Abstract
Objective
This study was performed to assess the clinical value of measuring the intact parathyroid hormone (iPTH) concentration 1 day after total thyroidectomy to estimate the occurrence of permanent hypoparathyroidism (pHPP).
Methods
Data of 546 patients who underwent total thyroidectomy from February 2008 to December 2018 were retrospectively analyzed. Calcium and iPTH concentrations were collected preoperatively and at 1 day and 6 months postoperatively. Logistic regression was used to analyze the correlation between clinical indexes and postoperative pHPP.
Results
Of the 546 patients, 22 (4.03%) developed pHPP. Multivariate analysis showed that the iPTH and serum calcium concentrations measured 1 day after surgery were independent predictors of the risk of pHPP. An iPTH concentration of 5.51 pg/mL measured 1 day postoperatively was used as the cut-off value, and the area under the curve was 0.956. The risk of pHPP was identified with a sensitivity of 100%, specificity of 85.1%, positive predictive value of 22%, and negative predictive value of 100%.
Conclusions
The iPTH concentration measured 1 day after total thyroidectomy is closely related to the occurrence of pHPP postoperatively and is an independent predictive risk factor. The postoperative iPTH concentration can be helpful in identifying patients at risk for developing pHPP.
Keywords: Total thyroidectomy, postoperative hypocalcemia, permanent hypoparathyroidism, intact parathyroid hormone, serum calcium, predictive factor
Introduction
Although the incidence of permanent hypoparathyroidism (pHPP) after thyroidectomy is low, it is a serious complication after thyroid surgery with an incidence rate of 1% to 7%.1 Measurement of the postoperative calcium and/or vitamin D concentration is a reasonable method to predict the occurrence of pHPP and is conducive to determining early and safe discharge of patients, reducing perioperative complications, and avoiding excessive treatment during hospitalization.2–5 In the past, the postoperative serum calcium concentration was used for assessment, but postoperative hypocalcemia is influenced by many factors.6 Thus, its accuracy as a predictive indicator has been disputed. Serum intact parathyroid hormone (iPTH) is characterized by a short half-life (3–5 minutes), and the serum calcium status can be evaluated according to the early postoperative iPTH concentration.7–9 However, although the iPTH concentration has been measured as a reference, no clinical agreement has been reached on its cut-off value. Many studies have shown that a low iPTH concentration in the early postoperative period is the most reliable risk indicator for hypocalcemia after thyroidectomy. In view of this situation, the present study was performed to analyze the correlation between the occurrence of pHPP and the iPTH and serum calcium concentrations measured 1 day after total thyroidectomy among patients in our hospital and to evaluate the predictive value of the iPTH concentration. These data will provide a reference for clinical screening and accurate treatment of high-risk patients who may develop pHPP after total thyroidectomy.
Materials and methods
Patient selection
We retrospectively reviewed consecutive patients who underwent surgical treatment of benign and malignant thyroid diseases in the Department of General Surgery, Beijing Chaoyang Hospital affiliated to Capital Medical University, Beijing, China from February 2008 to December 2018. The inclusion criteria were performance of total thyroidectomy for treatment of benign or malignant thyroid disease or on a prophylactic basis because of a BRAF mutation, monitoring of the serum calcium and iPTH concentrations 1 day postoperatively, and complete postoperative follow-up. The exclusion criteria were one-sided thyroid gland lobectomy or total excision, incomplete postoperative serum calcium and iPTH monitoring data, interruption of postoperative follow-up, and known parathyroid or metabolic bone disease, renal failure, previous neck surgery, or calcium and/or vitamin D supplementation. This study was conducted in accordance with the latest version of the Declaration of Helsinki of the World Medical Association. The study was approved by the Ethics Committee of Beijing Chaoyang Hospital. Verbal consent was obtained from the patients after they had received an explanation of the procedure and potential risks encountered. Written informed consent was subsequently obtained using a form approved by the Ethics Committee and is available upon request.
Surgical procedure
All operations were performed by four thyroid specialists in the department of general surgery. According to the institutional protocol, the surgical resection range was determined by the preoperative pathological diagnosis. All patients with thyroid cancer underwent total thyroidectomy plus central compartment lymphadenectomy of the affected side, and patients with multinodular thyroid goiter or thyroid follicular tumor underwent total thyroidectomy. The patients underwent general anesthesia in the supine position. Total thyroidectomy was then performed as follows. After the thyroid gland had been completely exposed, the upper pole of the thyroid gland was cut off with an ultrasonic knife. The dorsal side of the thyroid gland was separated near the inferior corner of the thyroid cartilage, and the parathyroid gland was preserved in situ by fine capsule dissection. If the parathyroid gland was mistakenly cut, the cut parathyroid gland was immediately transplanted into the sternocleidomastoid muscle. Another method of parathyroid autotransplantation was slicing of the gland into 0.5- to 1.0-mm pieces. The slices were implanted into two or three small pockets fashioned in the subcutaneous tissue of the non-dominant forearm through 2- to 3-mm skin incisions.10 This procedure required that the venipuncture for measurement of the serum PTH concentration be placed in the forearm without reimplantation. During surgery, the superior parathyroid gland located behind the upper pole of the thyroid was carefully identified, cautiously protected, and separated from the thyroid gland. Before treatment of the thyroid gland inferior pole vessels, the inferior parathyroid gland located on the dorsal side of the inferior pole of the thyroid gland was identified. The parathyroid gland and its nourishing vessels were attached to the capsule and separated laterally, and the thyroid gland was then lifted. After routine exposure of the recurrent laryngeal nerve, the whole thyroid gland was removed. For additional lymph node dissection in the central region of the neck, the parathyroid gland located at the inferior pole of the thyroid and its nourishing vessels were separated to the lateral side of the recurrent laryngeal nerve. After fully exposing the recurrent laryngeal nerve, the lymphatic fat tissues in the tracheoesophageal groove and at the front of the trachea were cleaned.
