Abstract
Background
The most common chemotherapeutic drug for triple-negative breast cancer (TNBC) treatment is 5-fluorouracil (5-FU), but its therapeutic index is low due to its toxicity. β-Elemene (ELE) possesses antitumor activity against different cancers, but it has never been used in combination with 5-FU to improve its chemotherapeutic effect against TNBC.
Materials and Methods
We treated MDA-MB-231 and BT549 cells of TNBC with ELE alone, 5-FU alone, or their combination to investigate their treatment effects on cell viability, proliferation, migration, invasion, and colony formation. We verified the molecular mechanisms of our results through confocal immunofluorescence, immunohistochemistry, and Western blot analysis in vitro and in vivo.
Results
Our result revealed that ELE enhanced the 5-FU effect against cell viability, proliferation, migration, invasion, and colony formation through different mechanisms in MDA-MB-231 and BT549 cell lines. In molecular mechanisms, ELE and 5-FU in combination enhances apoptosis in both cell lines through Bl-2 family protein and caspase cascade modulation, thereby inhibiting NF-kB pathway through IKKβ, IKKα, and p65 downregulation in the cytoplasm and p50 and p65 downregulation in the nucleus. ELE and 5-FU in combination regulated the PI3K/AKT pathway through p-AKT, P-85, p110r, p-PDK1, and p110a protein and RAF-MEK-ERK pathway inhibition through the p-c-raf and p-ERK downregulation. The PI3K inhibitor LY294002 or RAF-MEK-ERK inhibitor U0126 in combination with ELE and 5-FU decreased cell viability in both cell lines significantly, thereby showing the involvement of these pathways in cell apoptosis. In mouse xenograft model, ELE and 5-FU in combination inhibited the tumor growth and modulated its molecular markers.
Conclusion
The conclusion obtained, considering that the results suggest that the combination may be important specifically in the treatment of TNBC.
Keywords: 5-fluorouracil, 5-FU, β-elemene, triple-negative breast cancer, PI3K/AKT, NF-kB, COX2
Introduction
Worldwide Cancer has become the foremost cause of mortality worldwide, with approximately 8.2 million reported deaths and 14 million new cases;1 therefore, the precise treatment of this disease is necessary.2 Breast cancer is a common malignant tumor among females, with approximately 1,700,000 cases and 521,900 deaths in 2012 worldwide.3 Breast cancer is an extremely complex disease that shows a large degree of intra- and intertumoral heterogeneity.4–7 The breast cancer incidence is progressively increasing, particularly in the urban regions of China. Official data predicted a continuing increase in mortality rates for the next 5 years.8 To our knowledge, tumor metastasis remains the dominant cause of cancer-associated mortality.9 Therefore, identifying or developing drugs with antimetastatic ability for breast cancer therapy is necessary.10,11 Elemenes are a group of different natural compounds, including α-, β-, γ-, and δ-elemene, that derived from a number of different medicinal plants and herbs, such as Rhizomazedoariae, which is a dry rhizome derived from Curcuma wenuyujin, Curcuma phaeocaulis, and Curcuma kwangsiensis.2,12 Among these elemenes, β-elemene (ELE) possesses potent anticancer activities that had attracted the interest of scientists.13–15 Following several low-quality and small-scale clinical trials, ELE has been approved for cancer treatment by the Drug and Food Administration of China. ELE have been used to treat different cancers, such are leukemia, liver cancer, breast cancer, and brain carcinoma. ELE acts as an anticancer agent through different molecular mechanisms. For example, ELE induces cell cycle arrest and apoptosis16–18 and reverses multidrug resistance19–21 in various types of cancers, including breast cancer.
5-Fluorouracil (5-FU) is used as a single palliative treatment in combination with other antineoplastic agents for breast cancer treatment. 5-Fluorouracil (5-FU) has been investigated due to its short half-life (6–20 min) and activity duration upon exposure. In early trials, traditional bolus delivery was used in the standard cyclophosphamide, methotrexate, and 5-FU regimens.22–24 5-FU is an antimetabolite chemotherapeutic drug that acts primarily through thymidine synthetase inhibition, thereby resulting in nonfunctional DNA synthesis and causing deoxythymidine monophosphate shortage.25
The two main barriers to 5-FU treatment include its toxicity to normal cells and cancer cell resistance. Therefore, the combined use of 5-FU, along with other natural compounds, can sensitize 5-FU to cancer cells and reduce its toxicity to normal body cells.26
The phosphatidylinositol 3 kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway plays an important role in cell growth and survival through different molecular pathways.27,28 Any disturbance in the PI3K/PTEN/AKT/mTOR and Ras-Raf-MEK-ERK pathways causes genetic alterations, thereby increasing cell proliferation and decreasing apoptosis.28 The PI3K/PTEN/AKT/mTOR and Ras-Raf-MEK-ERK pathway inhibition is useful in cancer treatment.28
Nuclear factor kappa B (NF-κB) is involved in different activities in the body, including activation and development of innate immune cells, negative and positive selection of thymocyte, cytokine production, Ig class switching, and hematopoiesis.29,30 NF-κB regulate cellular network of aging, cancer, and anticancer therapies.31
In the present study, we investigated the combined effects of ELE and 5-FU against TNBC in vitro and in vivo. We hypothesized that ELE may sensitize TNBC cells’ response to 5-FU. To understand its underlying molecular mechanisms, we explored ELE in combination with 5-FU on TNBC proliferation, invasion, migration and apoptosis in BT549 and MDA-MB-231 cells. We also checked the combined effects of ELE and 5-FU on different key proteins, which play pivotal roles in cancer progression through different mechanisms.
Our study suggested that ELE enhances the effect of 5-FU in TNBC cells and mouse xenograft model through the regulation of different signaling pathways and revealed that combined therapy may be effective in TNBC treatment.
Materials and Methods
Cell Line and Cell Culture
MDA-MB-231 and BT549 TNBC cell lines were purchased from the American Type Culture Collection (ATCC Manassas, VA, USA). A monolayer of MDA-MB-231 cells was cultured in 1640 medium supplemented with 10% fetal bovine serum (FBS), penicillin, and 100 streptomycin µg/mL). Similarly, a monolayer of BT459 cells was grown in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with streptomycin, 10 µg/mL penicillin, and 10% FBS. Both cell types were cultured under standard culture conditions in an incubator with 5% CO2 at 37 °C.
Reagents and Antibodies
5-FU was obtained from Sigma Aldrich (St. Louis, MO, USA), and ELE (99.2% purity) was acquired from the Chinese National Institute for Drugs and Food Control (Liaoning, China). The primary antibodies of vimentin, N-cadherin, E-cadherin, mitochondrial membrane potential (MMP)-9, cyclooxygenase 2 (COX-2), p-110α, p-110β, AKT, p- AKT (Ser473), p-PDK (Ser421), p-cRaf (Ser 259), ERK, p-ERK, p38, p-P38, IkB-α, p-IkB-α, p-IKKα/β, IKKα, p-50, p65, and p-P65 were acquired from Cell Signaling Technology, Inc. (USA). Cleaved (cl) PARP, cl-caspase-3, Bcl-2, Bax, CD133, Nanog, CD44, OCT4, β-actin, and GAPDH were purchased from Protein tech (Wuhan, China). Other chemicals were ordered from Sigma unless specified otherwise.
Cell Viability Assay
We used MTT assay to determine the cell’s viability. We cultured 3000 cells/well in 96 well plates for 24 h. After 24 h, fresh medium was added to each well with different dimethyl sulfoxide (DMSO) concentrations, with the final concentration of 0.1%, and dissolved ELE or 5-FU alone or in combination. The control group was treated with 0.1% DMSO. Following 48 h of treatment, MTT that was diluted in medium was added to the cells. After 4 h MTT incubation, we added 150 µL of DMSO in each well and the absorbance values were detected at a wavelength of 490 nm. The data of three independent experiments were presented as mean±SD. The significance level was presented as *P<0.05, **P<.01, ***P<.001. The drug concentration that inhibits 50% of cell growth (IC50) was calculated from the dose-dependent curve, through CurveExpert1.3 software.
Colony Formation Assay
The TNBC cells (2×103 cells/well) were incubated in a 6-well plate for 24 h. Following incubation, we added fresh medium containing ELE (50 µM), 5-FU (10 µM), or their combination. After 48 h of treatment, the cells were washed, trypsinized, and seeded into new 6-well plates (500 cells/well) and incubated for 14 days. After incubation, the colonies were washed with PBS 3 times and fixed with fixation solution (water, glacial acetic:methanol, 1:8:1) for 10 min and stained for 30 min in 0.1% crystal violet solution. The clones with >80 cells were calculated.
Scratch Healing/Wound Healing Assay
Cell migration ability was detected by scratch healing assay/wound healing assay. The cells were cultured in 6-well plates to 80% to 90% confluence. The monolayer cells were scratched with a 100 µL pipette sterile tip and washed through starvation medium to remove the detached cells. The cells were incubated with certain DMSO, ELE, and 5-FU concentrations and incubated at 73 °C in CO2 (5%) in an incubator for 48 h. Following 48 h of treatment washed with PBS three times, wound gaps were studied under a microscope (Leica DM 14000B), and photos were taken. Each scratch width was measured using the Image-Pro Plus 5.1 software.
