0.012 | |
| LDH (⩾245 versus <245 U/l) | 1.404 (1.088–1.812) | 0.009 | |
| ALB (⩾40 versus <40 g/l) | 0.727 (0.553–0.957) | 0.023 | |
| Treatment modality | <0.001 | ||
| (IMRT alone versus CCRT) | 1.827 (1.134–2.945) | 0.013 | |
| (NACT+IMRT alone versus CCRT) | 1.063 (0.756–1.493) | 0.727 | |
| (NACT+CCRT versus CCRT) | 0.652 (0.509–0.833) | 0.001 | |
| (CCRT+ACT versus CCRT) | 0.860 (0.540–1.369) | 0.525 | |
| DMFS | |||
| Gender (Male versus Female) | 0.747 (0.563–0.992) | 0.044 | |
| Cigarette consumption (Yes versus No) | 1.331 (1.087–1.628) | 0.006 | |
| LDH (⩾245 versus <245U/l) | 1.476 (1.146–1.900) | 0.003 | |
| ALB (⩾40 versus<40 g/l) | 0.708 (0.539–0.929) | 0.013 | |
| Treatment modality | <0.001 | ||
| (IMRT alone versus CCRT) | 1.588 (0.933–2.703) | 0.088 | |
| (NACT+IMRT alone versus CCRT) | 1.095 (0.776–1.545) | 0.606 | |
| (NACT+CCRT versus CCRT) | 0.677 (0.528–0.870) | 0.002 | |
| (CCRT+ACT versus CCRT) | 0.947 (0.603–1.487) | 0.813 | |
| LRRFS | |||
| T classification (T4 versus T3) | 1.581 (1.211–2.063) | <0.001 | |
High-risk cluster: mixed type NPC (T3–4N2–3) with high cf EBV DNA load (⩾4000 copies/ml).
ACT, adjuvant chemotherapy; ALB, albumin; CRP, C-reactive protein; CCRT, concurrent chemoradiotherapy; CI, confidence intervals; DMFS, distant metastasis-free survival; HR, hazard ratio; IMRT, intensity modulated radiation therapy; LDH, lactate dehydrogenase; LRRFS, locoregional relapse-free survival; NACT, neoadjuvant chemotherapy; OS, overall survival; PFS, progression-free survival.
Discussion
In this real-world study, we comprehensively evaluated the combined value of LEP-defined subtypes and pre-treatment cf EBV DNA load in 7227 cases of LA-NPC for risk-stratification and therapeutic strategies optimization. Taking these two factors into consideration, patients were classified into six groups (G1–G6). Using a supervised clustering approach, G1–G6 were further clustered into three risk clusters based on the differences in PFS. The constructed easy-to-use risk clusters showed good performance, with excellent discrimination capability for PFS, DMFS, OS, and LRRFS. The low, intermediate, and high-risk clusters demonstrated significantly different inter-group prognoses across all clinical endpoints. Importantly, compared with the UICC/AJCC TNM staging system, the advantage of our current study was that the respective risk clusters were associated with the efficacy of different treatment strategies. We presented a prognostic stratification system that could be used to stratify risk and for risk-adaption of treatment for patients with LA-NPC.
Several previous prognostic models have been proposed for LA-NPC.35–37 Most of these models focused on several clinicopathological variables or molecular markers. For example, Tang et al. developed a nomogram based on a gene signature consisting of 13 genes, gender, N stage, serum lactate dehydrogenase (LDH), and C-reactive protein (CRP) to predict DMFS in LA-NPC.35 Ouyang et al. built a nomogram that integrated TNM stage, dose volume histogram parameters and N classification, age, gross primary tumor volume, and body mass index to predict OS in LA-NPC.36 Compared with those studies, our current research has several advantages. First, those previous models were relatively complicated to handle in the clinical setting because not all the variables mentioned above were routinely and easily tested in clinical practice for NPC before treatment. In our study, since the information of the LEP-defined subtypes and pre-treatment cf EBV DNA load is available in routine clinical practice, it can be used as a non-invasive, cost-effective, and convenient method for risk stratification. Second, a nomogram provides graphical depictions of variables in the predictive statistical model and requires the user to manually compute output individual risk scores. Compared with previous nomogram-based predictive models,35–37 our constructed risk clusters are a simpler and easy-to-use prognosis prediction tool for LA-NPC. Last, compared with serological markers such as LDH and CRP, which were included in the analyses in previous studies and could have been strongly influenced by inflammation or other diseases, cf EBV-DNA is relatively stable.