Perioperative management
All patients with symptomatic or severe hypocalcemia, even if asymptomatic, were initially treated with intravenous administration of 10% calcium gluconate as a calcium supplement at a dose of 1 to 2 g/day until the blood calcium concentration reached exceeded 1.90 mmol/L or symptoms disappeared. For patients with an iPTH concentration of <15 pg/mL at 1 day after surgery, calcium (1–3 g/day) and 1,25-hydroxyvitamin D (0.25–0.5 µg/day) were orally administered. Patients with mild asymptomatic hypocalcemia and an iPTH concentration of >15 pg/mL did not need to receive calcium supplement therapy.
Laboratory examinations and definitions
The blood calcium concentration was measured by conventional methods. The reference range of serum calcium was 2.13 to 2.65 mmol/L. The serum parathyroid hormone concentration was determined as iPTH, and the reference range was 15 to 65 pg/mL. iPTH was estimated using an automated electrochemiluminescent immunoassay analyzer (Modular Analytics E170; Roche Diagnostic GmbH, Mannheim, Germany). The threshold detection level was 1.2 pg/mL. The serum calcium, phosphate, magnesium, and albumin concentrations were determined using an automatic analyzer (cobas c 711; Roche Diagnostics GmbH).
Hypocalcemia was defined as a blood calcium concentration of <2.00 mmol/L. Mild hypocalcemia was defined as a blood calcium concentration of 1.90 to 2.00 mmol/L. Severe hypocalcemia was defined as a blood calcium concentration of <1.90 mmol/L the presence of facial numbness, foot numbness, or other symptoms of hypocalcemia. Biochemical hypoparathyroidism is defined as a low iPTH level, below the lower limit of the laboratory standard (usually 15 pg/mL), accompanied by hypocalcemia. The reference range of the iPTH concentration varies depending upon the laboratory. Clinical hypoparathyroidism is defined as biochemical hypoparathyroidism accompanied by symptoms and/or signs of hypocalcemia.11–13 pHPP was defined as the need for calcium and/or vitamin D supplementation at 6 months postoperatively to maintain a normal blood calcium concentration and was usually accompanied by a serum iPTH concentration of <15 pg/mL.12
Follow-up method
Follow-up mainly involved laboratory examinations. Signs and symptoms of an abnormal blood calcium concentration were also taken into account. The serum calcium and iPTH levels were recorded 1 day and 6 months after surgery. In patients with postoperative hypocalcemia, the blood calcium concentration was measured daily until a normal level was reached with calcium and vitamin D supplementation. Renal function and the alkaline phosphatase, albumin, phosphate, magnesium, and vitamin D concentrations were recorded before and 6 months after surgery to exclude other diseases or confirm vitamin D deficiency. The patients were closely monitored postoperatively for any signs or symptoms of hypocalcemia. The blood calcium level was measured at regular outpatient reexaminations to check for signs and symptoms of hypocalcemia. All patients who could not stop taking calcium and/or vitamin D were followed up at 6 months postoperatively; the follow-up time was then extended by another 6 to 12 months, during which time the serum calcium and iPTH concentrations were monitored.
Statistical analysis
The statistical analysis was performed using a commercially available software package (SPSS version 22.0 for Windows; IBM Corp., Armonk, NY, USA). Possible influencing factors were collected and recorded as follows: age, sex, preoperative diagnosis, surgical method, preoperative laboratory test results, and serum calcium and iPTH concentrations 1 day after surgery. Categorical variables are expressed as frequency and percentage, and measurement variables are expressed as mean ±standard deviation. An independent-samples t test was used to compare the differences between the measurement data groups. To compare categorical variables, the chi-square test or Fisher’s exact test was used as appropriate. Logistic regression was used to analyze the correlation between clinical parameters and postoperative pHPP. A two-sided P value of <0.05 was considered statistically significant.
