Abstract
Objective
Disulfiram is commonly used for alcohol abuse; however, recent studies have revealed its potential as an anti-cancer treatment. This study investigated the effects of disulfiram on gastric cancer and its underlying mechanisms of action.
Methods
The gastric cancer cell lines MKN-45 and SGC-7901 were used for all experiments. Cell proliferation was investigated using cell counting kit-8, cell migration and invasion were examined using Transwell assays, the proliferation and metastasis related proteins PCNA and MMP-2, respectively, were detected by ELISA. To explore the underlying molecular mechanisms, we also examined levels of proteins involved in the Wnt and NF-κB pathways by ELISA.
Results
Disulfiram significantly inhibited the proliferation, migration, and invasion of gastric cancer cells and decreased PCNA and MMP-2 levels. Additionally, disulfiram-treated MKN-45 and SGC-7901 cells showed reduced expression of Wnt, β-catenin, and NF-κB.
Conclusion
Disulfiram regulates the Wnt and NF-κB pathways, and thus could be a potential treatment for managing gastric cancer.
Keywords: Disulfiram, cancer stem cell, gastric cancer, Wnt, NF-kB, β-catenin
Introduction
Gastric cancer (GC) is a leading cause of cancer-related death; according to statistics from the American Cancer Society, the incidence of gastric cancer ranks fifth among digestive system cancers.1 Meanwhile, in East Asia, it has been estimated that gastric cancer is the second most commonly diagnosed cancer, and it ranks third among tumor-related deaths for both genders.2
Disulfiram, also known as Antabuse, is used as an anti-alcohol dependency drug due to its inhibitory effect on acetaldehyde dehydrogenase.4,5 Recent preclinical studies have revealed the potential role of disulfiram in targeting malignancies such as breast cancer, myeloma, and pancreatic cancer.4–7 Additionally, it has been reported that disulfiram can reverse drug resistance, inhibit DNA methylation, and induce apoptosis.8,9 However, its effects on gastric cancer have yet to be elucidated. Therefore, we performed this study on disulfiram as a candidate anti-tumor agent, with the aim of exploring its role in the proliferation, migration, and invasion of GC cells; to further explore its molecular mechanisms, we also investigated components of the Wnt and NF-κB pathways.
Materials and methods
Cell lines
The gastric cancer cell lines GES, MNK-45, and SGC-7901 were obtained from Xiangya Medical College at Central South University.
Reagents
Disulfiram was purchased from Sigma-Aldrich (St. Louis, MO, USA). RPMI-1640 medium, fetal bovine serum (FBS), and Matrigel were purchased from Gibco (Gaithersburg, MD, USA). Matrigel and Transwell chambers were purchased from Costar Inc. (Corning, NY, USA). Rabbit antibodies against MMP-2, PCNA, Wnt, β-catenin, and NF-κB were purchased from Santa Cruz Biotechnology (Dallas, TX, USA), and anti-rabbit secondary antibody and cell counting kit-8 (CCK-8) were obtained from Beyotime Biotechnology (Haimen, China).
Cell culture
Cells were cultured in complete medium (RPMI-1640 with 10% FBS) at 37°C in 5% CO2 and 100% relative humidity.
CCK-8 proliferation assay
To investigate the effects of disulfiram on the proliferation of gastric cancer cells, CCK-8 assays were performed. Briefly, cells in exponential growth phase were collected and processed into a single cell suspension using 0.25% trypsin, and then were seeded at 1 × 104 cells/well in 96-well plates with culture medium containing disulfiram. After 24, 48, 72, and 96 hours, 10 μL of CCK-8 solution was added, and absorbance was measured after a 2-hour incubation at 37°C.
