Important Compound Classes
Title
Tropomyosin Receptor Kinase (TRK) Degradation Compounds and Methods of use.
Patent Application Number
WO 2020/038415 A1
Publication Date
February 27, 2020
Priority Application
CN PCT/CN2018/101715 and PCT/CN2019/086894
Priority Date
August 22, 2018, and May 14, 2019
Inventors
Liu, J.; Plewe, M. B.; Wang, J.; Han, X.; Chen, L.
Assignee Company
Cullgen (Shanghai), Inc. [CN/CN], 230 Chuan Hong Road, Bldg. 6, Pu Dong New Area, Shanghai 201202 (CN).
Disease Area
Cancer
Biological Target
Tropomyosin Kinase (TRK)
Summary
The tropomyosin receptor kinase (TRK) family of enzymes belong to the transmembrane receptor tyrosine kinases (RTKs) subfamily that regulate plasticity and synaptic strength in the mammalian nervous system. There are three well differentiated TRK families (TRKA, TRKB, and TRKC that are encoded by NTRK1, NTRK2, and NTRK3 genes, respectively), which play an important role in regulating cell differentiation, proliferation, pain, and survival. In addition, they all have been implicated in the initiation and progression of malignancies, however, with TRKA being clinically relevant for pain and oncology therapy. The major difference between all three isoforms is the ligand that activates the receptor, which is the nerve growth factor (NGF) for TRKA, brain-derived neurotrophic factor (BDNF) for TRKB, and neurotrophin 3 (NT3) for TRKC. The binding of ligands to the extracellular domains of TRKs induces dimerization and activation of the receptors, which invariably induces downstream signal transduction, such as PI3K/ATK, RAF/MEK/ERK, and PLCγ pathways. The residues that interact directly with ATP in the active site share 95% sequence identity between TRKA and TRKB, while TRKB and TRKC are indistinguishable. For the TRK domain, the TRKA hinge is more structurally constrained when compared to TRKB and TRKC.
The primary molecular mechanism activating TRKs in cancer is by in-frame fusions of NTRK genes; however, the incidence of NTRK fusion genes in each specific tumor type overall is rare, which generates difficulties for patient identification and adequate recruitment for clinical trials. The frequency of TRK fusions is relatively low and 0.5% to 2.7% colon cancers are affected by TRK fusions, while in secretory breast carcinoma, TRK fusions can be found in the vast majority of cases. NTRK fusions are found in about 50% of pediatric glioma and nonbrainstem glioblastoma, 16.7% of thyroid cancers, 7.1% of pediatric gliomas, 3.3% of lung cancers, and 2.2% of colorectal cancers. In addition, TRK mutations and deletions have been observed in diseases such as acute myeloid leukemia, anhidrosis syndrome, pulmonary neuroendocrine tumors, obesity, and congenital heart defects. Furthermore, the amplification of TRK is associated with several diseases, including uterine cancer, prostate cancer, liver cancer, invasive breast cancer, urinary bladder cancer, and so forth.
Protein kinases catalyze phosphoryl group transfer to downstream substrate but only have micromolar affinity for ATP, weak kinase activity for ATP, and rapid turnover. As a result, small molecules can effectively bind and block activity of TRK despite a high endogenous concentration of ATP. TRK inhibitors can be broken down into four: type I are ATP competitive and bind to the ATP-binding site, and the majority of these TRK inhibitors are under clinical investigation. The type II are ATP noncompetitive and exhibit noncompetitive or pseudocompetitive binding kinetics. They bind at the ATP-binding site and also to an adjacent allosteric pocket. Type III TRK inhibitors bind to the kinase domain outside of the ATP-binding site. These are true allosteric inhibitors; unlike the Type II inhibitors, they can exploit unique kinase-specific functionality, which may help achieve selectivity among TRK isoforms. Finally, the Type IV TRK inhibitors bind to other regions apart from the kinase domain.
TRKA is the main receptor for the never growth factor (NGF), which plays important roles in central and peripheral pain. The nociceptive neurons express TRKA and mediate pain sensation via transmission of pain signals to the central nervous system (CNS). The efficacy of NGF antibodies in pain relief has been documented in the clinics. In addition, multiple NGF-neutralizing antibodies such as tanezumab are undergoing clinical trial for lower back pain, cancer pain, osteoarthritis, neuropathic pain, etc. However, administration of NGF neutralizing antibodies has shown adverse effects, such as rapid joint destruction, which may be related to sustained exposure to NGF antibodies. Consequently, targeting TRK provides another therapeutic strategy in which the NGF/TRK signaling pathway is blocked in pain management. This strategy is not without problem as currently available pan-TRK kinase inhibitors may induce off-target adverse effects by modulating TRK family members present in the CNS. Nonspecific side effects and resistance to TRK kinase inhibitors remain a formidable challenge for an effective treatment. Currently available selective small-molecule TRK kinase inhibitors reported include entrectinib, altiratinib, cabozantinib, sitravatinib, dovitinib, milciclib, pegcantratinib, larotrectinib, and pegcantratinib.
Another therapeutic strategy involves the peripheral restricted TRK bifunctional degraders, which should selectively block the NGF/TRK pathway in the peripheral nerves and avoid targeting those present in the CNS. Consequently, small-molecule targeting TRK’s functions through degradation may provide an effective alternative therapy. The selective degradation is achieved by recruiting an E3 ubiquitin ligase and mimicking protein misfolding with a hydrophobic tag. The one end of the small molecule binds to an E3 ubiquitin ligase and another binds the protein target of interest. The proximity leads to ubiquitination of the target, which is followed by its degradation via proteasome-mediated proteolysis.