Receiver operating characteristic curve analysis was performed using MedCalc version 15.2.2 (MedCalc Software, Mariakerke, Belgium). The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the serum calcium and iPTH concentrations were calculated by the area under the curve (AUC) to find the threshold values for predicting pHPP. With respect to the accuracy of the diagnostic test with an appropriate reference provided, an AUC of 0.5 to 0.6 suggests poor accuracy of the diagnostic test, 0.6 to 0.7 suggests sufficient accuracy, 0.7 to 0.8 suggests good accuracy, 0.8 to 0.9 suggests very good accuracy, and >0.9 suggests excellent accuracy.13
Results
Patient characteristics
Among 647 consecutive patients who underwent total thyroidectomy, 101 were excluded because of incomplete postoperative data and interruption of follow-up; therefore, 546 patients were included in the final analysis. The 546 patients comprised 158 men and 388 women with a mean age of 50.9 ± 13.2 years (range, 19.0–79.2 years). The postoperative pathological diagnoses were thyroid nodular goiter (n = 123), thyroid follicular tumor (n = 83), and malignant tumor (n = 340). The 340 malignant tumors comprised thyroid papillary carcinoma (n = 322), thyroid follicular carcinoma (n = 12), medullary carcinoma (n = 4), and anaplastic carcinoma (n = 2). Total thyroidectomy was performed in 230 patients, and total thyroidectomy plus central compartment lymphadenectomy was performed in 316 patients. The mean preoperative baseline iPTH concentration was 38.9 ± 13.7 pg/mL (range, 13.2–73.1 pg/mL), and the mean preoperative baseline serum calcium concentration was 2.23 ± 0.093 mmol/L (range, 1.95–2.47 mmol/L) (see Table 1).
Table 1.
Patient characteristics (n = 546).
| Variable | |
|---|---|
| Sex | |
| Male | 158 (28.9) |
| Female | 388 (71.1) |
| Age, years | 50.9 ± 13.2 |
| Preoperative diagnosis | |
| Thyroid nodular goiter | 123 (22.5) |
| Thyroid follicular tumor | 83 (15.2) |
| Malignant tumor | 340 (62.3) |
| Thyroid papillary carcinoma | 322 (94.7) |
| Thyroid follicular carcinoma | 12 (3.5) |
| Thyroid medullary carcinoma | 4 (1.2) |
| Thyroid anaplastic carcinoma | 2 (0.6) |
| Surgical procedure | |
| Total thyroidectomy | 230 (42.1) |
| Total thyroidectomy plus central dissection | 316 (57.9) |
| Preoperative baseline iPTH concentration, pg/mL | 38.9 ± 13.7 |
| Preoperative baseline calcium concentration, mmol/L | 2.23 ± 0.093 |
Data are expressed as number (percentage) or mean ± standard deviation.
iPTH, intact parathyroid hormone.
Changes in serum calcium and iPTH concentrations 1 day postoperatively and development of pHPP 6 months postoperatively
Within 24 hours after surgery, 203 (37.1%) patients had hypocalcemia; among these patients, 47 (23.2%) had symptoms. Most patients had mild hypocalcemia. The iPTH concentration was low (<15 pg/mL) in 37.7% (206/546) of patients 1 day after surgery; of these patients, 43.7% (90/206) had hypocalcemia. This incidence was significantly higher than the 4.1% (14/340) of patients with a normal iPTH concentration 1 day after surgery (P < 0.001). One day after surgery, 46.3% (94/203) of the patients with hypocalcemia had a normal iPTH concentration, which was higher than the 28.3% (97/343) of patients with a normal blood calcium concentration (P < 0.001). The mean blood calcium concentration of patients with normal iPTH was 2.10 ± 0.01 mmol/L, while that of patients with low iPTH 1.89 ± 0.12 mmol/L; the difference between the two groups was statistically significant (P < 0.001) (Table 2).
Table 2.
Comparison of relevant factors between patients with normal and low serum calcium and iPTH concentrations 1 day after total thyroidectomy (n = 546).