Cell Invasion Assay
The Matrigel-coated Transwell chambers with a pore size of 80 µm were used to perform the cell invasion assay. The TNBC cells were cultured overnight in 1649 medium without FBS. After overnight incubation, the cells were collected and resuspended. The upper side membrane of the Transwell chamber was coated through 30 mg/l Matrigel in 50 µL of ice-cold, serum free medium and dried at room temperature (RT). A total of 250 µL of cell suspension with a density of 1×105 cells/well and 500 µL of FBS (20%) containing 1640 medium were added in the upper and lower chambers, respectively. The cells were treated for the next 48 h with certain concentration of 50 µM ELE or 10 µM 5-FU or in combination, and control cells were incubated with DMSO (0.1%). Following the treatment, transwell chamber were taken out. From the upper chamber, the medium was washed three times with FBS. The invasive cells were stained with crystal violet (0.1%). Invasiveness is the total number of cells in the different microscopic fields.
Acridine Orange (AO)/Ethidium Bromide (EB) Fluorescence Staining
The cells were cultured for 24 h in 6-well plates and incubated in the presence of 50 µM ELE or 10 µM 5-FU or their combination for 48 h. Following treatment, the control and treated cells were collected and washed with PBS. After washing, the cells were fixed in ethanol (95%) for 15 min and dried. In each well, 10 µL of AO/EB dye (AO 10 µg/mL and EB 10 µg/mL in PBS) were added, and the live and dead cells were studied under a fluorescence microscope (IX81, Olympus).
Confocal Immunofluorescence (IF)
For the performance of confocal microscopy, the cells were grown on chamber slides and treated with 50 µM ELE, 10 µM 5-FU, or their combination and incubated for the next 48 h. Following incubation and washing, the cells were fixed in the paraformaldehyde (4%) at RT for 20 min. Afterward, the samples were incubated using 0.2% (w/v) Triton X-100 in PBS for 10 min to permeabilize. Afterward, the cells were blocked for 30 min through 10% BSA in PBS and incubated overnight at 4 °C with primary antibodies (cytochrome-c [cyt-c], p50, and p-65). Following 10 min of washing through PBS for three times, the cells were again incubated in florescence-labeled secondary antibodies at RT for 1 h. The corresponding localizations of p50, p65, and cyto-c were analyzed using a Leica confocal microscope, and the images were taken and processed through Image-Pro Plus 5.1 software (Frederick, MD, USA).
Sphere Formation
A total of 2000 cells were cultured in 35 mm ultralow attachment plates (Corning, Corning, NY) and supplemented in DMEM/F-12 containing B27 (2%), EGF (20 ng/mL), and bFGF (10 ng/mL) for 15–20 days. The growth factor-containing medium was changed after every 3 days. Then, the sphere formation of cells (>25) with the diameters of >50 μm was counted.
Protein Extraction and Western Blot Analysis
We extracted the whole cell proteins from control and treated cells by using lysis buffer (150 mM NaCl, 0.2 mM Na3VO4, 1 mM EDTA, pH of 7.4, 1 mM EGTA, pH of 8.0, 1% Triton X-100, 0.5 Nonidet P-40, 0.2 phenylmethylsulfonyl fluoride), and the proteins were collected. Next, for cytoplasmic and nuclear proteins, the treated and control cells were lysed through 250 µL of cytoplasmic lysis buffer (300 mM sucrose, 0.5% NP-40, 1.5 mM MgCl2, 10 mM KCl, 10 mM HEPES, pH of 7.9) with different inhibitors (1 gm/mL leupeptin, 10mM NaF, 0.1 mM PMSF, β-glycerophosphate, 0.5 mM dithiothreitol, and 1 mM Na3VO4) on ice for 15 min. In brief, the samples were vortexed and centrifuged for 10 min at 3000 g at 4 °C. The supernatant was collected to determine the expression levels of the cytoplasmic proteins. Then, we used nuclei lysis buffer (420 mM NaCl, 1.5 mM MgCl2, 0.1 mM EDTA, 2.5% glycerol, 20 mM HEPES, pH of 7.9) with 70–80 µL of multiple protease inhibitors. Then, the samples were resuspended and incubated on ice for 30 min. Following incubation on ice, the samples were centrifuged at 1400 ×g for 30 min. The supernatant was collected for nuclear protein expression, and its concentration was determined through BCA assay.
In Western blot, each protein samples with equal amounts (30 µg) were separated through 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrotransferred on a nitrocellulose membrane. Following electrotransfer, the membranes were blocked in 10% of nonfat milk in Tris-buffered saline supplemented with 0.1% Tween 20 (TBST) at RT for 2 h and in the presence of primary antibodies incubated overnight at 4 °C. Following overnight incubation, we washed the membranes three times with cold TBST and incubated again in secondary anti-bodies at RT for 1 h. After incubation, the membranes were washed through TBST, and the protein expression were detected using high chemiluminescence. Each band densitometry was quantified using a Scion Image 4.03.
Animal Experiments
Female nude mice (3–4 weeks old) were purchased and housed in standard environmental conditions in the Animal Centre of Dalian Medical University (DMU). The DMU Laboratory animal use and care protocol was followed during all operations. MDA-MB-231 cells (5×106 cells in 100 µL of PBS) were subcutaneously injected to the left flank of each mouse. As the tumor size became 150 mm3 after cell inoculation, the mice were divided to four groups (n=6). The control group received the intratumor injection of PBS, the second one was treated with ELE (20 mg/kg), the third one with 5-FU (10 mg/kg), and the fourth one was treated with the combination of ELE and 5-FU every day for 13 days. The tumor sizes were measured using a vernier caliper once in every 2 days, and the tumor volume was calculated using the following formula: V=(width2×length)/2. After 21 days of tumor cell inoculation, the experiment was terminated, the mice were sacrificed, tumors were excised, and the weights were measured.
Immunohistochemistry (IHC) Assay
Several parts of the mouse tumors were fixed in formalin (10%) and embedded in paraffin wax for IHC with antibodies against p-ERK (1:100), p-AKT (1:100), p-P65 (1:50), vimentin (1:100), and OCT4 (1:100). One part of the tumors was used for Western blot analysis.
Densitometry Analysis
The densities of Western blot protein bands were determined through Scion Image software (Frederick, MD). Data were expressed as an arbitrary unit.
Statistical Analysis
All experiments were performed three times. The results of at least three measurements were obtained and expressed as mean±SD. For all statistical analysis we used GraphPad prism and SPSS-16. The values of control and test samples were determined through ANOVA and Tukey’s test. P<0.05 was significantly different.
Results
ELE Sensitized with 5-FU to Inhibit Tumor Growth and Proliferation and Induce Morphological Changes in MDA-MB-231 and BT-549 Cells
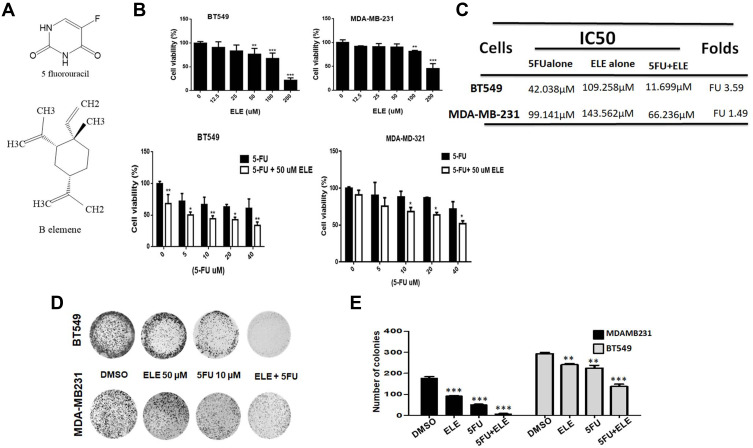
The chemical structures of ELE and 5-FU is shown in Figure 1A. To determine whether ELE increases 5-FU-mediated cell growth inhibition and enhances its chemotherapeutic effect in TNBC, we first examined the effects of ELE, 5-FU, or their combination on cell viability and colony formation in BT-549 and MDA-MB-231 cell lines. To determine the effect of ELE and 5-FU cell viability, we perform MTT assay for which we treat the cells with different concentrations of ELE and 5-FU. After evaluation of different concentration of ELE and 5-FU, we select the dose of ELE (50 µM) and 5-FU (10 µM) for further experiment due to its significant and time dependent effect in both cell lines. Furthermore, ELE significantly inhibited cell viability dose-dependently in BT549 (25–200 µM) and MDA-MB-231 (50–200 µM) cells, as shown in Figure 1B. The cells treated with 5-FU (5–40 µM) alone also suppressed BT549 and MDA-MB-231 cell viability dose-dependently, as shown in Figure 1B. However, the combined treatment of ELE (50 µM) and 5-FU (5–40 µM) significantly increased the 5-FU-mediated cell viability inhibition in both cell lines, as shown in Figure 1B.
