Important Compound Classes
Title
Proteolysis targeting chimeric (PROTAC) compound with E3 ubiquitin ligase binding activity and targeting alpha-synuclein protein for treating neurovegetative diseases.
Patent Application Number
WO 2020/041331 A1
Publication Date
February 27, 2020
Priority Application
US 62/719,937
Priority Date
August 20, 2018
Inventors
Crew, A. P.; Dong, H.; Berlin, M.; Sparks, S. M.
Assignee Company
Arvinas Operations, Inc., 5 Science Park, New Haven, Connecticut 06511, U.S.
Disease Area
Neurodegenerative Diseases
Biological Target
α-Synuclein Protein
Summary
α-Synuclein (α-syn) is a presynaptic 140 amino acid, intrinsically disordered without secondary structure protein found in many tissues but predominantly expressed in human brain cells and cells of the central nervous system. The misfolding and aggregation of these proteins is a pathological characteristic of Parkinson’s disease (PD) and many other neurodegenerative disorders. The aggregation of α-syn into highly stable amyloid fibrils results in the formation of cytoplasmic Lewy body inclusions, and excreted α-syn can be both monomeric and aggregated and has been found in cerebrospinal fluid and blood plasma. Also, there is evidence that small amounts of α-syn are released from neuronal cells that contribute to the extracellular pool resulting in neurodegeneration as well as to the progressive spreading of α-syn cell pathology that is characteristic of PD. The propensity for α-syn to aggregate is influenced by various factors, including post-translational modifications. There is evidence that the presence of αS phosphorylated at Tyr39, resulting from c-Abl kinase activity, is found in vivo and PD patients display elevated levels of c-Abl compared to healthy individuals.
The α-syn amino acid sequence can be divided into three distinct domains: a central, hydrophobic region which drives the assembly of α-syn into amyloid fibrils, which is flanked by an N-terminal region, and a highly acidic C-terminal region with an overall negative net charge to the α-syn protein.
Spread of the misfolded and aggregated α-syn species is found to be in a prion-like fashion from cell to cell. This process eventually leads to the amplification of fibrils and the spread and progression of specific fibril strain dependent synucleopathies. It is known that α-syn amyloid deposits appear in enteric nerves prior to deposition in the brain; as a consequence, the pathogenesis of synucleinopathies is believed to begin in the enteric nervous system. Clinical studies have supported this hypothesis, and patients suffering from PD experience gastrointestinal problems years before motor deficits emerge.
Alzheimer’s disease (AD) is the most common neurodegenerative disease characterized by the loss of hippocampal neurons and forebrain cholinergic neurons. AD pathology is caused by the aggregation of the 40 or 42 residue peptide, β-amyloid (Aβ), leading to the formation of the characteristic extracellular amyloid plaques found in the brains of AD patients. Although AD pathology predominates in the cerebral cortex and hippocampus, and PD pathology mostly affects the substantia nigra, there is overlap in the symptoms and pathologies of the two diseases. Many patients with AD develop signs of PD, and vice versa, however, PD patients may exhibit more pronounced cognitive dysfunction than AD patients. These findings have raised questions about pathological synergy between Aβ and αS. Expression levels of αS are high in brain regions where AD lesions are abundant, and αS load is associated with Aβ plaques in cortical areas.
This patent highlight discloses bifunctional (PROTAC) compounds, which find utility as modulators of α-syn target protein. The PROTAC compound has one end a Von Hippel–Lindau (VHL), cereblon (CRBN), inhibitors of apoptosis proteins, or mouse double-minute homologue 2 ligand that binds to the respective E3 ubiquitin ligase and the other end a moiety that binds the target protein. The binding allows target protein to be placed in proximity to the ubiquitin ligase to effect degradation of the target protein. Consequently, disorders or diseases such as alpha-synucleinopathies or neurogenerative diseases associated with α-syn accumulation and aggregation (AD, PD, dementia, etc.) are treated or prevented with the representative PROTAC compounds found in this patent highlight.