|
Serum calcium |
iPTH |
||||||
|---|---|---|---|---|---|---|---|
| Normal | Low | Normal | Low | ||||
| Variable | Total | (n = 343) | (n = 203) | P-value | (n = 340) | (n = 206) | P-value |
| Sex | 0.960 | 0.787 | |||||
| Female | 388 | 244 | 144 | 243 | 145 | ||
| Male | 158 | 99 | 59 | 97 | 61 | ||
| Age, years | 51.2 ± 13.6 | 50.0 ± 12.3 | 0.307 | 50.6 ± 9.9 | 51.3 ± 9.5 | 0.454 | |
| Preoperative diagnosis | 0.771 | 0.709 | |||||
| Thyroid nodular goiter | 123 | 78 | 45 | 75 | 48 | ||
| Thyroid follicular tumor | 83 | 53 | 30 | 49 | 34 | ||
| Thyroid carcinoma | 340 | 212 | 128 | 216 | 124 | ||
| Surgical procedure | 0.088 | 0.266 | |||||
| Total thyroidectomy plus central dissection | 316 | 189 | 127 | 190 | 126 | ||
| Total thyroidectomy | 230 | 154 | 76 | 150 | 80 | ||
| Preoperative laboratory data | |||||||
| Calcium, mmol/L | 2.23 ± 0.09 | 2.22 ± 0.09 | 0.952 | 2.27 ± 0.08 | 2.26 ± 0.09 | 0.888 | |
| 25-hydroxyvitamin D3, ng/mL | 21.82 ± 8.38 | 20.94 ± 7.71 | 0.221 | 20.47 ± 8.38 | 19.19 ± 7.96 | 0.079 | |
| Magnesium, mmol/L | 0.87 ± 0.07 | 0.89 ± 0.30 | 0.224 | 0.88 ± 0.31 | 0.84 ± 0.25 | 0.070 | |
| Phosphate, mmol/L | 1.16 ± 0.18 | 1.13 ± 0.22 | 0.096 | 1.16 ± 0.16 | 1.13 ± 0.26 | 0.114 | |
| Alkaline phosphatase, pg/mL | 73.4 ± 23.7 | 71.2 ± 21.6 | 0.256 | 62.1 ± 23.1 | 65.1 ± 18.7 | 0.111 | |
| TSH, mIU/L | 1.87 ± 1.01 | 1.91 ± 0.95 | 0.653 | 1.95 ± 1.04 | 2.09 ± 1.04 | 0.147 | |
| iPTH, pg/mL | 38.8 ± 11.9 | 39.4 ± 11.1 | 0.586 | 37.34 ± 15.5 | 39.07 ± 10.4 | 0.119 | |
| 24 hours postoperatively | |||||||
| iPTH, pg/mL | 24.37 ± 10.6 | 21.23 ± 10.6 | 0.001 | 26.72 ± 8.45 | 9.07 ± 3.38 | <0.001 | |
| Calcium, mmol/L | 2.16 ± 0.05 | 1.95 ± 0.05 | <0.001 | 2.10 ± 0.01 | 1.89 ± 0.12 | <0.001 | |
Data are expressed as number or as mean ± standard deviation.
iPTH, intact parathyroid hormone; TSH, thyroid-stimulating hormone.
A P value of <0.05 was considered statistically significant.
At 6 months postoperatively, The iPTH concentration was still <15 pg/mL in 22 patients, and they required oral calcium and vitamin D supplementation to maintain a normal blood calcium concentration. Therefore, the incidence of pHPP after total thyroidectomy was in 4.03% (22/546) of the total sample. Among the 340 patients with a normal iPTH concentration 1 day after surgery, the iPTH concentration was within the reference range at 6 months after surgery, and all 22 patients with pHPP were among those with a low iPTH concentration 1 day after surgery.
Analysis of risk factors for pHPP at 6 months after thyroidectomy
A univariate analysis was conducted based on the possible risk factors associated with pHPP after thyroid surgery.11 The results showed that a low iPTH concentration (P = 0.001) and a low serum calcium concentration (P = 0.009) 1 day after surgery were risk factors for pHPP, and a low vitamin D concentration was a potential risk factor (Table 3). These factors were included in the logistic regression model for the multivariate analysis. At 6 months after thyroid surgery, the patients were divided into those with pHPP (n = 22) and those without pHPP (n = 524). The results showed that the iPTH concentration (OR = 2.932, 95% confidence interval [CI]: 1.129–7.616, P = 0.027) and serum calcium concentration (OR = 2.584, 95% CI: 1.017–6.567, P = 0.046) 1 day after surgery were independent predictors of the risk of pHPP (Table 4). Lower iPTH and serum calcium concentrations 1 day postoperatively were correlated with a higher likelihood of pHPP.
Table 3.
Univariate analysis of pHPP at 6 months after total thyroidectomy.
| Variable | Total546 | No pHPP(n = 524) | pHPP(n = 22) | P |
|---|---|---|---|---|
| Age, years | 0.795 | |||
| <45 | 262 | 252 (48.1) | 10 (45.5) | |
| ≥45 | 284 | 272 (51.9) | 12 (54.5) | |
| Sex | 0.865 | |||
| Male | 92 | 88 (16.8) | 4 (18.2) | |
| Female | 454 | 436 (83.2) | 18 (81.8) | |
| Surgery procedure | 0.576 | |||
| Total thyroidectomy plus central dissection | 316 | 302 (57.6) | 14 (63.6) | |
| Total thyroidectomy | 230 | 222 (42.4) | 8 (36.4) | |
| Preoperative vitamin D | 0.054 | |||
| Normal | 396 | 384 (73.3) | 12 (54.5) | |
| Low | 150 | 140 (26.7) | 10 (45.5) | |
| Preoperative magnesium | 0.550 | |||
| Normal | 365 | 349 (66.6) | 16 (72.7) | |
| Low | 181 | 175 (33.4) | 6 (27.3) | |
| iPTH at 24 hours postoperatively | 0.001 | |||
| Normal | 340 | 334 (63.7) | 6 (27.3) | |
| Low | 206 | 190 (36.3) | 16 (72.7) | |
| Calcium at 24 hours postoperatively | 0.009 | |||
| Normal | 343 | 335 (63.9) | 8 (36.4) | |
| Low | 203 | 189 (36.1) | 14 (63.6) |
Data are presented as n (%).
pHPP, permanent hypoparathyroidism; intact parathyroid hormone.
Table 4.
