Abstract
Background
Alveolar echinococcosis (AE) is a zoonotic parasitic disease caused by Echinococcus multilocularis larval tapeworm infections in humans that severely impairs the health of affected patients in the northern hemisphere.
Methods
The expression levels of 20 cytokines associated with AE infection were measured by enzyme-linked immunosorbent assay, and the correlations between these cytokines were analysed in the R programming language.
Results
Serum cytokine levels differed among individuals in both the AE patient and healthy control groups. The results of the correlations among the cytokines showed obvious differences between the two groups. In the AE patients group, Th1 and Th2 cytokines formed a more complicated network than that in the healthy control group.
Conclusions
The altered correlations between Th1 and Th2 cytokines may be closely associated with AE infection, which may provide a new explanation for the essential differences between AE patients and healthy individuals.
Keywords: Alveolar echinococcosis, Echinococcus multilocularis, Th1 cytokines, Th2 cytokines, Correlation analysis
Background
Alveolar echinococcosis (AE) is a severe parasitic disease caused by Echinococcus multilocularis larval tapeworm infection in humans that is fatal if left untreated [1, 2]. The liver is the primary target of the disease and is affected in nearly 95% of cases; this disease can also spread and affect other organs, including the lungs, brain and bone [1, 3]. AE causes severe damage or dysfunction of target organs [4, 5]. This disease is restricted to the northern hemisphere, principally in rural areas of western, northern and eastern Europe; the highest disease prevalence is in central Asia, China and Kyrgyzstan [6, 7]. Epidemiological investigations have shown that pastoral regions on the Tibetan Plateau appear to be high-risk areas for AE disease due to specific landscape features and husbandry practices. Specifically, a range of different wildlife hosts, especially small mammals, are involved in the transmission of E. multilocularis in a pastoral region of Qinghai province [5–7].
The World Health Organization (WHO) has listed echinococcosis as one of the 17 neglected diseases targeted for control or elimination by 2050 (http://whqlibdoc.who.int/hq/2012/WHO_HTM_NTD_2012.1_eng.pdf). To date, surgery is the only potentially curative option for the treatment of AE; however, AE recurrence after hepatectomy is high, and many patients present with inoperable disease [8]. Recently, immunotherapy has been used to complement anti-infective drug approaches, and this approach was suggested to be highly effective in treating echinococcosis; however, there is no accepted immunotherapy against AE infection due to the complicated interactions between the parasites and host immunity.
The type of immune response impacts disease development, and T helper (Th) cells can selectively differentiate into the Th1 or Th2 subtype in response to an E. multilocularis antigen. A Th1/Th2 imbalance has been suggested to play an important role in controlling the immunological response to AE infection [9, 10]. AE patients with Th1-oriented immunity are more likely to harbour fewer parasites or even aborted parasites, whereas AE patients with Th2-oriented immunity are more likely to develop chronic AE [11]. In mice, the Th1 response was shown to dominate at the early stage of AE, and the immune response gradually shifted towards a Th2-dominated response at the late stage of AE to prevent Th1-mediated damage [11, 12]. The imbalance between Th1-type cytokines and Th2-type cytokines in AE is not completely understood due to the limited number of studies, regional differences and complex interactions between parasites and host immunological and genetic factors [9, 12].
In this study, 20 cytokines, including Th1 and Th2 cytokines, were selected according to the related literature [13–15]. The expression levels of these cytokines were compared and analysed by bioinformatics and statistical analysis methods to explore the correlations among Th1- and Th2-type cytokines in AE patients and healthy controls from Qinghai Province in China.