Figure 1.
The combined effects of ELE and 5-FU on MDA-MB-231 and BT549 cell proliferation, morphology, and colony formation.
Notes: (A) ELE and 5-FU structures. (B) MDA-MB-231 and BT549 cell lines were treated with ELE alone, 5-FU alone, or their combination with indicated concentrations; MTT assay was used to test cell viability. (C) The IC50 values of 5-FU alone or the combination of 5-FU and ELE were calculated using the CVXPT32 software. (D) MDA-MB-231 and BT549 were treated with 5-FU alone, ELE alone, or their combination. An inverted microscope with 200 μm scale bars was used, and the representative photos of cell morphology were captured. (E) The colony formation assay of BT549 and MDA-MB-231 cells treated with 5-FU, ELE, or their combination was performed to test cell proliferation capacity. The number of colonies was calculated, and photographs were taken. The results from three different experiments were expressed as mean ± SD. *P<0.05, **P<0.01, and ***P<0.001 were considered significant.
Next, we examined the IC50 values of 5-FU for cell viability inhibition compared with those of the control cells. The cotreatment of ELE significantly increased MDA-MB-231 and BT549 cell sensitization to 5-FU treatment, which decreased the IC50 value of 5-FU compared with the 5-FU alone treatment of BT-549 and MDA-MB-231 cells. The BT549 cells were more sensitive to 5-FU compared with MDA-MB-231 cells (Figure 1C).
The combined treatment of ELE and 5-FU significantly increased the inhibition of colony formation in MDA-MB-231 and BT549 cells compared with individual treatments, as shown in Figure 1D and E.
ELE Combined with 5-FU Enhanced Migration Inhibition and TNBC Cell Invasion
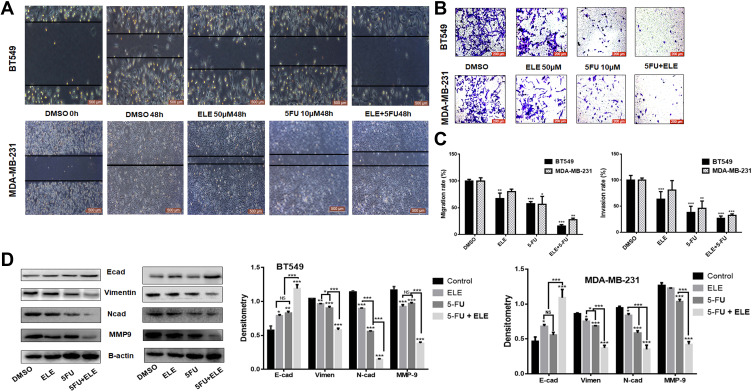
We examined whether ELE can increase the 5-FU-mediated invasion and migration inhibition in MDA-MB-231 and BT549 cell lines. Thus, we performed a wound healing assay for BT549 and MDA-MB-231 cells. The result showed that the individual treatments of ELE or 5-FU inhibited cell migration, but cell migration was significantly inhibited after the combination treatment with ELE and 5-FU. The wound space or gap between the cell layers in the control group was approximately filled by migrated cells in both cell lines after 48 h. However, the cells treated with the combination of 5-FU and ELE were unable to migrate and fill the space in both cell lines, as depicted in Figure 2A. The quantitative analysis showed that the combined treatment of 5-FU and ELE significantly inhibited the migration in both cell lines, as depicted in Figure 2C.
Figure 2.
ELE enhanced 5-FU inhibition activity on MDA-MB-231 and BT549 cell line invasion, migration, and related pathways.
Notes: (A) Wound-healing assay was performed in both cell lines to test cellular migration after treatment with 5-FU, ELE, or their combination. (B) We tested the capacity of the two types of cell invasion following 48 h of treatment with 5-FU, ELE, or their combination via transwell assay. Crystal violet was used to stain the cancer cells, and an inverted microscope was used to capture the images. (C) We used a software to calculate the rate of cell migration and invasion (*P<0.05, **P<0.01, ***P<0.001). (D) We performed Western blot to determine the expression levels of the main EMT concern proteins N-cadherin, E-cadherin, vimentin, and MMP9 of the two cell lines after treatment with ELE alone, 5-FU alone, or their combination. The data obtained from three independent experiments were presented as mean ± SD, and the significance levels were *P<0.05, **P<0.01, and ***P<0.001.
Next, we performed the transwell invasion assay for the evaluation of combined effects of 5-FU and ELE on the invasive capability of BT549 and MDA-MB-231 cell lines. The invasive cells were stained on the lower surface of the filter and counted. The individual treatments of 5-FU and ELE significantly decreased the penetrated cell number compared with that in the control group. Meanwhile, the combined effects of 5-FU and ELE further significantly inhibited the cells from penetrating the Matrigel of both cell lines, as shown in Figure 2B. These quantitative analyses revealed that the combined treatment of ELE and 5-FU significantly increased cell invasion inhibition compared with the individual treatments of ELE or 5-FU in both cell lines, as illustrated in Figure 2C. We also verified our results by checking the expression of the protein marker from cell migration and invasion. Vimentin and MMP-9, which are the key proteins for cell migration, were significantly inhibited by the combined treatment of ELE and 5-FU compared with the individual treatments of ELE or 5-FU alone in both cell lines, as shown in Figure 2D. E-Cadherin and N-cadherin are the key proteins associated with invasion. The combined treatment of 5-FU and ELE considerably promoted the E-cadherin expression while inhibiting the N-cadherin expression, as shown in Figure 2D. Therefore, ELE enhances the 5-FU-induced inhibition of invasion and migration in MDA-MB-231 and BT549 cells.
ELE Increased 5-FU Induced Apoptosis by Modulating Mitochondrial-Dependent and Caspase Signaling Pathways
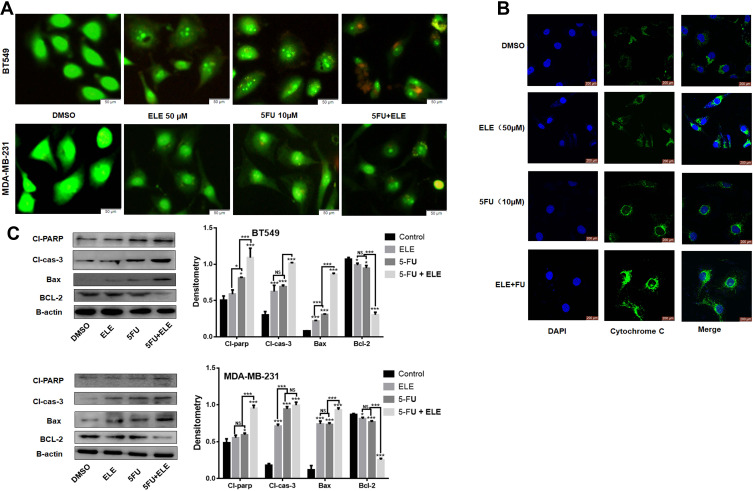
The apoptosis pathway is the potential therapeutic target for cancer treatment.32,33 To determine whether ELE can increase the growth inhibition effects of in MDA-MB-231 and MB-549 cell lines by activating certain apoptotic pathways, we used the AO/EB assay to test the status of tumor cell apoptosis. In this phenomenon, additional EB passed through the cell membrane of damaged cells and embedded in DNA in nucleus, thereby resulting in a bright orange-red color fluorescence in the nuclear region in cells treated with ELE and 5-FU compared with those treated with ELE or 5-FU alone and the control group, as shown in Figure 3A. These results indicated that apoptosis was induced in both cell lines when exposed to combined treatment of ELE and 5-FU but not due to their individual treatments.
Figure 3.
ELE enhanced 5-FU-mediated apoptosis in MDA-MB-231 and BT549 cell lines.
Notes: (A) Both cell lines were treated with indicated concentrations of ELE, 5-FU, their combinations and incubated for 48 h. Following incubation, the cells were stained with AO and EB, and images were captured. (B) We checked the cyt-c release from the mitochondrial intermembrane space after MDA-MB-231 cells were treated with ELE, 5-FU, or their combination for 48 h via the immunofluorescence assay. (C) The expression levels of major proteins, including those of Bcl-2, Bax, cl-caspase-3, and cl-PARP, were determined in both cell lines through Western blot after the treatment with ELE, 5-FU, or their combination. The data obtained from three independent experiments were presented as mean ± SD, and the significance levels were *P<0.05 and ***P<0.001.
We also detected the expression levels of several key proteins involved in apoptosis (eg, Bcl-2, Bax, Cl-caspase-3,9, and Cl-Parp) induced by ELE and 5-FU in both cell lines, as shown in Figure 3C. The Bcl-2 expression significantly decreased in the combined ELE and 5-FU treatment compared with that in their individual treatments. By contrast, the expression levels of Bax, cl-caspase-3, and cl-parp significantly increased in the combined treatments of ELE and 5-FU compared with their individual treatments in both cell lines.