CCRT is the standard treatment protocol for LA-NPC (stage III and IVa NPC).5–8 With the use of CCRT, although the rates of locoregional relapse have decreased, distant metastasis has become the leading source of treatment failure.38,39 Thus, the value of additional therapy has been explored. However, controversy remains regarding the additional benefit of adding NACT or ACT to CCRT because of the inconsistent results of several prospective randomized trials.40–46 One possible reason was that the treatment decisions were based mainly on the TNM staging system, which has the limitation of being anatomy-based rather than reflecting the intrinsic biological heterogeneity of tumors. Although LEP of NPC is also classified based on anatomical T and N staging, it can divide patients with LA-NPC into three subtypes with markedly different clinical biological behaviors, which suggests that the LEP-defined subtypes might have the potential to provide additional biological information for NPC.
Existing TNM staging cannot guide stratified treatment for patients with LA-NPC (stage III and IVa NPC). Several previous studies demonstrated survival benefits gained from treatment intensification in the “high-risk” subgroup for LA-NPC, identified by the construction of their respective prognostic models.47–49 Here, the constructed risk clusters corresponded to the efficacy of different treatment strategies; we did not observe a superiority for treatment modality over CCRT in the low and intermediate-risk clusters. Notably, within the low-risk cluster, there was no significant difference in prognosis between IMRT alone and CCRT in terms PFS, OS, DMFS, and LRRFS. For the intermediate-risk cluster, CCRT was superior to IMRT alone in terms of PFS, OS, and DMFS. Patients in the high-risk group were likely to benefit from therapy intensification with NACT+CCRT over CCRT alone in terms of PFS, OS, and DMFS. Based on our findings for the three risk clusters, the following risk-adapted therapeutic strategies were recommended for patients with LA-NPC: for the low-risk cluster: IMRT alone or CCRT; for the intermediate-risk cluster: CCRT; for the high-risk cluster: NACT+CCRT. Our results suggested a potential method of individualizing treatment in clinical practice.
The present study had a few limitations. First, the retrospective nature of this study and the possible selection bias mean that a prospective study is still warranted to confirm our findings. However, considering the large patient sample size and that the collected real-word data have the advantage of reflecting the real situation, we believe that our results are credible. Second, our results are from a single-center and were not validated in another dataset. In the future, a well-designed, multicenter study is needed to validate our findings.
Conclusion
In conclusion, we performed a combined analysis of the prognostic value of LEP-defined subtypes and pre-treatment cf EBV DNA load in 7227 cases of LA-NPC in the endemic area and generated three risk clusters, which presented excellent discrimination capability for PFS, DMFS, OS, and LRRFS rates. This prognostic stratification system indicated risk-adapted treatment in LA-NPC: IMRT alone or CCRT for low-risk cluster; CCRT for intermediate-risk cluster; NACT followed by CCRT for high-risk cluster.
Supplemental Material
Supplemental material, Clean_Supplementary_materials for Risk stratification for nasopharyngeal carcinoma: a real-world study based on locoregional extension patterns and Epstein-Barr virus DNA load by Lu-Lu Zhang, Meng-Yao Huang, Fei-Xu, Ke-Xin Wang, Di Song, Ting Wang, Li-Yue Sun and Jian-Yong Shao in Therapeutic Advances in Medical Oncology
Acknowledgments
We sincerely thank Wei Liang (Yidu Cloud Technology Ltd., Beijing, China) for providing technical support in extracting study data from their big data intelligence database platform.
Footnotes
Availability of data and materials: The authenticity of this article has been validated by uploading the key raw data onto the Research Data Deposit (RDD) public platform (http://www.researchdata.org.cn), with the RDD approval number of RDDA2020001441.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Natural Science Foundation of China (No. 81472522) and the China Postdoctoral Science Foundation Grant (No. 2019M663305).
ORCID iD: Jian-Yong Shao  https://orcid.org/0000-0002-0171-908X
https://orcid.org/0000-0002-0171-908X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Lu-Lu Zhang, Department of Molecular Diagnostics, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Collaborative Innovation Center for Cancer Medicine, Guangzhou, People’s Republic of China.
Meng-Yao Huang, School of Mathematics, Sun Yat-Sen University, Guangzhou, People’s Republic of China.
Fei-Xu, Department of Molecular Diagnostics, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Collaborative Innovation Center for Cancer Medicine, Guangzhou, People’s Republic of China.
Ke-Xin Wang, School of Basic Medicine, GanNan Medical University, Guangzhou, People’s Republic of China.
Di Song, Department of Molecular Diagnostics, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Collaborative Innovation Center for Cancer Medicine, Guangzhou, People’s Republic of China.