Multivariate analysis of pHPP at 6 months after total thyroidectomy (n = 546).
| Variables | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Postoperative calcium | |||
| Normal | 1.000 | ||
| Low | 2.584 | 1.017–6.567 | 0.046 |
| Postoperative iPTH | |||
| Normal | 1.000 | ||
| Low | 2.932 | 1.129–7.616 | 0.027 |
| Surgical procedure | |||
| Total thyroidectomy | 1.000 | ||
| Total thyroidectomy plus central dissection | 1.674 | 0.637–4.394 | 0.296 |
| Age, years | |||
| <45 | 1.000 | ||
| ≥45 | 1.012 | 0.421–2.430 | 0.979 |
| Sex | |||
| Male | 1.000 | ||
| Female | 1.420 | 0.582–3.465 | 0.442 |
| Preoperative vitamin D | |||
| Normal | 1.000 | ||
| Low | 1.194 | 0.487–2.925 | 0.698 |
| Preoperative magnesium | |||
| Normal | 1.000 | ||
| Low | 0.611 | 0.225–1.658 | 0.333 |
pHPP, permanent hypoparathyroidism; intact parathyroid hormone; CI, confidence interval.
Use of 1-day postoperative iPTH and serum calcium concentrations to predict occurrence of pHPP at 6 months after surgery
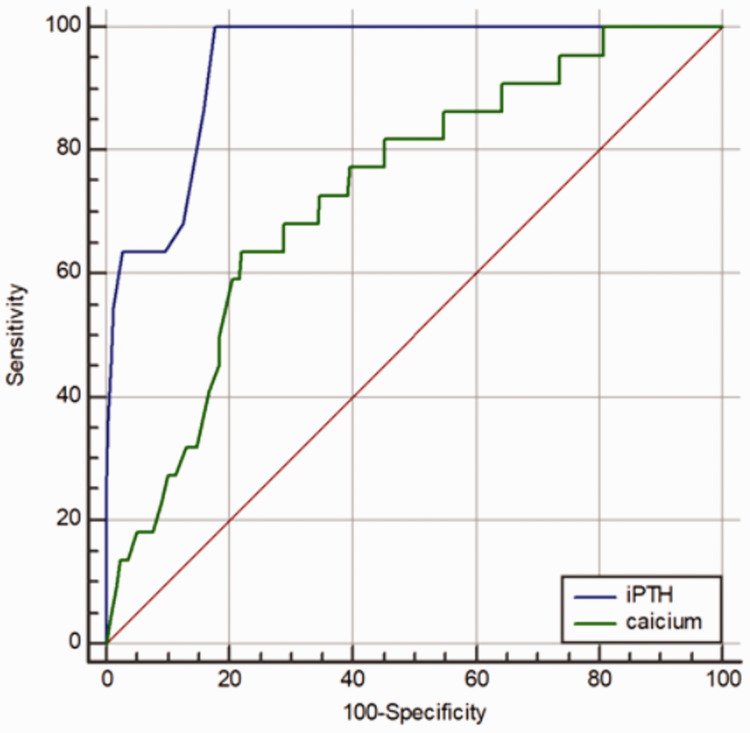
As shown in Figure 1, the receiver operating characteristic curve to predict the occurrence of postoperative pHPP revealed a threshold iPTH concentration of 5.51 pg/mL at 1 day after surgery, with an AUC of 0.956 (95% CI, 0.936–0.972; P < 0.001). Its predictive value was high, with a sensitivity of 100%, specificity of 85.1%, PPV of 22%, and NPV of 100%. When the cut-off serum calcium concentration was set at 1.93 mmol/L, the AUC was 0.733 (95% CI, 0.694–0.770; P < 0.001). Its predictive value was moderate, with a sensitivity of 63.6%, specificity of 78.1%, PPV of 10.8%, and NPV of 98.1%. In contrast, the predictive value of measuring the iPTH concentration was significantly higher than that of measuring the serum calcium concentration (calculated AUC, 0.956 vs. 0.733; 95% CI, 0.936–0.972 vs. 0.694–0.770; P < 0.001).
Figure 1.
Comparison of sensitivity and specificity between serum calcium and intact parathyroid hormone (iPTH) concentrations to predict permanent hypoparathyroidism (pHPP). An iPTH concentration of ≤5.51 pg/mL at 24 hours after total thyroidectomy had 100% sensitivity and 85.1% specificity to predict pHPP. A postoperative serum calcium concentration ≤1.93 mmol/L was 63.6% sensitive and 78.1% specific to predict pHPP. The predictive value of iPTH was significantly higher than that of serum calcium.
Discussion
This study showed that pHPP is not a common complication when total thyroidectomy is performed by an experienced surgical team and that a postoperative iPTH concentration of >5.51 pg/mL may help to predict which patients will not develop this complication.