Cyt-c is an upstream regulator of the caspase-dependent pathway. Therefore, we performed IF assay to determine the cyt-c subcellular localization. We performed IF analysis to determine the cyt-c subcellular localization. Treatment with ELE or 5-FU alone triggered the cyt-c release from the inner mitochondrial space to the cytosol in BT549 and MDA-MB-231 cells, but the combined treatment of ELE and 5-FU significantly increased the cyt-c release from the inner mitochondrial space to cytosol, as shown in Figure 3B.
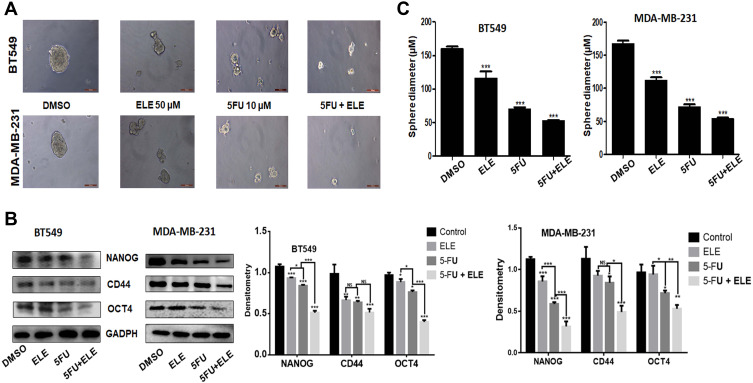
ELE Combined with 5-FU Inhibited Triple-Negative Breast Cancer Cell Sphere Formation
Tumor cell regeneration, reproduction, malignancy, and clinical healing effects are involved in tumor formation. Therefore, to determine whether ELE can enhance the ability of 5-FU in inhibiting BT549 and MDA-MB-231 cell formation, we must pass the sphere formation experiment. The results of the sphere formation experiments are shown in Figure 4A. The results showed that ELE combined with 5-FU can significantly reduce sphere formation in both cell types compared with that of ELE or 5-FU alone. To determine the action mechanisms of ELE in combination with 5-FU, we verified the expression levels of several proteins that are closely related to tumor growth inhibition. As illustrated in Figure 4B, the protein expression levels of Nanog, CD44, and OCT4 were significantly decreased when ELE was used in combination with 5-FU. Figure 4C shows the diameters of MDA-MB-231 and BT549 cells after the treatments with ELE alone, 5-FU alone, and ELE combined with 5-FU. After combination therapy, the diameter of the cancer cell spheres was significantly reduced, which was completely consistent with the conclusions obtained in Figure 4A. According to the results above, the treatment with the combination of ELE and 5-FU can enhance the ability to decrease the sphere formation in TNBC cells.
Figure 4.
Effects of ELE and 5-FU on TNBC MDA-MB-231 and BT549 cells.
Notes: (A) Both cell types were grown in 3D treated with drugs for 14 days, and we used inverted microscope to take photos and quantitative for structural integrity of cancer cells. (B) The CD44, OCT-4, and Nanog protein expression levels were determined through Western blot in MDA-MB-231 and BT549 cells after ELE alone, 5-FU alone, or their combinations. The data obtained from three independent experiments were presented as mean ± SD, and the significance levels were *P<0.05, **P<0.01, and ***P<0.001. (C) Sphere were calculated of three independent experiment, and the data were presented as mean ± SD with the significance level of ***P<0.001.
ELE and 5-FU Combination Suppressed PI3K/AKT Signaling
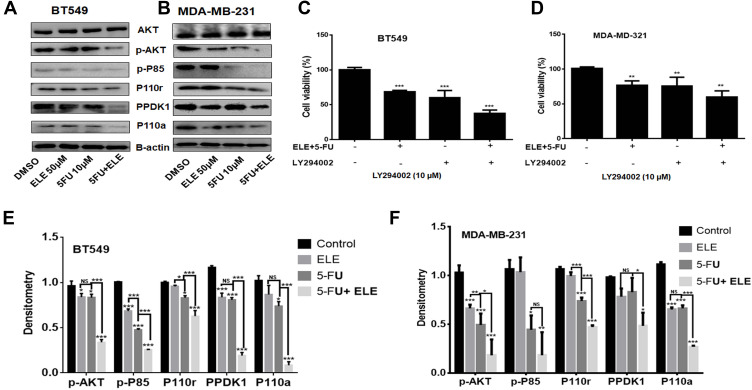
The PI3K/AKT pathway is involved in death and proliferation of cancer cells.34,35 ELE targets different cancer types through different mechanisms, including the PI3K/AKT/mTOR pathway.2 Melatonin synergizes the anticancer activity of 5-FU through PI3K/AKT pathway modulation.36 In the present study, we analyzed the effects of ELE and 5-FU on the expression levels of several proteins that are associated with PI3K/AKT signaling in MDA-MB-231 and BT549 cell lines through Western blot analysis. In both cell lines, the combination of ELE and 5-FU considerably inhibited p-AKT, P-85, p110r, p-PDK1, and p110a phosphorylation compared with the individual treatments of ELE or 5-FU. The protein band quantitative analysis results also revealed that the combined treatment of ELE and 5-FU suppressed p-AKT, P-85, p110r, p-PDK1, and p110a protein phosphorylation significantly in BT549 and MDA-MB-231 cell lines, as shown in Figure 5A,B,E, and F. Next, we confirmed the involvement of PI3K/AKT signaling pathway in the ELE and 5-FU combine inhibition of BT549 and MDA-MB-231 cells. The cells were treated with the combination of ELE and 5-FU after 8 h of pretreatment with the PI3K-specific inhibitor LY294002. Following 48 h incubation, MTT assay was performed to analyze the cell proliferation, as shown in Figure 5C and D. PI3K-specific inhibitor LY294002 alone inhibited cell viability. However, the combination treatment of ELE and 5-FU significantly decreased cell viability. These results suggested that the combination of ELE and 5-FU inhibited the PI3K/AKT pathway, thereby effectively inhibiting cell proliferation.
Figure 5.
ELE and 5-FU combined effects on MDA-MB-231 and BT549 cell lines.
Notes: (A and B) Both cell lines were treated with ELE alone, 5-FU alone, or their combination. The AKT, p-AKT, p-P85, p110r, PPDK1, and P110a expression levels were checked following treatment via Western blot analysis. We used GAPDH as the loading control. (C and D) Both cell lines were pretreated with PI3K inhibitor (LY294002) and then cultured with combination of ELE and 5-FU at an indicated dose. After 48 h, cell viability was performed through MTT assay. The data obtained from three independent experiments were presented as mean ± SD, and the significance levels were ***P<0.001 and **P<0.01 respectively. (E and F) All proteins were quantitatively analysed. The data obtained from three independent experiments were presented as mean ± SD, and the significance levels were *P<0.05, **P<0.01, and ***P<0.001.
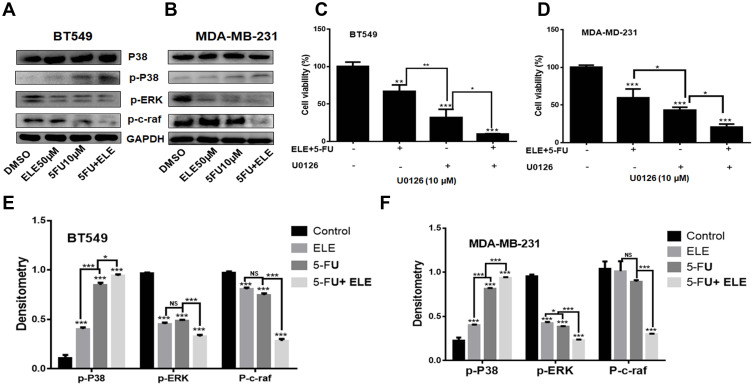
ELE Combined with 5-FU Enhanced Cell Proliferation Inhibition and RAF-MEK-ERK Signaling Pathway Modulation
We investigated the relationship between ELE and 5-FU in MDA-MB-231 and BT549 cells and on the RAF-MEK-ERK signaling pathway-related proteins via Western blot experiments. As shown in Figure 6A,B,E, and F, compared with the use of only 5-FU or ELE, the treatment with the combination of ELE and 5-FU treatment of BT549 and MDA-MB-231cells can significantly inhibit phosphorylated p-ERK and pc-raf protein expression and increased the p-p38 protein expression. To investigate whether the RAF-MEK-ERK signaling pathway is associated with ELE-mediated 5-FU inhibition of the growth of both cell lines, we performed MTT assays to test cell proliferation. First, both cell lines were treated with the RAF-MEK-ERK specific inhibitor U0126 for 8 h and with the combination of ELE and 5-FU for 48 h, as shown in Figure 6C and D. The MDA-MB-231 and BT549 cell treatment with U0126 alone can inhibit cell viability, but U0126 combined with ELE and 5-FU can significantly decrease cell viability. The experimental results above showed that the combination of ELE and 5-FU can inhibit the RAF-MEK-ERK pathway, thereby interfering with tumor cell proliferation.