Ting Wang, Department of Molecular Diagnostics, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Collaborative Innovation Center for Cancer Medicine, Guangzhou, People’s Republic of China.
Li-Yue Sun, Department of Molecular Diagnostics, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Collaborative Innovation Center for Cancer Medicine, Guangzhou, People’s Republic of China.
Jian-Yong Shao, Department of Molecular Diagnostics, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Collaborative Innovation Center for Cancer Medicine, 651 Dongfeng East Road, Guangzhou, 510060, People’s Republic of China.
References
- 1. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87e108. [DOI] [PubMed] [Google Scholar]
- 2. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 3. Mao YP, Xie FY, Liu LZ, et al. Re-evaluation of 6th edition of AJCC staging system for nasopharyngeal carcinoma and proposed improvement based on magnetic resonance imaging. Int J Radiat Oncol Biol Phys 2009; 73: 1326–1334. [DOI] [PubMed] [Google Scholar]
- 4. Wei KR, Zheng RS, Zhang SW, et al. Nasopharyngeal carcinoma incidence and mortality in China in 2010. Chin J Cancer 2014; 33: 381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin JC, Jan JS, Hsu CY, et al. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol 2003; 21: 631–637. [DOI] [PubMed] [Google Scholar]
- 6. Wee J, Tan EH, Tai BC, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American joint committee on cancer/international union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol 2005; 23: 6730–6738. [DOI] [PubMed] [Google Scholar]
- 7. Lee AW, Tung SY, Chua DT, et al. Randomized trial of radiotherapy plus concurrent adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst 2010; 102: 1188–1198. [DOI] [PubMed] [Google Scholar]
- 8. Blanchard P, Lee A, Marguet S, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol 2015; 16: 645–655. [DOI] [PubMed] [Google Scholar]
- 9. Su SF, Han F, Zhao C, et al. Intensity-modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two-dimensional radiotherapy: a 10-year experience with a large cohort and long follow-up. Eur J Cancer 2015; 51: 2587–2595. [DOI] [PubMed] [Google Scholar]
- 10. Zhang MX, Li J, Shen GP, et al. The seventh edition of the UICC/AJCC staging system for nasopharyngeal carcinoma is prognostically useful for patients treated with intensity-modulated radiotherapy from an endemic area in China. Radiother Oncol 2012; 104: 331–337. [DOI] [PubMed] [Google Scholar]
- 11. Lee AWM, Ng WT, Chan JYW, et al. Management of locally recurrent nasopharyngeal carcinoma. Cancer Treat Rev 2019; 79: 101890. [DOI] [PubMed] [Google Scholar]
- 12. Chan OS, Ngan RK. Individualized treatment in stage IVC nasopharyngeal carcinoma. Oral Oncol 2014; 50: 791–797. [DOI] [PubMed] [Google Scholar]
- 13. Xie ZG, Li ZQ. Natural development of nasopharyngeal carcinoma and clinical types of late stage cases. Tientsin Med J 1963; 1: 129–131. [Google Scholar]
- 14. Li ZQ, Xia YF, Liu Q, et al. Radiotherapy-related typing in 842 patients in Canton with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2006; 66: 1011–1016. [DOI] [PubMed] [Google Scholar]
- 15. Yao JJ, Qi ZY, Liu ZG, et al. Clinical features and survival outcomes between ascending and descending types of nasopharyngeal carcinoma in the intensity-modulated radiotherapy era: a big-data intelligence platform-based analysis. Radiother Oncol 2019; 137: 137–144. [DOI] [PubMed] [Google Scholar]
- 16. Jahr S, Hentze H, Englisch S, et al. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res 2001; 61: 1659–1665. [PubMed] [Google Scholar]
- 17. Giacona MB, Ruben GC, Iczkowski KA, et al. Cell-free DNA in human blood plasma: length measurements in patients with pancreatic cancer and healthy controls. Pancreas 1998; 17: 89–97. [DOI] [PubMed] [Google Scholar]
- 18. Stroun M, Lyautey J, Lederrey C, et al. About the possible origin and mechanism of circulating DNA apoptosis and active DNA release. Clin Chim Acta 2001; 313: 139–142. [DOI] [PubMed] [Google Scholar]