Identification and protection of the parathyroid gland is a necessary skill for surgeons who perform thyroid surgery and should not be ignored. Total thyroidectomy still has a risk of hypocalcemia and pHPP, even when performed by the most experienced hands.14–16 Almquist et al.17 performed total thyroidectomy in 519 patients with thyroid disease and closely followed them up for a median of 2.7 years (range, 1.2–10.3 years). The multivariate analysis showed that surgical experience, intraoperative accidental parathyroid excision, and the serum PTH and calcium concentrations measured 1 day after total thryoidectomy were independent predictors of the risk of pHPP after thyroid surgery. In the present study, the incidence of pHPP after total thyroidectomy was 4.03%, which is consistent with the results of other studies.18–20 Our multifactor analysis showed that the iPTH and serum calcium concentrations measured 1 day postoperatively were closely related to pHPP after thyroid surgery and were independent risk factors.
Several studies showed that a cut-off iPTH value of 15 pg/mL measured 1 day after thyroid surgery could not predict whether the patient would develop pHPP.21–23 The receiver operating characteristic curve analysis showed that the optimal iPTH threshold was 5.51 pg/mL, which could be expanded to between 5 and 6 pg/mL; this is far lower than the reference data threshold of 15 pg/mL. When an iPTH concentration of 5.51 pg/mL was used as the cut-off point, the AUC was 0.956. In patients with an iPTH concentration of ≤5.51 pg/mL measured at 24 hours after surgery, the sensitivity was 100% for a high risk of pHPP, with a PPV of 22%. The reason for this may be that the final incidence of pHPP was only 4.03%, with a higher predicted false-positive rate. After the operation, the parathyroid function remains in a state of dynamic recovery. Over time, the potentially impaired parathyroid gland function is likely to recover, and it is difficult to predict whether pHPP will develop. An iPTH concentration of ≤5.51 pg/mL indicates to some extent that the iPTH concentration is not likely to return to the reference range within a short period of time and that there is a high risk of pHPP. However, in patients with an iPTH concentration of >5.51 pg/mL, the specificity was 85.1% and NPV was 100%; this was predictive of normal parathyroid function 6 months after surgery. Almost all patients who were excluded would have a risk of pHPP. The reason may be that a higher iPTH concentration on day 1 postoperatively is associated with less damage to the parathyroid during the operation, a better the blood supply to the parathyroid gland, and more rapid return of parathyroid function to the normal level after surgery. Julián et al.24 conducted a prospective study of 70 patients with thyroid surgery and came to a similar conclusion. At 24 hours after surgery, when the iPTH concentration was ≤5.8 pg/mL, the PPV of pHPP was 30% and NPV was 100%. Hermann et al.25 found that the determination of PTH after total thyroidectomy was more advantageous in estimating the occurrence of hypocalcemia than the determination of the serum calcium concentration. The PTH concentration largely determined the occurrence of hypocalcemia. Compared with determination of the iPTH concentration in the present cohort, when the postoperative serum calcium concentration was ≤1.93 mmol/L, the sensitivity was 63.6%, the specificity was 78.1%, and the PPV was only 10.8%. The predictive value of the iPTH concentration was significantly higher than that of the serum calcium concentration. The AUC of >0.9 for the iPTH level measured 1 day after total thyroidectomy suggests the excellent accuracy of this diagnostic test for assessing the occurrence of pHPP after the operation. The serum calcium concentration 1 day postoperatively had a lower accuracy of predicting the occurrence of pHPP.
In thyroid surgery, whether the serum iPTH concentration should be conducted intraoperatively, several hours postoperatively, or 1 day postoperatively is controversial.26–28 In published studies, the timing of iPTH measurements has ranged from 10 minutes to 24 hours after surgery. McLeod et al.29 found that a PTH concentration of <12 pg/mL measured 20 minutes after the operation could predict the occurrence of hypocalcemia, with a sensitivity of 100% and a specificity of 92%. Sywak et al.30 reported that a low PTH concentration of 3 to 10 pg/mL measured 4 hours after surgery had a sensitivity of 90% and a specificity of 84% for predicting postoperative hypocalcemia. Asari et al.31 reported that an iPTH concentration of ≤15 pg/mL at 1 day after surgery predicted hypoparathyroidism with a sensitivity of 97.7% and a specificity of 82.6%. In this study, we determined an iPTH threshold of ≤5.51 pg/mL at 1 day after total thyroidectomy for predicting postoperative pHPP with a sensitivity of 100% and a specificity of 85.1%. Therefore, the best time to measure the serum iPTH concentration is 1 day after surgery to predict whether pHPP will occur after thyroidectomy; this strategy has good feasibility and effectiveness. However, our observation in the present study was limited by a certain deviation in the postoperative time interval (16% loss of information; e.g., some patients might have been tested 20 hours postoperatively and other sooner than that) and by great variability in the time to iPTH measurement.
The present study has some limitations. The study was a retrospective analysis and lacked randomization control. This highlights the risk factors for predicting postoperative pHPP in thyroid surgery and demonstrates the need for more prospective studies in this field. Several parameters have been associated with the risk of pHPP, including the postoperative PTH concentration, decline in PTH concentration between the preoperative and postoperative measurements, degree of decrease in the magnesium concentration, T stage, and whether parathyroid autotransplantation was carried out.32–35 Our results may be biased because the risk of unwanted parathyroid removal is higher when the resection is larger. The percentage of parathyroid glands removed at surgery and/or autotransplanted was likewise not analyzed. Further studies are required to include these relevant factors, all of which may help to assess the risk of developing pHPP postoperatively.