Transwell assays
To evaluate the migration and invasion abilities of cells treated with disulfiram, Transwell assays were conducted. Briefly, Transwell chambers were added to 24-well plates containing 0.1% BSA-RPMI 1640, followed by the addition of 100 μL of cell suspension into the chambers with disulfiram at different concentrations (0, 12.5, 25, 50, and 100 μmol/L). After 12 hours, the chambers were removed, fixed in formalin, and stained. Subsequently, cells on the surface of the permeable membrane were removed, and samples were processed in neutral balsam. The number of cells penetrating from the chambers was counted from five different randomly selected fields of each membrane. To evaluate the invasiveness of cancer cells, 5 μg of Matrigel was added to the surface of a permeable membrane to form a basement membrane. Subsequently, the Transwell assay was performed as above.
ELISA analysis
ELISAs were performed in accordance with the instructions supplied by the manufacturer. Briefly, cell culture supernatants and standard proteins were added to a multi-well plate, coated, and then incubated for 2 hours. Plates were then washed with washing buffer (Tween 20 in phosphate-buffered saline; Sigma-Aldrich). Next, a biotin-labeled antibody was added to each well and incubated for another 2 hours. ELISA plates were then washed, and a streptavidin-horseradish peroxidase solution was added. After adding tetramethylbenzidine (Sigma-Aldrich), a color reaction was achieved. Optical density was measured at 450 nm on an ELISA plate-reader.
Statistical analysis
All experiments were repeated at least three times. Acquired data were analyzed with SPSS Statistics for Windows, version 24.0 (IBM Corp., Armonk, NY, USA) and plotted using GraphPad Prism 6.0 (GraphPad Software, Inc., San Diego, CA, USA). One-way ANOVA or Student’s t-tests were used to compare the experimental and control groups. A p-value <0.05 was considered statistically significant.
Results
Disulfiram inhibited the proliferation of gastric cancer cells
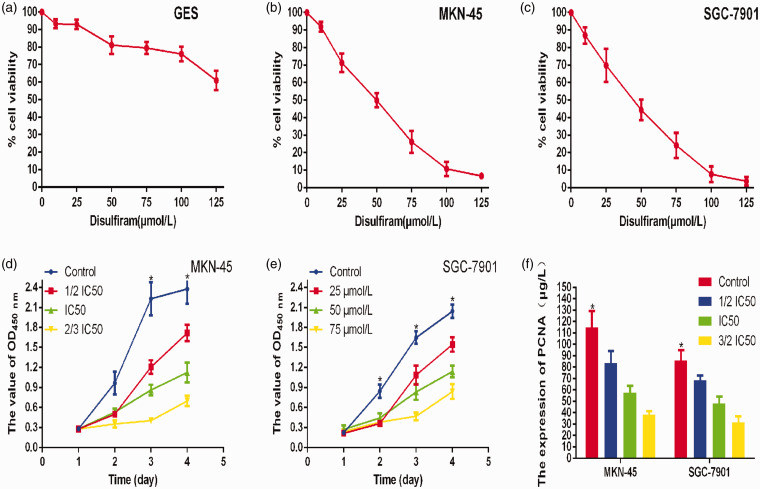
To determine the effective concentration of disulfiram, we tested the inhibition rate of different disulfiram concentrations (0, 10, 25, 50, 75, 100, and 125 μmol/L) on GES, MKN45 and SGC-7901 cells using the CCK-8 assay. The results showed that 50 μmol/L disulfiram was the IC50 value. Then, the CCK-8 assay was used to evaluate the effect of different disulfiram concentrations (0, 25, 50, and 75 μmol/L) on the proliferation of gastric cancer cells. As shown in Figure 1, disulfiram significantly inhibited the proliferation of gastric cancer cells in vitro (p<0.05), and exhibited a corresponding concentration-dependent inhibition of cell growth with increasing disulfiram concentrations. Finally, we evaluated PCNA levels, a biomarker of proliferation, by ELISA. The results showed that disulfiram significantly inhibited PCNA expression.
Figure 1.
a–c: To determine the effective concentration of disulfiram, we measured the inhibition rate of different disulfiram concentrations (0, 10, 25, 50, 75, 100, and 125 μmol/L) on GES, MKN45, and SGC-7901 cells by the CCK-8 assay. The results showed that 50 μmol/L was the IC50 value for disulfiram in gastric cancer cells. d–f: CCK-8 result showed that disulfiram significantly inhibited the proliferation of gastric cancer cells in vitro, and ELISA showed decreased PCNA expression.