Figure 6.
The combined effects of ELE and 5-FU on MDA-MB-231 and BT549 cell lines.
Notes: (A and B) Both cell lines were treated with ELE alone, 5-FU alone, or their combination. Following treatment, we used Western blot to check the expression levels of the major Raf-MEK-ERK signaling pathway proteins, including p-p38, p-38, p-c-Raf, and p-Erk1/2. We used GAPDH as a loading control. (C and D) MDA-MB-231 and BT549 cells were pretreated with the MEK inhibitor U0126 and cultured with the combination of ELE and 5-FU at an indicated dose. After 48 h, the cell viability was checked through MTT assay. The results from three different experiments were expressed as mean ± SD, and the significance level were *P<0.05, **P<0.01, ***P<0.001. (E and F) The protein quantitative analyses were performed. The data obtained from three independent experiments were presented as mean ± SD, and the significance levels were *P<0.05 and ***P<0.001.
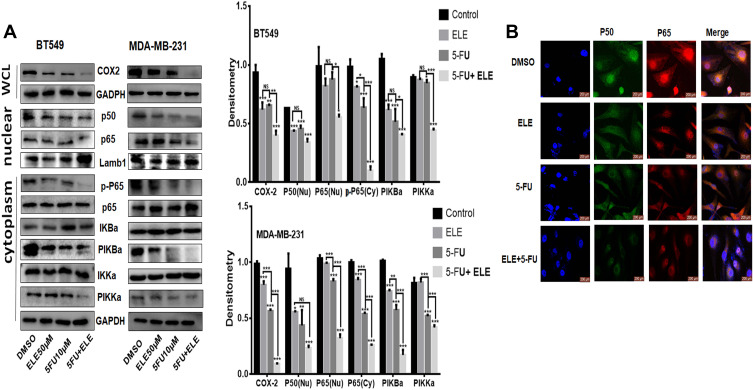
ELE and 5-FU Combination Suppressed NF-κB/COX2 Signaling Pathway
NF-κB is evolutionary conserved, and it controls immune and inflammatory responses. Previous reports showed that NF-κB controls the cellular network of aging, anticancer therapies, and cancer.31 We want to determine the combined effects of ELE and 5-FU on the NF-κB pathway. The cells were treated for 48 h with ELE alone, 5-FU alone, or their combination, and cytoplasmic and nuclear proteins were isolated to determine the expression levels of the key proteins that are involved in the NF-κB signaling pathway. Our results revealed that the combined treatment of ELE and 5-FU suppressed IKKα, IkBα, and p65 phosphorylation in the cytoplasm. However, the total IKKα, IκBα, and p65 expression levels were unaffected, as illustrated in Figure 7A. The combined treatment of ELE and 5-FU significantly inhibited the translocation of p50 and p65 into the nucleus compared with that of the alone treatment in both cell lines, as shown in Figure 7A. Next, we performed IF assay to determine the NF-κB localization in cells. The results suggested that cotreatment with ELE and 5-FU inhibited NF-κB p50/p65 translocation from the cytoplasm to the nucleus, as shown in Figure 7B. The expression level of COX-2 is not only associated with the different cancer types but also with the increase in metastasis; therefore, its inhibition can lead to cancer reduction.37 In the present study, we detected the effects of ELE and 5-FU on the COX-2 expression in TNBC cells. The results indicated that the combined treatment with ELE and 5-FU significantly inhibited the COX-2 expression level in both cell lines compared with that of treatment with ELE or 5-FU alone, as shown in Figure 7A. The results above indicated that ELE combined with 5-FU can interfere with the growth of tumor cells by interfering with the NF-κB/COX2 signaling pathway in TNBC.
Figure 7.
The combined effects of ELE and 5-FU on NF-κB/COX-2 signaling pathway.
Notes: (A) MDA-Mb-231 and BT459 cells were treated with ELE alone, 5-FU alone, or their combination. We used Western blot to check the p65 and p50 expression levels in the nucleus from the nuclear lysate, p-P65, p65, p-IKKα/β, p-IκBα, and IκBα in the cytoplasmic lysate while detecting the COX-2 expression level from the whole cell lysate. We used GAPDH as the loading control for whole cell and cytoplasmic proteins, while Lamb1 was used for nuclear proteins. The data obtained from three independent experiments were presented as mean ± SD, and the significance levels were *P<0.05, **P<0.01, and ***P<0.001. (B) The subcellular localization of p65 and p50 was detected through immunofluorescence assay of BT549 cells after treatment with ELE alone, 5-FU alone, or their combination.
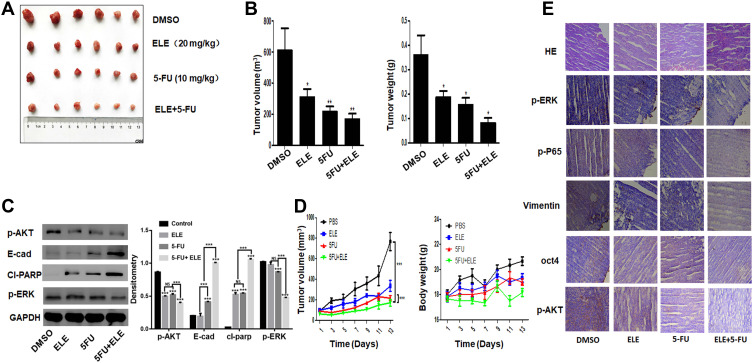
ELE and 5-FU Combination Inhibited Tumor Growth in TNBC Mouse Model
We obtained our in vitro results and investigated the potential therapeutic activity of ELE and 5-FU in the TNBC mouse xenograft model. We injected the MDA-MB-231 cells to left flank of nude mice. After 7 days, the visible tumors were developed with average tumor size 150 mm3. The mice were randomly divided into four different groups. Following ELE and 5-FU administration for 15 days, and the tumor volume and weight (Figure 8A,B, and D) were decreased by treatment with ELE or 5-FU alone. The combined treatment of ELE and 5-FU decreased tumor growth in the xenograft model compared with the treatment with ELE or 5-FU alone. The combined treatment did not cause any signs of toxicity or significantly altered the mouse body weight (Figure 8D). The xenograft tumor lysate on the Western blot analysis revealed that the combined treatment of ELE and 5-FU decreased the expression levels of key proteins that are involved in tumor growth, such as p-AKT and p-ERK, while increasing the E-cadherin and cl-parp expression levels (Figure 8C). Hematoxylin and eosin staining revealed that the control mouse tumor cells were large, irregularly shaped with additional cytoplasm, and had deformed nuclei (Figure 8E). However, the ELE- and 5-FU-cotreated mouse tumor cells were regularly shaped with small nuclei (Figure 8E). The IHC assay was used to determine the expression levels of p-ERK, p-P65, p-AKT, vimentin, and OCT4. The p-ERK, p-P65, p-AKT, vimentin, and OCT4 expression levels were decreased by the cotreatment of ELE and 5-FU compared with those treated with ELE or 5-FU alone and control group. The result supports our in vitro result that ELE enhancing the effect of 5-FU and enhances the inhibition of TNBC xenograft.
Figure 8.
The combined effects of ELE and 5-FU on TNBC tumor xenograft mouse model.
Notes: Female athymic nude mice (3–4 weeks old) were used in the present study. In the left flank of each mouse, MDA-MB-231 cells (5×106 in 100 μL of PBS) were subcutaneously injected. When the tumor sizes reached 150 mm3, the mice were divided randomly into four groups. The control, second, third, and fourth groups were treated with PBS, ELE (20 mg/kg), 5-FU (10 mg/kg), and combined ELE (20 mg/kg) and 5-FU (10 mg/kg), respectively. The mice were treated daily from 08:00 to 9:00 a.m. To maintain the biological activity of mice, they were provided with 12 h light/dark cycle (light, 8:00 am to 20:00 pm; dark, 20:00 pm to 08:00 am). The mice body weight and tumor volume (E) were measured once every 3 days. Tumor volume was calculated using the following formula: V=(width2×length)/2. At the end of experiment, the tumors from all mice were excised, and pictures were taken (A). The tumor weight and volume (B) were also calculated. The data obtained from three independent experiments were presented as mean ± SD, and the significance levels were *P<0.05 and **P<0.01. (C) The p-ERK, p-AKT, cl-parp, and E-cadherin expression level were checked via Western blot analysis. The data obtained from three independent experiments were presented as mean ± SD, and the significance levels were ***P<0.001. (D) The data of tumour volume were presented as mean ± SD, and the significance levels were ***P<0.001. (E) Immunohistochemical analysis was performed for p-ERK, p-AKT, p-P65, vimentin, and OCT4.
Discussion
5-FU is a potential chemotherapeutic agent and often used for breast cancer treatment.38,39 5-FU is the most commonly used agent for breast cancer treatment but resisted by cancer cells. Therefore, the rapid recovery of 5-FU is needed to overcome this resistance.