Conclusions
The iPTH concentration measured 1 day after total thyroidectomy is closely related to the occurrence of pHPP postoperatively and is an independent predictive risk factor. Postoperative measurement of the iPTH concentration can be helpful in identifying patients at risk for developing pHPP. The predictive value of the iPTH concentration measured after surgery is much higher than that of the serum calcium concentration. If the concentration of iPTH is >5.51 pg/mL, patients are less likely to develop pHPP after surgery. In contrast, if the measured iPTH concentration is ≤5.51 pg/mL, there is a higher risk of pHPP postoperatively.
Acknowledgements
The authors wish to thank the patients and their families for participating in the study.
Abbreviations
iPTH = intact parathyroid hormone, PTH = parathyroid hormone, pHPP = permanent hypoparathyroidism, AUC = area under the curve, CI = confidence interval, PPV = positive predictive value, NPV = negative predictive value.
Author contributions
Conceptualization: Cai SY, Gao ZG
Data curation: Zheng JW, Song HM, Han G
Investigation: Wang YL, Han XF
Methodology: Zheng JW, Cai SY
Project administration: Wu HL
Visualization: Wang YL
Writing (original draft): Zheng JW
Writing (review and editing): Cai SY, Gao ZG
All authors read and approved the final manuscript.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available because of privacy or ethical restrictions.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Shuyan Cai https://orcid.org/0000-0003-4275-8963
References
- 1.Shoback DM, Bilezikian JP, Costa AG, et al. Presentation of hypoparathyroidism: etiologies and clinical features. J Clin Endocrinol Metab 2016; 101: 2300–2312. [DOI] [PubMed] [Google Scholar]
- 2.Selberherr A andNiederle B.. Avoidance and management of hypoparathyroidism after thyroid gland surgery. Chirurg 2015; 86: 13–16. (in German) [DOI] [PubMed] [Google Scholar]
- 3.Promberger R, Ott J, Bures C, et al. Can a surgeon predict the risk of postoperative hypoparathyroidism during thyroid surgery? A prospective study on self-assessment by experts. Am J Surg 2014; 208: 13–20. [DOI] [PubMed] [Google Scholar]
- 4.Selberherr A, Scheuba C, Riss P, et al. Postoperative hypoparathyroidism after thyroidectomy: efficient and cost-effective diagnosis and treatment. Surgery 2015; 157: 349–353. [DOI] [PubMed] [Google Scholar]
- 5.Prichard RS, Edhouse PJ, Sidhu SB, et al. Post-operative partial hypoparathyroidism: an under-recognized disorder. ANZ J Surg 2011; 81: 524–527. [DOI] [PubMed] [Google Scholar]
- 6.Nawrot I, Pragacz A, Pragacz K, et al. Total thyroidectomy is associated with increased prevalence of permanent hypoparathyroidism. Med Sci Monit 2014; 20: 1675–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lazzara S, Barbera A, Zanghi GN, et al. Prevention, identification and management of postoperative hypoparathyroidism. J Endocr Surg 2018; 18: 121–131. [Google Scholar]
- 8.Sitges-Serra A, Ruiz S, Girvent M, et al. Outcome of protracted hypoparathyroidism after total thyroidectomy. Br J Surg 2010; 97: 1687–1695. [DOI] [PubMed] [Google Scholar]
- 9.Ji YB, Song CM, Sung ES, et al. Postoperative hypoparathyroidism and the viability of the parathyroid glands during thyroidectomy. Clin Exp Otorhinolaryngol 2017; 10: 265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavallaro G, Iorio O, Centanni M, et al. Parathyroid reimplantation in forearm subcutaneous tissue during thyroidectomy: a simple and effective way to avoid hypoparathyroidism. World J Surg 2015; 39: 1936–1942. [DOI] [PubMed] [Google Scholar]
- 11.Orloff LA, Wiseman SM, Bernet VJ, et al. American Thyroid Association statement on postoperative hypoparathyroidism: diagnosis, prevention, and management in adults. Thyroid 2018; 28: 309–349. [DOI] [PubMed] [Google Scholar]
- 12.Ponce de León-Ballesteros G, Velázquez-Fernández D, Hernández-Calderón FJ, et al. Hypoparathyroidism after total thyroidectomy: importance of the intraoperative management of the parathyroid glands. World J Surg 2019; 43: 1728–1735. [DOI] [PubMed] [Google Scholar]
- 13.Šimundić AM. Measures of diagnostic accuracy: basic definitions. EJIFCC 2009; 19: 203–211. [PMC free article] [PubMed] [Google Scholar]
- 14.Carr AA, Yen TW, Fareau GG, et al. A single parathyroid hormone level obtained 4 hours after total thyroidectomy predicts the need for postoperative calcium supplementation. J AM Coll Surg 2014; 219: 757–764. [DOI] [PubMed] [Google Scholar]