This Patent Highlight contains representative novel bifunctional TRK degraders that are useful in the treatment of TRK-mediated cancers, inflammatory diseases, bone-related diseases, neurodegenerative diseases, neuroblastoma, lung carcinoma, ovarian cancer, and so forth.
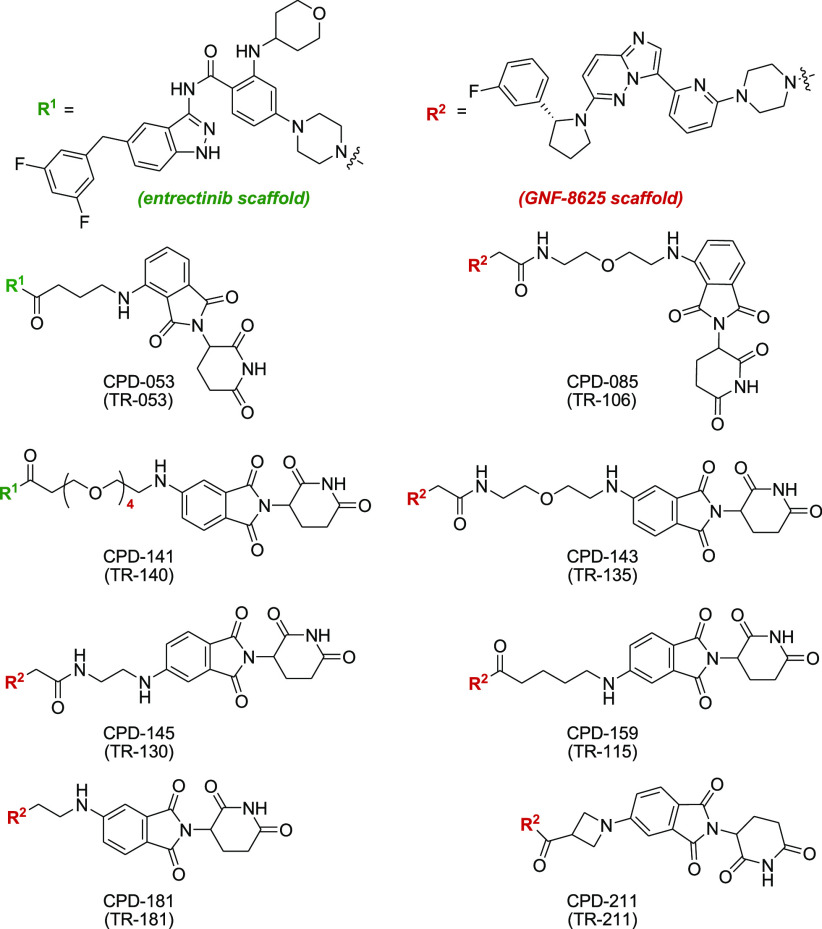
Key Structures
Biological Assay
Total protein concentrations were determined by BCA assays (Beyotime Biotechnology). Cell viability was determined using CellTiter-Glo assay kit (Promega).
Biological Data
The table below shows exemplary compounds
that demonstrated inhibitory activities using cell viability assay
and reported IC50 values. Tumor xenograft and mouse pharmacokinetic
studies were carried out, in addition to monoiodoacetate (MIA) induced
pain models.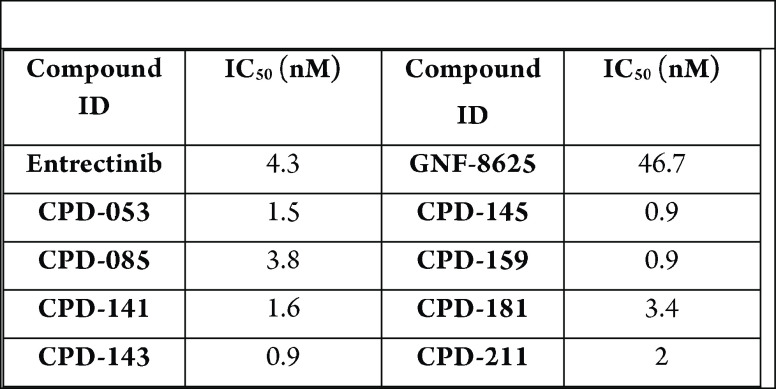
Recent Review Articles
-
1.
Farago A. F.; Demetri G. D.. Future Oncol. 2020, 16, 417.
-
2.
Gambella A.; Collemi G.; Cassoni P.; Bertero L.; Senetta R.; Papotti M.; Vallero S. G.; Fagioli F.; Monticelli M.. Int. J. Mol. Sci. 2020, 21, 753.
-
3.
Jin W.Cancers 2020, 12, 147.
-
4.
Mamdani H.; Jalal S. I.. Ann. Transl. Med. 2019, 7, S155.
-
5.
Yan W.; Lakkaniga N. R.; Carlomagno F.; Santoro M.; McDonald N. Q.; Lv F.; Gunaganti N.; Frett B.; Li H.. J. Med. Chem. 2019, 62, 1731.
-
6.
Miao Q.; Chen D.; Ma K.; Wu X.; Jiang S.. Eur. J. Med. Chem. 2019, 175, 129.
The author declares no competing financial interest.