ELE has antitumor activity in different types of cancers, including breast, lung, and gastric cancers.40–44 ELE exerts proapoptotic and antiproliferative activities in breast cancer through targeting multiple signaling pathways.43,44 A 5-FU and ELE have antitumor effects in different cancers but their combined effects have never been studied. In this study, we investigated the response of TNBC cells to the combined effects of ELE and 5-FU. Our results indicated that ELE and 5-FU in combination enhanced the inhibitory effects on TNBC cell growth, which was mediated through the simultaneous modulation of the mitochondrial-dependent pathway (Bcl-2 and Bax) and the activation of the apoptosis inhibition cascade, PI3K/AKT, RAF-MEK-ERK, and NF-κB/COX-dependent signaling pathways). To the best of our knowledge, this study is the first to determine the combined effects of ELE and 5-FU in TNBC cells. Our findings may serve as a guide for the use of combined natural antitumor compounds to increase the efficacy of available drugs in TNBC treatment.
In cancer cells, the mitochondrial dysfunction increases glycolysis, induces resistance, and decreases apoptosis;45 if it is reversed, then a patient can lead a normal life. Apoptosis is an important physiological process that play essential role in tissue homeostasis and development.46 The Bcl-2 family proteins are accompanied by caspase activation of caspases, which are the main features of mitochondrial-mediated apoptosis in cancer cells.47–49 In the present study, ELE significantly increased the 5-FU-mediated Bcl-2 inhibition and Bax activation. These results revealed that the decrease in cell proliferation induced by the combined treatment with ELE and 5-FU was associated with the mitochondrial dependent pathway through the modulation of Bcl-2 family proteins. The released cyt-c can bind to ATP and then to pro-caspase-9 for apoptosome formation, which induces the cleavage of pro-caspase-9 to cleaved caspase-9 and activates effector caspase-3 Parp activation.46,50 In the present study, ELE increased the 5-FU mediated caspase-3 and parp activation. Therefore, our result showed that the inhibition of cells proliferation is induced through caspase dependent pathway.
The PI3K/AKT/mTOR signaling pathway increases cell survival and growth through different mechanisms.28 The PI3K/Akt pathway controls the COX-2 and AKT expression and may play an essential role in tumor growth.51 ELE modulates the PI3K/AKT/mTOR pathway via PI3K inhibition, which further inhibits Akt, mTOR, and p70S6K1, thereby leading to apoptosis.52 ELE also inhibits the iNOS and COX-2 expression.53,54 The targeting of PI3K/AKT and COX-2 signaling may provide promising therapeutic implications in cancer treatment. Our present study showed that the cotreatment with ELE and 5-FU inhibited PI3K/AKT signaling in TNBC cells.
The mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) pathway, which is also called Ras/Raf/MEK/ERK pathway, possesses several cascades but are mostly deregulated (eg, Ras/Raf/MEK/ERK1/2) in human cancers.55 The MAPK/ERK pathway controls several cell functions, including cell growth, differentiation, apoptosis, proliferation, migration, senescence, and apoptosis.56 ELE alone57,58 and the combination of 5-FU and Lachnum exopolysaccharide inactivate the Ras/Raf/MEK/ERK pathway.59 The present results showed that the combined effects of ELE and 5-FU controlled the Raf-MEK-ERK signaling pathway in TNBC cells.
COX-2 is a causative agent of carcinogenesis and inflammatory diseases60–63 and is resistant to apoptosis;64,65 its inhibition with nonsteroidal anti-inflammatory drugs effectively inhibits angiogenesis and proliferation and increases apoptosis in cancer cells. COX-2 overexpression is involved in many types of tumor development.66 ELE inhibits the NF-κB pathway by inhibiting NF-κB and NF-κB p65, thereby further inhibiting COX-2; hence, PGE2 is downregulated, and cell proliferation is inhibited.53,54 Our results demonstrated that ELE markedly increased the 5-FU-induced inhibition of COX-2 in TNBC cells. The results also indicated that the molecular mechanism of the NF-κB/COX-2 signaling pathway may be due to the combined effects of ELE and 5-FU. Our results indicated that the ELE increased the 5-FU-mediated inhibition of COX-2 by suppressing the IKKα/β and IκBα phosphorylation, which inhibited the NF-κB p65/p50 protein translocation from cytoplasm into nucleus and abrogated the binding of p65 to the promoter region of COX-2. Thus, COX-2 expression is suppressed. COX-2 has a critical role in different pathophysiological processes, including tumorigenesis and NF-κB deregulated activation to chemoresistance; thus, inhibiting COX-2 expression via the inactivation of the NF-κB signaling pathway mediated through the combined treatment of ELE and 5-FU is a considerable improvement in chemotherapy sensitivity. The inhibition of PI3K/AKT, RAF-MEK-ERK, and NF-κB/COX-2 signaling partially contributed to the ELE and 5-FU-mediated suppression of cell proliferation. Thus, ELE can enhance the therapeutic ability of 5-FU in TNBC cells through the PI3K/AKT, RAF-MEK-ERK, and NF-κB/COX-2 signaling pathways.
Our in vitro experiment also validated the combined effects of ELE and 5-FU in the TNBC mouse model. The combined treatment of ELE (20 mg/kg) and 5-FU (10 mg/kg) significantly inhibited tumor growth through PI3K/AKT, NF-κB, and apoptosis signaling pathway suppression. During the entire experiment, we did not observe any sign of in vivo toxicity, which may be due to the fact that low 5-FU or ELE dose decreased or eliminated the toxicity of 5-FU. Further studies are needed to investigate and improve the combined treatment of ELE and 5-FU.
Conclusions
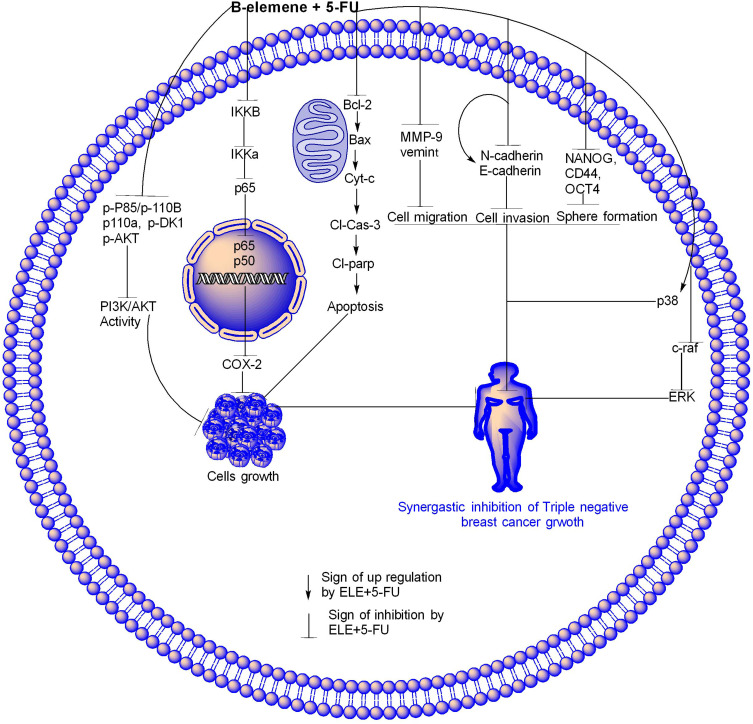
ELE enhanced the 5-FU effect through different molecular pathways in TNBC. In apoptosis pathway, ELE and 5-FU synergistically inhibited Bcl-2 and increased Bax due to which the cyt-c release from mitochondria into cytoplasm where they increase the expression of cl-caspase-3 and cl-parp due to which cell apoptosis happen.In the NF-kB/COX-2 pathway, ELE and 5-FU synergistically downregulated IKKβ, IKKα, and p65 expression levels in the cytoplasm, thereby decreasing p50 and p65 translocation, downregulating COX-2 expression, and inhibiting the cells. The activity of PI3K/AKT pathway was also inhibited by ELE and 5-FU in combination through inhibition of p-AKT, P-85, p110r, p-PDK1, and p110a proteins, thereby causing triple-negative cancer inhibition. ELE and 5-FUin combination modulated the Raf-MEK-Erk pathway through p-38 upregulation and c-raf and ERK downregulation. Hence, sphere formation is inhibited through inhibition of CD44, OCT4, and Nanog; cell invasion is also inhibited through E-cadherin upregulation and N-cadherin downregulation. ELE and 5-FU in combination inhibited cells migration through MMP-9 and vimentin downregulation, as shown in Figure 9. The modulation of all these pathways showed that, the ELE and 5-FU in combination inhibit the TNBC. Our results also suggested that the combination of ELE and 5-FU may be a potential therapy for TNBC. Further research is required to confirm the combined efficacy of ELE and 5-FU through different mechanisms.
Figure 9.
ELE enhanced 5-FU effect through different molecular pathways.