- 15.Díaz-Soto G, Mora-Porta M, Nicolau J, et al. Efficacy and safety of long term treatment of unresponsive hypoparathyroidism using multipulse subcutaneous infusion of teriparatide. Horm Metab Res 2012; 44: 708–710. [DOI] [PubMed] [Google Scholar]
- 16.Meola A, Vignali E, Matrone A, et al. Efficacy and safety of long-term management of patients with chronic post-surgical hypoparathyroidism. J Endocrinol Invest 2018; 41: 1221–1226. [DOI] [PubMed] [Google Scholar]
- 17.Almquist M, Hallgrimsson P, Nordenström M, et al. Prediction of permanent hypoparathyroidism after total thyroidectomy. World J Surg 2014; 38: 2613–2620. [DOI] [PubMed] [Google Scholar]
- 18.Hicks G George R andSywak M.. Short and long-term impact of parathyroid autotransplantation on parathyroid function after total thyroidectomy. Gland Surg 2017; 6: S75–S85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serpell JW. Preventing hypoparathyroidism after total thyroidectomy. ANZ J Surg 2018; 88: 127–128. [DOI] [PubMed] [Google Scholar]
- 20.Lorente-Poch L, Sancho JJ, Muñoz-Nova JL, et al. Defining the syndromes of parathyroid failure after total thyroidectomy. Gland Surg 2015; 4: 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcinkowska M, Sniecikowska B, Zygmunt A, et al. Postoperative hypoparathyroidism in patients after total thyroidectomy - retrospective analysis. Neuro Endocrinol Lett 2017; 38: 488–494. [PubMed] [Google Scholar]
- 22.Park YM, Kim JR, Oh KH, et al. Comparison of functional outcomes after total thyroidectomy and completion thyroidectomy: hypoparathyroidism and postoperative complications. Auris Nasus Larynx 2018: S0385814617308465. [DOI] [PubMed] [Google Scholar]
- 23.Ritter K, Elfenbein D, Schneider DF, et al. Hypoparathyroidism after total thyroidectomy: incidence and resolution. J Surg Res 2015; 197: 348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Julián MT, Balibrea JM, Granada ML, et al. Intact parathyroid hormone measurement at 24 hours after thyroid surgery as predictor of parathyroid function at long term. Am J Surg 2013; 206: 783–789. [DOI] [PubMed] [Google Scholar]
- 25.Hermann M, Ott J, Promberger R, et al. Kinetics of serum parathyroid hormone during and after thyroid surgery. Bri J Surg 2008; 95: 1480–1487. [DOI] [PubMed] [Google Scholar]
- 26.Vasileiadis I, Charitoudis G, Vasileiadis D, et al. Clinicopathological characteristics of incidental parathyroidectomy after total thyroidectomy: the effect on hypocalcemia. A retrospective cohort study. Int J Surg 2018; 55: 167–174. [DOI] [PubMed] [Google Scholar]
- 27.Lee YM, Cho JY, Sung TY, et al. Clinicopathological risk factors and biochemical predictors of safe discharge after total thyroidectomy and central compartment node dissection for thyroid cancer: a prospective study. Int J Endocrinol 2015; 2015: 214525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puzziello A, Gervasi R, Orlando G, et al. Hypocalcaemia after total thyroidectomy: could intact parathyroid hormone be a predictive factor for transient postoperative hypocalcemia? Surgery 2015; 157: 344–348. [DOI] [PubMed] [Google Scholar]
- 29.McLeod IK, Arciero C, Noordzij JP, et al. The use of rapid parathyroid hormone assay in predicting postoperative hypocalcemia after total or completion thyroidectomy. Thyroid 2006; 16: 259–265. [DOI] [PubMed] [Google Scholar]
- 30.Sywak MS, Palazzo FF, Yeh M, et al. Parathyroid hormone assay predicts hypocalcaemia after total thyroidectomy. Anz J Surg 2007; 77: 667–670. [DOI] [PubMed] [Google Scholar]
- 31.Asari R, Passler C, Kaczirek K, et al. Hypoparathyroidism after total thyroidectomy: a prospective study. Arch Surg 2008; 143: 132–137. [DOI] [PubMed] [Google Scholar]
- 32.Lee DR, Hinson AM, Siegel ER, et al. Comparison of intraoperative versus postoperative parathyroid hormone levels to predict hypocalcemia earlier after total thyroidectomy. Otolaryngol Head Neck Surg 2015; 153: 343–349. [DOI] [PubMed] [Google Scholar]
- 33.Alqahtani A, Parsyan A, Payne R, et al. Parathyroid hormone levels 1 hour after thyroidectomy: an early predictor of postoperative hypocalcemia. Can J Surg 2014; 57: 237–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vanderlei FA, Vieira JG, Hojaij FC, et al. Parathyroid hormone: an early predictor of symptomatic hypocalcemia after total thyroidectomy. Arq Bras Endocrinol Metabol 2012; 56: 168–172. [DOI] [PubMed] [Google Scholar]
- 35.Lecerf P, Orry D, Perrodeau E, et al. Parathyroid hormone decline 4 hours after total thyroidectomy accurately predicts hypocalcemia. Surgery 2012; 152: 863–868. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available because of privacy or ethical restrictions.