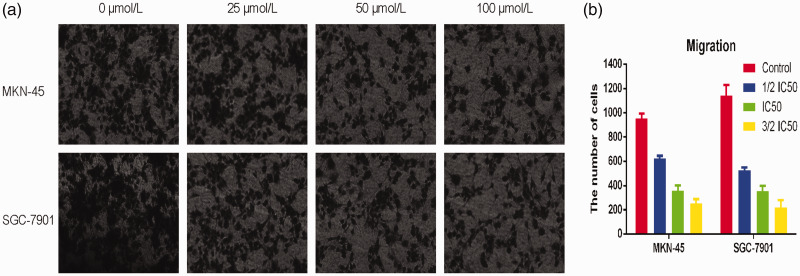
Disulfiram inhibited the migration and invasion potential of gastric cancer cells
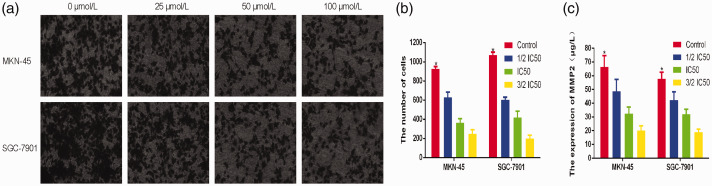
To determine the effect of disulfiram on migration and invasion, Transwell assays were performed. Consequently we observed that disulfiram significant inhibited the migration and invasion of gastric cancer cells. As shown in Figure 2, cell migration was significantly decreased at higher disulfiram concentrations (p<0.05). Furthermore, the invasion ability of gastric cancer cells was also significantly inhibited at higher disulfiram concentrations (p<0.05). Additionally, we evaluated MMP-2 expression, a biomarker for migration and invasion, by ELISA. These results showed that disulfiram significantly inhibited MMP-2 expression (Figure 3).
Figure 2.
a,b: The migration of gastric cancer cells was inhibited by disulfiram in a dose-dependent manner (*p<0.05).
Figure 3.
a,b: The invasion of gastric cancer cells was inhibited by disulfiram in a dose-dependent manner. c: ELISA results showed that disulfiram significantly inhibited MMP-2 expression (*p<0.05).
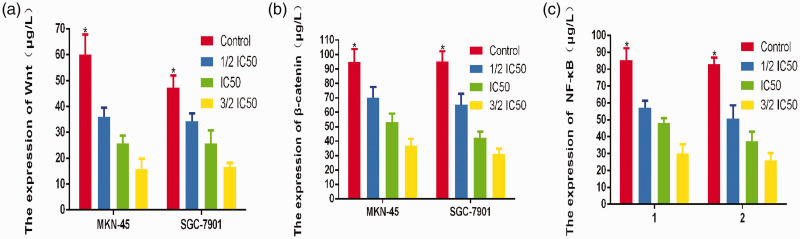
Disulfiram regulated the Wnt and NF-κB pathways
To investigate whether disulfiram regulates Wnt and NF-κB signaling in GC cells, cellular proteins were extracted from cells treated with disulfiram at different concentration as well as from the cells in the control group. The levels of specific proteins were measured by ELISA. The results showed that disulfiram inhibited the expression of Wnt, β-catenin, and NF-κB in both cell lines compared with the control groups (Figure 4).
Figure 4.
a-c: ELISA results showed decreased Wnt, β-catenin, and NF-κB expression in both gastric cancer cell lines following disulfiram treatment.
Discussion
In this in vitro study, we found that disulfiram inhibited the proliferation of both the GC cell lines MKN-45 and SGC 7901. This finding was supported by ELISA, which showed decreased expression of PCNA, which is a marker of proliferation,10 after disulfiram treatment. We also observed inhibitory effects of disulfiram on the migration and invasion of GC cells, which was supported by also finding decreased MMP-2 expression, which is correlated with migration and invasive potential.11 As we further explored the molecular mechanism, we found that the Wnt and NF-κB signaling pathways were downregulated by disulfiram.