- 19. Jiang N, Reich CF, Pisetsky DS. Role of macrophages in the generation of circulating blood nucleosomes from dead and dying cells. Blood 2003; 102: 2243–2250. [DOI] [PubMed] [Google Scholar]
- 20. Chan KCA, Woo JKS, King A, et al. Analysis of plasma Epstein–Barr virus DNA to screen for nasopharyngeal cancer. N Engl J Med 2017; 377: 513–522. [DOI] [PubMed] [Google Scholar]
- 21. Tang LQ, Li CF, Li J, et al. Establishment and validation of prognostic nomograms for endemic nasopharyngeal carcinoma. J. Natl Cancer Inst 2015; 108: pii: djv291. [DOI] [PubMed] [Google Scholar]
- 22. Guo R, Tang LL, Mao YP, et al. Proposed modifications and incorporation of plasma Epstein–Barr virus DNA improve the TNM staging system for Epstein–Barr virus related nasopharyngeal carcinoma. Cancer 2019; 125: 79–89. [DOI] [PubMed] [Google Scholar]
- 23. Leung SF, Chan KCA, Ma BB, et al. Plasma Epstein–Barr viral DNA load at midpoint of radiotherapy course predicts outcome in advanced-stage nasopharyngeal carcinoma. Ann Oncol 2014; 25: 1204–1208. [DOI] [PubMed] [Google Scholar]
- 24. Huang CL, Sun ZQ, Guo R, et al. Plasma Epstein–Barr viral DNA load after induction chemotherapy predicts outcome in locoregionally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2019; 104: 355–361. [DOI] [PubMed] [Google Scholar]
- 25. Lo YM, Chan LY, Chan AT, et al. Quantitative and temporal correlation between circulating cell-free Epstein–Barr virus DNA and tumor recurrence in nasopharyngeal carcinoma. Cancer Res 1999; 59: 5452–5455. [PubMed] [Google Scholar]
- 26. Wang WY, Twu CW, Lin WY, et al. Plasma Epstein–Barr virus DNA screening followed by ¹⁸F-fluoro-2-deoxy-D-glucose positron emission tomography in detecting posttreatment failures of nasopharyngeal carcinoma. Cancer 2011; 117: 4452–4459. [DOI] [PubMed] [Google Scholar]
- 27. Chan AT, Lo YM, Zee B, et al. Plasma Epstein–Barr virus DNA and residual disease after radiotherapy for undifferentiated nasopharyngeal carcinoma. J Natl Cancer Inst 2002; 94: 1614–1619. [DOI] [PubMed] [Google Scholar]
- 28. Lo YMD, Chan LY, Lo KW, et al. Quantitative analysis of cell-free Epstein–Barr virus DNA in plasma of patients with nasopharyngeal carcinoma. Cancer Res 1999; 59: 1188–1191. [PubMed] [Google Scholar]
- 29. Lo YMD, Leung SF, Chan LY, et al. Plasma cell-free Epstein–Barr virus DNA quantitation in patients with nasopharyngeal carcinoma. Correlation with clinical staging. Ann N Y Acad Sci 2000; 906: 99–101. [DOI] [PubMed] [Google Scholar]
- 30. Fan H, Nicholls J, Chua D, et al. Laboratory markers of tumor burden in nasopharyngeal carcinoma: a comparison of viral load and serologic tests for Epstein–Barr virus. Int J Cancer 2004; 112: 1036–1041. [DOI] [PubMed] [Google Scholar]
- 31. Lin L, Liang W, Li CF, et al. Development and implementation of a dynamically updated big data intelligence platform from electronic health records for nasopharyngeal carcinoma research. Br J Radiol 2019; 92: 20190255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee JY, Sun JM, Oh DR, et al. Comparison of weekly versus triweekly cisplatin delivered concurrently with radiation therapy in patients with locally advanced nasopharyngeal cancer: a multicenter randomized phase II trial (KCSG-HN10-02). Radiother Oncol 2016; 118: 244–250. [DOI] [PubMed] [Google Scholar]
- 33. Shao JY, Li YH, Gao HY, et al. Comparison of plasma Epstein–Barr virus (EBV) DNA levels and serum EBV immunoglobulin A/virus capsid antigen antibody titers in patients with nasopharyngeal carcinoma. Cancer 2004; 100: 1162–1170. [DOI] [PubMed] [Google Scholar]
- 34. Leung SF, Zee B, Ma BB, et al. Plasma Epstein–Barr viral deoxyribonucleic aciquantitation complements tumor-node-metastasis staging prognostication in nasopharyngeal carcinoma. J Clin Oncol 2006; 24: 5414–5418. [DOI] [PubMed] [Google Scholar]
- 35. Tang XR, Li YQ, Liang SB, et al. Development and validation of a gene expression-based signature to predict distant metastasis in locoregionally advanced nasopharyngeal carcinoma: a retrospective, multicentre, cohort study. Lancet Oncol 2018; 19: 382–393. [DOI] [PubMed] [Google Scholar]