Notes: In apoptosis pathway, ELE and 5-FU synergistically inhibited Bcl-2, increased Bax due to which the cyt-c was released from the mitochondria to cytoplasm where they increased the cl-caspase-3 and cl-parp expression levels due to which cell the apoptosis happened. NF-kB/COX-2 pathway ELE and 5-FU synergistically downregulated the expression IKKβ, IKKα and p65 in cytoplasm due to which the translocation of p50 and p65 decreased and led to COX-2 expression downregulation and cell inhibition. The activity of PI3K/AKT pathway were also inhibited by ELE and 5-FU in combination through the inhibition of p-AKT, P-85, p110r, p-PDK1, and p110a proteins, thereby causing triple-negative cancer inhibition. ELE and 5-FU in combination modulated the MAPK pathway through p-38 upregulation and c-raf and ERK downregulation, thereby inhibiting sphere formation through CD44, OCT4, and Nanog. Cell invasion was promoted through E-cadherin upregulation and N-cadherin downregulation. ELE and 5-FU also in combination inhibit cell migration through MMP-9 and vimentin downregulation. The modulation of all of these pathways showed that ELE and 5-FU in combination inhibit triple-negative TNBC.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (grant nos. 81703904 KZ and 81473452 LZ).
Ethics Statement
This study had been approved by the Institutional Animal Care and Use Committee of The Dalian Medical University, People’s Republic of China.
Disclosure
The authors have declared no competing interest.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–386. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Pengyu SAB, Lijuan Z. Natural β-elemene:advances in targeting cancer through different molecular pathways. North Am J Acad Res. 2018;1(4):27. [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 4.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093 [DOI] [PubMed] [Google Scholar]
- 5.Curtis C, Shah SP, Chin SF, et al. The genomic and transcriptomic architecture of 2000 breast tumours reveals novel subgroups. Nature. 2012;486(7403):346–352. doi: 10.1038/nature10983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marusyk A, Almendro V, Polyak K. Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer. 2012;12(5):323–334. doi: 10.1038/nrc3261 [DOI] [PubMed] [Google Scholar]
- 8.Shi XJ, Au WW, Wu KS, Chen LX, Lin K. Mortality characteristics and prediction of female breast cancer in China from 1991 to 2011. Asian Pac J Cancer Prev. 2014;15(6):2785–2791. doi: 10.7314/APJCP.2014.15.6.2785 [DOI] [PubMed] [Google Scholar]
- 9.Guan X. Cancer metastases: challenges and opportunities. Acta Pharm Sin B. 2015;5(5):402–418. doi: 10.1016/j.apsb.2015.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown GT, Murray GI. Current mechanistic insights into the roles of matrix metalloproteinases in tumour invasion and metastasis. J Pathol. 2015;237(3):273–281. doi: 10.1002/path.4586 [DOI] [PubMed] [Google Scholar]
- 11.Vlodavsky I, Friedmann Y, Elkin M, et al. Mammalian heparanase: gene cloning, expression and function in tumor progression and metastasis. Nat Med. 1999;5(7):793–802. doi: 10.1038/10518 [DOI] [PubMed] [Google Scholar]
- 12.Edris AE. Anti-cancer properties of Nigella spp. essential oils and their major constituents, thymoquinone and β-elemene. Curr Clin Pharmacol. 2009;4(1):43–46. doi: 10.2174/157488409787236137 [DOI] [PubMed] [Google Scholar]
- 13.Lu JJ, Dang YY, Huang M, Xu WS, Chen XP, Wang YT. Anti-cancer properties of terpenoids isolated from Rhizoma Curcumae–a review. J Ethnopharmacol. 2012;143(2):406–411. doi: 10.1016/j.jep.2012.07.009 [DOI] [PubMed] [Google Scholar]
- 14.Zhao J, Li QQ, Zou B, et al. In vitro combination characterization of the new anticancer plant drug beta-elemene with taxanes against human lung carcinoma. Int J Oncol. 2007;31(2):241–252. [PubMed] [Google Scholar]
- 15.Tao L, Zhou L, Zheng L, Yao M. Elemene displays anti-cancer ability on laryngeal cancer cells in vitro and in vivo. Cancer Chemother Pharmacol. 2006;58(1):24–34. doi: 10.1007/s00280-005-0137-x [DOI] [PubMed] [Google Scholar]
- 16.Wang G, Li X, Huang F, et al. Antitumor effect of β-elemene in non-small-cell lung cancer cells is mediated via induction of cell cycle arrest and apoptotic cell death. Cell Mol Life Sci. 2005;62(7–8):881–893. doi: 10.1007/s00018-005-5017-3 [DOI] [PubMed] [Google Scholar]
- 17.Li QQ, Lee RX, Liang H, Zhong Y. Anticancer activity of β-elemene and its synthetic analogs in human malignant brain tumor cells. Anticancer Res. 2013;33(1):65–76. [PMC free article] [PubMed] [Google Scholar]
- 18.Li QQ, Wang G, Huang F, Li JM, Cuff CF, Reed E. Sensitization of lung cancer cells to cisplatin by β-elemene is mediated through blockade of cell cycle progression: antitumor efficacies of β-elemene and its synthetic analogs. Med Oncol. 2013;30(1):488. doi: 10.1007/s12032-013-0488-9 [DOI] [PubMed] [Google Scholar]
- 19.Xu HB, Li L, Fu J, Mao XP, Xu LZ. Reversion of multidrug resistance in a chemoresistant human breast cancer cell line by ß-elemene. Pharmacology. 2012;89(5–6):303–312. doi: 10.1159/000337178 [DOI] [PubMed] [Google Scholar]
- 20.Ding XF, Shen M, Xu LY, Dong JH, Chen G. 13,14-bis(cis-3,5-dimethyl-1-piperazinyl)-β-elemene, a novel β-elemene derivative, shows potent antitumor activities via inhibition of mTOR in human breast cancer cells. Oncol Lett. 2013;5(5):1554–1558. doi: 10.3892/ol.2013.1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Mu XD, Li EZ, et al. The role of E3 ubiquitin ligase cbl proteins in β-elemene reversing multi-drug resistance of human gastric adenocarcinoma cells. Int J Mol Sci. 2013;14(5):10075–10089. doi: 10.3390/ijms140510075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Canellos GP, Devita VT, Gold GL, Chabner BA, Schein PS, Young RC. Cyclical combination chemotherapy for advanced breast carcinoma. Br Med J. 1974;1(5901):218–220. doi: 10.1136/bmj.1.5901.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segaloff A, Hankey BF, Carter AC, Escher GC, Ansfield FJ, Talley RW. An evaluation of the effect of vincristine added to cyclophosphamide, 5-fluorouracil, methotrexate, and prednisone in advanced breast cancer. Breast Cancer Res Treat. 1985;5(3):311–319. doi: 10.1007/BF01806026 [DOI] [PubMed] [Google Scholar]
- 24.Muss HB, White DR, Cooper MR, Richards F, Spurr CL. Combination chemotherapy in advanced breast cancer.: a randomized trial comparing a three-vs a five-drug program. Arch Intern Med. 1977;137(12):1711–1714. doi: 10.1001/archinte.1977.03630240045015 [DOI] [PubMed] [Google Scholar]
- 25.Liu MN, Liu AY, Pei FH, et al. Functional mechanism of the enhancement of 5-fluorouracil sensitivity by TUSC4 in colon cancer cells. Oncol Lett. 2015;10(6):3682–3688. doi: 10.3892/ol.2015.3801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Yang ZR, Guo XF, et al. Synergistic effects of puerarin combined with 5-fluorouracil on esophageal cancer. Mol Med Rep. 2014;10(5):2535–2541. doi: 10.3892/mmr.2014.2539 [DOI] [PubMed] [Google Scholar]
- 27.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28(6):1075–1083. doi: 10.1200/JCO.2009.25.3641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steelman LS, Chappell WH, Abrams SL, et al. Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging (Albany NY). 2011;3(3):192–222. doi: 10.18632/aging.100296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerondakis S, Siebenlist U. Roles of the NF- B pathway in lymphocyte development and function. Cold Spring Harb Perspect Biol. 2010;2(5):a000182. doi: 10.1101/cshperspect.a000182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayden MS, West AP, Ghosh S. NF-κB and the immune response. Oncogene. 2006;25(51):6758–6780. doi: 10.1038/sj.onc.1209943 [DOI] [PubMed] [Google Scholar]
- 31.Tornatore L, Thotakura AK, Bennett J, Moretti M, Franzoso G. The nuclear factor kappa B signaling pathway: integrating metabolism with inflammation. Trends Cell Biol. 2012;22(11):557–566. doi: 10.1016/j.tcb.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 32.Abraha AM, Ketema EB. Apoptotic pathways as a therapeutic target for colorectal cancer treatment. World J Gastrointest Oncol. 2016;8(8):583–591. doi: 10.4251/wjgo.v8.i8.583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baig S, Seevasant I, Mohamad J, Mukheem A, Huri HZ, Kamarul T. Potential of apoptotic pathway-targeted cancer therapeutic research: where do we stand? Cell Death Dis. 2016;7(1):e2058. doi: 10.1038/cddis.2015.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lien GS, Lin CH, Yang YL, Wu MS, Chen BC. Ghrelin induces colon cancer cell proliferation through the GHS-R, Ras, PI3K, Akt, and mTOR signaling pathways. Eur J Pharmacol. 2016;776:124–131. doi: 10.1016/j.ejphar.2016.02.044 [DOI] [PubMed] [Google Scholar]