Disulfiram is clinically used to treat alcoholism due to its inhibitory effect on acetaldehyde dehydrogenase, causing strong and unpleasant symptoms such as flushing, tachycardia, and headache after drinking alcohol.12,13 More recently, disulfiram has been shown to regulate a wide range of malignancies. For example, Cong et al.6 reported that disulfiram was effective at inhibiting the growth of pancreatic ductal adenocarcinoma both in vitro and in vivo. In the human body, disulfiram is catalyzed into diethyldithiocarbamate, which has been shown to chelate into Cu2+-containing complexes. These complexes have been reported to inhibit proteasome activity and elevate radical oxygen species;14 thus, disulfiram influence the activities of cancer cells.
Abnormal activation of canonical Wnt/β-catenin signaling has been observed in carcinomas of the breast, prostate, lung, and other tissues.15,16 In this signaling pathway, Wnt ligand is a secreted glycoprotein that binds to membrane receptors. Following ligand engagement, β-catenin translocates to the nucleus and form a transcriptional complex, which subsequently activates Wnt target genes that facilitate cell proliferation and/or metastasis.17 In the absence of Wnt, β-catenin is degraded by a destruction complex comprising Axin, glycogen synthase kinase 3, adenomatosis polyposis coli, and other scaffold and adaptor proteins.18–21 When Wnt ligands bind to their membrane receptors, proteins including Dishevelled are recruited and activated, which leads to the polymerization and inactivate of the destruction complex. Together, these changes lead to the stabilization and accumulation of β-catenin. It has also been observed in breast cancer cells that the epithelial-to-mesenchymal transition-related protein SNAI2 is stabilized by Wnt ligands in the same manner.22 ASPP2 is a metastasis-related protein, and Wang et al.23 reported that ASPP2 directly binds to the β-catenin/E-cadherin complex. Additionally, they found that ASPP2 inhibited the N-terminal phosphorylation of β-catenin, preventing it from degradation.
The NF-κB pathway regulates apoptosis and is closely related to tumor initiation, growth, and metastasis.24 NF-κB controls the transcription of cellular DNA and participates in cellular responses to noxious stimuli. NF-κB is commonly overexpressed in cancer cells, and aberrant NF-κB activation reduces the eradication of cancer cells by the immune system and promotes metastasis. Additionally, NF-κB expression results in decreased apoptosis by regulating the anti-apoptotic gene TRAF (tumor necrosis factor receptor associated factor). We found that both the Wnt and NF-κB pathways, which are involved in cancer cell proliferation, apoptosis, and progression, were inhibited by disulfiram. Taken together, we conclude that the inhibitory effects of disulfiram on gastric cancer cells were associated with altering Wnt and NF-κB signaling.
In summary, these results indicated that disulfiram regulates the NF-κB and Wnt pathways, thereby influencing the behavior of gastric cancer cells. Disulfiram is a promising compound for treating gastric cancer, but future in vivo studies are needed to further test this hypothesis.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by the National Natural Science Foundation of China (81570783), the National Key R&D Program of China (2016YFC1302201 and 2016YFC0107006), and the Open Fund of State Key Laboratory of Cancer Biology, China (CBSKL201718).