- 36. OuYang PY, You KY, Zhang LN, et al. External validity of a prognostic nomogram for locoregionally advanced nasopharyngeal carcinoma based on the 8th edition of the AJCC/UICC staging system: a retrospective cohort study. Cancer Commun (Lond) 2018; 38: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang K, Tian J, Zhang B, et al. A multidimensional nomogram combining overall stage, dose volume histogramparameters and radiomics to predict progression-free survival in patients with locoregionally advanced nasopharyngeal carcinoma. Oral Oncol 2019; 98: 85–91. [DOI] [PubMed] [Google Scholar]
- 38. Chen L, Zhang Y, Lai SZ, et al. 10-Year results of therapeutic ratio by intensity-modulated radiotherapy versus two-dimensional radiotherapy in patients with nasopharyngeal carcinoma. Oncologist 2019; 24: e38–e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Au KH, Ngan RKC, Ng AWY, et al. Treatment outcomes of nasopharyngeal carcinoma in modern era after intensity modulated radiotherapy (IMRT) in Hong Kong: a report of 3328 patients (HKNPCSG 1301 study). Oral Oncol 2018; 77: 16–21. [DOI] [PubMed] [Google Scholar]
- 40. Hui EP, Ma BB, Leung SF, et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol 2009; 27: 242–249. [DOI] [PubMed] [Google Scholar]
- 41. Fountzilas G, Ciuleanu E, Bobos M, et al. Induction chemotherapy followed by concomitant radiotherapy and weekly cisplatin versus the same concomitant chemoradiotherapy in patients with nasopharyngeal carcinoma: a randomized phase II study conducted by the Hellenic cooperative oncology group (HeCOG) with biomarker evaluation. Ann Oncol 2012; 23: 427–435. [DOI] [PubMed] [Google Scholar]
- 42. Lee AW, Ngan RK, Tung SY, et al. Preliminary results of trial NPC-0501 evaluating the therapeutic gain by changing from concurrent-adjuvant to induction-concurrent chemoradiotherapy, changing from fluorouracil to capecitabine, and changing from conventional to accelerated radiotherapy fractionation in patients with locoregionally advanced nasopharyngeal carcinoma. Cancer 2015; 121: 1328–1338. [DOI] [PubMed] [Google Scholar]
- 43. Lee AW, Lau WH, Tung SY, et al. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally advanced nasopharyngeal carcinoma: NPC-9901 trial by the Hong Kong nasopharyngeal cancer study group. J Clin Oncol 2005; 23: 6966–6975. [DOI] [PubMed] [Google Scholar]
- 44. Lee AW, Tung SY, Chan AT, et al. Preliminary results of a randomized study (NPC-9902 Trial) on therapeutic gain by concurrent chemotherapy and/or accelerated fractionation for locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2006; 66: 142–151. [DOI] [PubMed] [Google Scholar]
- 45. Tan T, Lim WT, Fong KW, et al. Concurrent chemo-radiation with or without induction gemcitabine, carboplatin, and paclitaxel: a randomized, phase 2/3 trial in locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2015; 91: 952–960. [DOI] [PubMed] [Google Scholar]
- 46. Zhang Y, Chen L, Hu GQ, et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med 2019; 381: 1124–1135. [DOI] [PubMed] [Google Scholar]
- 47. Du XJ, Tang LL, Chen L, et al. Neoadjuvant chemotherapy in locally advanced nasopharyngeal carcinoma: defining high-risk patients who may benefit before concurrent chemotherapy combined with intensity-modulated radiotherapy. Sci Rep 2015; 5: 16664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. OuYang PY, Zhang LN, Xiao Y, et al. Validation of published nomograms and accordingly individualized induction chemotherapy in nasopharyngeal carcinoma. Oral Oncol 2017; 67: 37–45. [DOI] [PubMed] [Google Scholar]
- 49. Yang Q, Zhang MX, Zou X, et al. A prognostic bio-model based on SQSTM1 and N-Stage identifies nasopharyngeal carcinoma patients at high risk of metastasis for additional induction chemotherapy. Clin Cancer Res 2018; 24: 648–658. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Clean_Supplementary_materials for Risk stratification for nasopharyngeal carcinoma: a real-world study based on locoregional extension patterns and Epstein-Barr virus DNA load by Lu-Lu Zhang, Meng-Yao Huang, Fei-Xu, Ke-Xin Wang, Di Song, Ting Wang, Li-Yue Sun and Jian-Yong Shao in Therapeutic Advances in Medical Oncology