- 35.Zhu L, Derijard B, Chakrabandhu K, Wang BS, Chen HZ, Hueber AO. Synergism of PI3K/Akt inhibition and Fas activation on colon cancer cell death. Cancer Lett. 2014;354(2):355–364. doi: 10.1016/j.canlet.2014.08.038 [DOI] [PubMed] [Google Scholar]
- 36.Gao Y, Xiao X, Zhang C, et al. Melatonin synergizes the chemotherapeutic effect of 5-fluorouracil in colon cancer by suppressing PI3K/AKT and NF-κB/iNOS signaling pathways. J Pineal Res. 2017;62(2):e12380. doi: 10.1111/jpi.12380 [DOI] [PubMed] [Google Scholar]
- 37.Misra S, Sharma K. COX-2 signaling and cancer: new players in old arena. Curr Drug Targets. 2014;15(3):347–359. doi: 10.2174/1389450115666140127102915 [DOI] [PubMed] [Google Scholar]
- 38.Chen XX, Lam KH, Chen QX, et al. Ficus virens proanthocyanidins induced apoptosis in breast cancer cells concomitantly ameliorated 5-fluorouracil induced intestinal mucositis in rats. Food Chem Toxicol. 2017;110:49–61. doi: 10.1016/j.fct.2017.10.017 [DOI] [PubMed] [Google Scholar]
- 39.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3(5):330–338. doi: 10.1038/nrc1074 [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Jiang ZY, Zhou YL, et al. β-elemene regulates endoplasmic reticulum stress to induce the apoptosis of NSCLC cells through PERK/IRE1α/ATF6 pathway. Biomed Pharmacother. 2017;93:490–497. doi: 10.1016/j.biopha.2017.06.073 [DOI] [PubMed] [Google Scholar]
- 41.Liu J, Zhang Y, Qu J, et al. β-elemene-induced autophagy protects human gastric cancer cells from undergoing apoptosis. BMC Cancer. 2011;11(1):183. doi: 10.1186/1471-2407-11-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu JM, Mao BY, Hong S, Liu YH, Wang XJ. The postoperative brain tumour stem cell (BTSC) niche and cancer recurrence. Adv Ther. 2008;25(5):389–398. doi: 10.1007/s12325-008-0050-x [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Sun X, Nan N, et al. Elemene inhibits the migration and invasion of 4T1 murine breast cancer cells via heparanase. Mol Med Rep. 2017;16(1):794–800. doi: 10.3892/mmr.2017.6638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J, Zhang HD, Yao YF, Zhong SL, Zhao JH, Tang JH. β-elemene reverses chemoresistance of breast cancer cells by reducing resistance transmission via exosomes. Cell Physiol Biochem. 2015;36(6):2274–2286. doi: 10.1159/000430191 [DOI] [PubMed] [Google Scholar]
- 45.Yi X, Guo W, Shi Q, et al. SIRT3-dependent mitochondrial dynamics remodeling contributes to oxidative stress-induced melanocyte degeneration in vitiligo. Theranostics. 2019;9(6):1614. doi: 10.7150/thno.30398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Negroni A, Cucchiara S, Stronati L. Apoptosis, necrosis, and necroptosis in the gut and intestinal homeostasis. Mediators Inflamm. 2015;2015:250762. doi: 10.1155/2015/250762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maryam A, Mehmood T, Zhang H, Li Y, Khan M, Ma T. Alantolactone induces apoptosis, promotes STAT3 glutathionylation and enhances chemosensitivity of A549 lung adenocarcinoma cells to doxorubicin via oxidative stress. Sci Rep. 2017;7(1):6242. doi: 10.1038/s41598-017-06535-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khan M, Ding C, Rasul A, et al. Isoalantolactone induces reactive oxygen species mediated apoptosis in pancreatic carcinoma PANC-1 cells. Int J Biol Sci. 2012;8(4):533–547. doi: 10.7150/ijbs.3753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48(6):749–762. doi: 10.1016/j.freeradbiomed.2009.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang R, Cong WH, Guo G, et al. Synergism between carnosic acid and arsenic trioxide on induction of acute myeloid leukemia cell apoptosis is associated with modulation of PTEN/Akt signaling pathway. Chin J Integr Med. 2012;18(12):934–941. doi: 10.1007/s11655-012-1297-z [DOI] [PubMed] [Google Scholar]
- 51.Xia S, Zhao Y, Yu S, Zhang M. Activated PI3K/Akt/COX-2 pathway induces resistance to radiation in human cervical cancer HeLa cells. Cancer Biother Radiopharm. 2010;25(3):317–323. doi: 10.1089/cbr.2009.0707 [DOI] [PubMed] [Google Scholar]
- 52.Liu J, Hu XJ, Jin B, Qu XJ, Hou KZ, Liu YP. β-elemene induces apoptosis as well as protective autophagy in human non-small-cell lung cancer A549 cells. J Pharm Pharmacol. 2012;64(1):146–153. doi: 10.1111/j.2042-7158.2011.01371.x [DOI] [PubMed] [Google Scholar]
- 53.Patra S, Muthuraman MS, Meenu M, Priya P, Pemaiah B. Anti-inflammatory effects of royal poinciana through inhibition of toll-like receptor 4 signaling pathway. Int Immunopharmacol. 2016;34:199–211. doi: 10.1016/j.intimp.2016.02.027 [DOI] [PubMed] [Google Scholar]
- 54.Zheng CP, Tong XM, Yao HP, et al. beta-elemene enhances aclarubicin-induced apoptotic effect in HL-60 cells and its mechanism. Zhonghua Xue Ye Xue Za Zhi. 2009;30(12):821–824. [PubMed] [Google Scholar]
- 55.Santarpia L, Lippman SM, El-Naggar AK. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16(1):103–119. doi: 10.1517/14728222.2011.645805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang TH, Hsieh FL, Ko TP, Teng KH, Liang PH, Wang AH. Structure of a heterotetrameric geranyl pyrophosphate synthase from mint (Mentha piperita) reveals intersubunit regulation. Plant Cell. 2010;22(2):454–467. doi: 10.1105/tpc.109.071738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li P, Zhou X, Sun W, et al. Elemene induces apoptosis of human gastric cancer cell line BGC-823 via extracellular signal-regulated kinase (ERK) 1/2 signaling pathway. Med Sci Monit. 2017;23:809–817. doi: 10.12659/MSM.903197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhan YH, Liu J, Qu XJ, et al. β-elemene induces apoptosis in human renal-cell carcinoma 786-0 cells through inhibition of MAPK/ERK and PI3K/Akt/mTOR signalling pathways. Asian Pac J Cancer Prev. 2012;13(6):2739–2744. doi: 10.7314/APJCP.2012.13.6.2739 [DOI] [PubMed] [Google Scholar]
- 59.Zong S, Li J, Yang L, et al. Mechanism of bioactive polysaccharide from Lachnum sp. acts synergistically with 5-fluorouracil against human hepatocellular carcinoma. J Cell Physiol. 2019;234(9):15548–15562. doi: 10.1002/jcp.28202 [DOI] [PubMed] [Google Scholar]
- 60.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- 61.Shao HM, Tang YH, Jiang PJ, et al. Inhibitory effect of flavonoids of puerarin on proliferation of different human acute myeloid leukemia cell lines in vitro. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2010;18(2):296–299. [PubMed] [Google Scholar]
- 62.Seyfried TN, Flores R, Poff AM, D’Agostino DP, Mukherjee P. Metabolic therapy: a new paradigm for managing malignant brain cancer. Cancer Lett. 2015;356(2):289–300. doi: 10.1016/j.canlet.2014.07.015 [DOI] [PubMed] [Google Scholar]
- 63.Todoric J, Antonucci L, Karin M. Targeting inflammation in cancer prevention and therapy. Cancer Prev Res (Phila). 2016;9(12):895–905. doi: 10.1158/1940-6207.CAPR-16-0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang B, Tang F, Wang Z, et al. Upregulation of Akt/NF-κB-regulated inflammation and Akt/Bad-related apoptosis signaling pathway involved in hepatic carcinoma process: suppression by carnosic acid nanoparticle. Int J Nanomedicine. 2016;11:6401–6420. doi: 10.2147/IJN.S101285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kamalakannan V, Shiny A, Babu S, Narayanan RB. Autophagy protects monocytes from Wolbachia heat shock protein 60-induced apoptosis and senescence. PLoS Negl Trop Dis. 2015;9(4):e0003675. doi: 10.1371/journal.pntd.0003675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perdiki M, Korkolopoulou P, Thymara I, et al. Cyclooxygenase-2 expression in astrocytomas. Relationship with microvascular parameters, angiogenic factors expression and survival. Mol Cell Biochem. 2007;295(1–2):75–83. doi: 10.1007/s11010-006-9275-7 [DOI] [PubMed] [Google Scholar]