Definitions
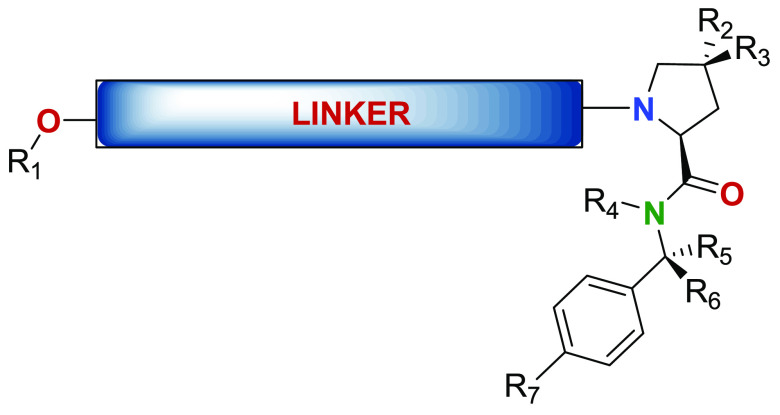
R1 = representative compounds,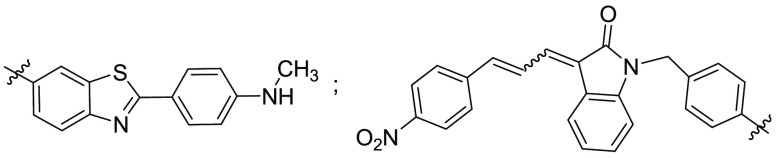
R2 = R3 = H or OH;
R4 = H;
R5 = R6 = H or CH3;
R7 = representative
compounds,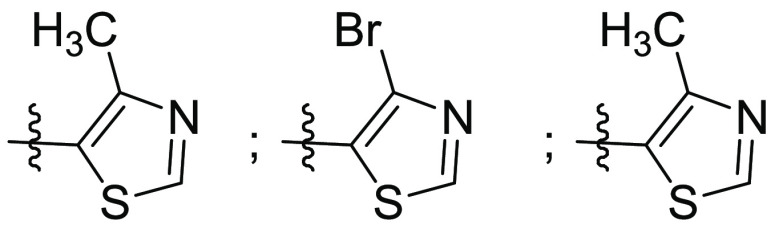
Linker comprising one or more covalently connected structural units.
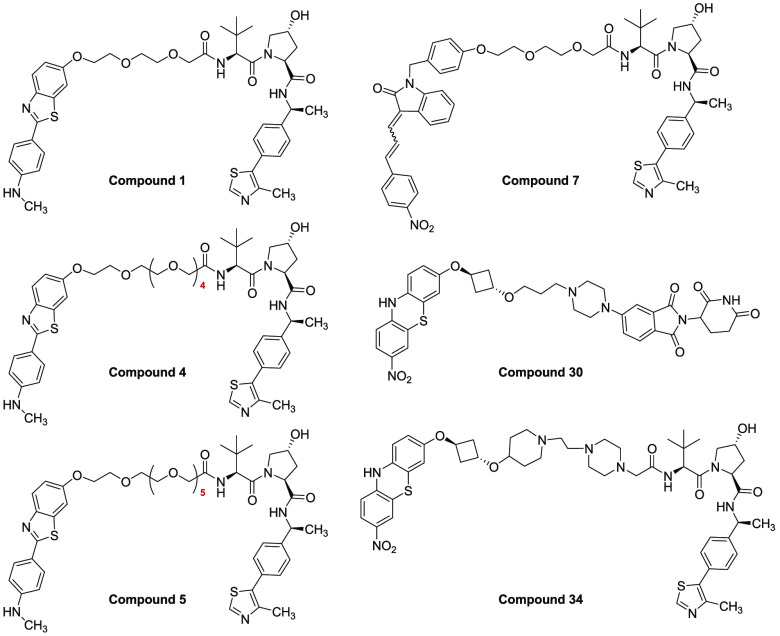
Key Structures
Biological Assay
ELISA was used to evaluate degradation α-synuclein activity in HEK293 TREX α-syn A53T cells.
Biological Data
The table below shows exemplary compounds
that demonstrated α-synuclein target protein degradation. The
α-synuclein-degradations were measured by the amount of protein
remaining relative to DMSO control, where A = less than 35% protein
remaining, B = between 35% and 70% protein remaining, and C = between
70% and 120% protein remaining.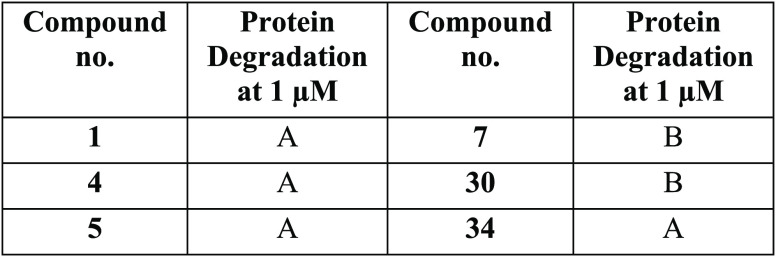
Recent Review Articles
-
1.
Schwab A. D.; Thurston M. J.; Machhi J.; Olson K. E.; Namminga K. L.; Gendelman H. E.; Mosley R. L.. Neurobiol. Dis. 2020, 137, 104760.
-
2.
Fatoba O.; Ohtake Y.; Itokazu T.; Yamashita T.. Front. Mol. Neurosci. 2020, 11, 337.
-
3.
Fernandez-Valle T.; Gabilondo I.; Gomez-Esteban J.. Int. Rev. Neurobiol. 2019, 146, 281. 10.1016/bs.irn.2019.06.014.
-
4.
Savitt D.; Jankovic J.. Drugs 2019, 79, 797. 10.1007/s40265-019-01104-1.
-
5.
Fields C. R.; Bengoa-Vergniory N.; Wade-Martins R.. Front. Mol. Neurosci. 2019, 12, 299.
The author declares no competing financial interest.