ORCID iDs
Jun Zhang https://orcid.org/0000-0002-7993-2235
Yukui Peng https://orcid.org/0000-0003-2770-377X
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. World Cancer Report 2014. Geneva: WHO Press. Lyon: International Agency for Research on Cancer; 2014. pp. 16–53 [Google Scholar]
- 3.Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Sstem Cell 2014; 14: 275–291. [DOI] [PubMed] [Google Scholar]
- 4.He H, Markoutsa E, Li J, et al. Repurposing disulfiram for cancer therapy via targeted nanotechnology through enhanced tumor mass penetration and disassembly. Acta Biomater 2018; 68: 113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu X, Xue X, Wang L, et al. Suppressing autophagy enhances disulfiram/copper-induced apoptosis in non-small cell lung cancer. Eur J Pharmacol 2018; 827: 1–12. [DOI] [PubMed] [Google Scholar]
- 6.Cong J, Wang Y, Zhang X, et al. A novel chemoradiation targeting stem and nonstem pancreatic cancer cells by repurposing disulfiram. Cancer Lett 2017; 409: 9–19. [DOI] [PubMed] [Google Scholar]
- 7.Skrott Z, Mistrik M, Andersen KK, et al. Alcohol-abuse drug disulfiram targets cancer via p97 segregase adaptor NPL4. Nature 2017; 552: 194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sauna ZE, Shukla S, Ambudkar SV. Disulfiram, an old drug with new potential therapeutic uses for human cancers and fungal infections. Mol Biosyst 2005; 1: 127. [DOI] [PubMed] [Google Scholar]
- 9.Lin J, Haffner MC, Zhang Y, et al. Disulfiram is a DNA demethylating agent and inhibits prostate cancer cell growth. Prostate 2011; 71: 333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choe KN, Moldovan GL. Forging ahead through darkness: PCNA, still the principal conductor at the replication fork. Mol Cell 2017; 65: 380–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsieh MJ, Chen JC, Yang WE. Dehydroandrographolide inhibits oral cancer cell migration and invasion through NF-κB-, AP-1-, and SP-1-modulated matrix metalloproteinase-2 inhibition. Biochem Pharmacol 2017; 130: 10–20. [DOI] [PubMed] [Google Scholar]
- 12.Jin N, Zhu X, Cheng F, et al. Disulfiram/copper targets stem cell-like ALDH(+) population of multiple myeloma by inhibition of ALDH1A1 and Hedgehog pathway. J Cell Biochem 2018; 119: 6882–6893. [DOI] [PubMed] [Google Scholar]
- 13.Shin YS, Cha HY, Lee BS, et al. Anti-cancer effect of luminacin, a marine microbial extract, in head and neck squamous cell carcinoma progression via autophagic cell death. Cancer Res Treat 2016; 48: 738–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kona F, Buac D, Burger A. Disulfiram, and disulfiram derivatives as novel potential anticancer drugs targeting the ubiquitin proteasome system in both preclinical and clinical studies. Curr Cancer Drug Targets 2011; 11: 338–346. [DOI] [PubMed] [Google Scholar]
- 15.Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discovery 2006; 5: 997–1014. [DOI] [PubMed] [Google Scholar]
- 16.Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene 2017; 36: 1461–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tenbaum SP, Ordóñez-Morán P, Puig I, et al. β-catenin confers resistance to PI3K and AKT inhibitors and subverts FOXO3a to promote metastasis in colon cancer. Nat Med 2012; 18: 892–901. [DOI] [PubMed] [Google Scholar]
- 18.Zimmerli D, Hausmann G, Cantu C, et al. Pharmacological interventions in the Wnt pathway: inhibition of Wnt secretion versus disrupting the protein-protein interfaces of nuclear factors. Br J Pharmacol 2017; 174: 4600–4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nusse R. Wnt signaling in disease and in development. Cell Res 2005; 15: 28–32. [DOI] [PubMed] [Google Scholar]
- 20.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 2004; 20: 781–810. [DOI] [PubMed] [Google Scholar]
- 21.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nature Rev Cancer 2008; 8: 387–398. [DOI] [PubMed] [Google Scholar]
- 22.Wu ZQ, Li XY, Hu CY, et al. Canonical Wnt signaling regulates Slug activity and links epithelial-mesenchymal transition with epigenetic Breast Cancer 1, Early Onset (BRCA1) repression. Proc Natl Acad Sci USA 2012; 109: 16654–16659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Bu F, Royer C, et al. ASPP2 controls epithelial plasticity and inhibits metastasis through β-catenin-dependent regulation of ZEB1. Nat Cell Biol 2014; 16: 1092–1104. [DOI] [PubMed] [Google Scholar]
- 24.Brasier AR. The NF-kappaB regulatory network. Cardiovasc Toxicol 2006; 6: 111–130. [DOI] [PubMed] [Google Scholar]