Methods
Study groups and sample collection
The participants consisted of 45 AE patients (29 females/16 males) and 45 healthy people (27 females/18 males). The mean age of the AE patients was 38 years (range, 21–52 years), and the mean age of the healthy controls was 39 years (range, 26–53 years). All the recruited participants were Tibetan and lived in the Guoluo Tibetan Autonomous Prefecture of Qinghai Province, and 91% were herdsmen. The diagnosis of AE was according to the People’s Republic of China Health Industry Standard—Diagnostic Criteria for Hydatid Disease (WS257–2006) by a professional doctor. The classification of AE patients in different clinical stages of AE was accomplished according to the World Health Organization- (WHO-) PNM (P: Hepatic localization of the metacestode; N: Extrahepatic involvement of neighbouring organs; and M: Presence or absence of distant metastases), detailed in Table 1. No patients had received any anti-inflammatory drugs or anti-parasitic drugs, and none had undergone a curative hepatectomy or a liver transplantation before the study. All healthy controls showed normal abdominopelvic cavity images as detected by B-mode ultrasound. Written consent was obtained from all participants, and this study was approved by the Ethics Committee of Qinghai Institute of Endemic Disease Control and Prevention.
Table 1.
The classification of AE patients
| Classification | Percentage (%) | ||
|---|---|---|---|
| Lesion numbers | Single (%) | 27 (60%) | |
| Double(%) | 14 (31.1%) | ||
| Multiple (%) | 4 (8.8%) | ||
| PNM classification | P | P1 (%) | 10 (22.2%) |
| P2 (%) | 15 (33.3%) | ||
| P3 (%) | 18 (40%) | ||
| P4 (%) | 2 (4.4%) | ||
| N | 0 | not detected | |
| M | undetected | ||
| Lesion classification (67 lesions in 45 patients) | Infiltrating type (%) | 49 (73.1%) | |
| Calcification type (%) | 5 (7.5%) | ||
| Liquefied cavity type (%) | 13 (19.4%) | ||
Note: No adjacent organs or tissues were found to be infected in all patients; M classification was not provided due to poor medical conditions and remote areas
Five millilitres of peripheral venous blood was harvested from each participant after an 8- to 12-h fast under strict precautions in sterile tubes containing EDTA anticoagulation, and 1 mL of serum was immediately separated from the blood and preserved at − 80 °C for the measurement of cytokines.
Serum analysis
The serum levels of 20 cytokines in AE patients and healthy controls were measured by an enzyme-linked immunosorbent assay (ELISA) kit (Thermo Scientific) according to the manufacturer’s protocols. All samples were measured three times for each cytokine, and the mean value was taken for analysis. The 20 analysed cytokines are listed in Table 2.
Table 2.
The Th1 and Th2 cytokines analysed in the study
| Cytokine type | Specific cytokines |
|---|---|
| Th1 cytokines | IL-8, IL-2, IL-12, IL-1β, IFN-γ-inducible protein 10 (IP-10), MIP-1β, MCP-1α, and IFN-γ. |
| Th2 cytokines | IL-4, IL-5, IL-6, IL-13, IL-18, GRO-α, and eotaxin. |
| Both Th1 and Th2 cytokines | Stromal cell-derived factor (SDF-1α),TNF-α, GM-CSF, MIP-1α, and regulated on activation, normal T cell expressed and secreted (RANTES). |
Literature review
We searched related studies in the PubMed database using the keywords “cytokine” and “alveolar echinococcosis” to explore data on cytokine expression in previous studies associated with AE from 1990 to 2019. After a literature review, a total of 56 studies were identified, 16 of which reported cytokine levels in human AE.
Statistical methods
The relationships between the AE patient group and the healthy group were analysed by a principal component analysis (PCA) performed based on the Bray-Curtis distance matrix across the samples using the vegan package in R (version 3.4.4). Student’s t-test was performed to compare PC1/PC2 between groups using the stats package in R. Spearman’s correlations between cytokines were calculated using the Hmisc package in R. The correlation networks between cytokines were constructed using the GeneNet package in R and were further visualized using Cytoscape 3.4.0 [16].
Results
Cytokine analysis
The expression levels of 20 cytokines were compared between the AE patient group and the healthy control group, while the ELISA results showed that cytokine expression levels differed among individuals in both the AE patient and healthy control groups.
PCA of AE patients and healthy controls
The PCA showed that most samples in the AE patient group had obviously different cytokine compositions than those in the healthy control group, while a few samples showed similarity (Fig. 1). The differences in cytokine composition between the two groups were further analysed by comparing their PC1 and PC2 values (Fig. 2); these values were significantly different between the two groups (P < 0.001).
Fig. 1.
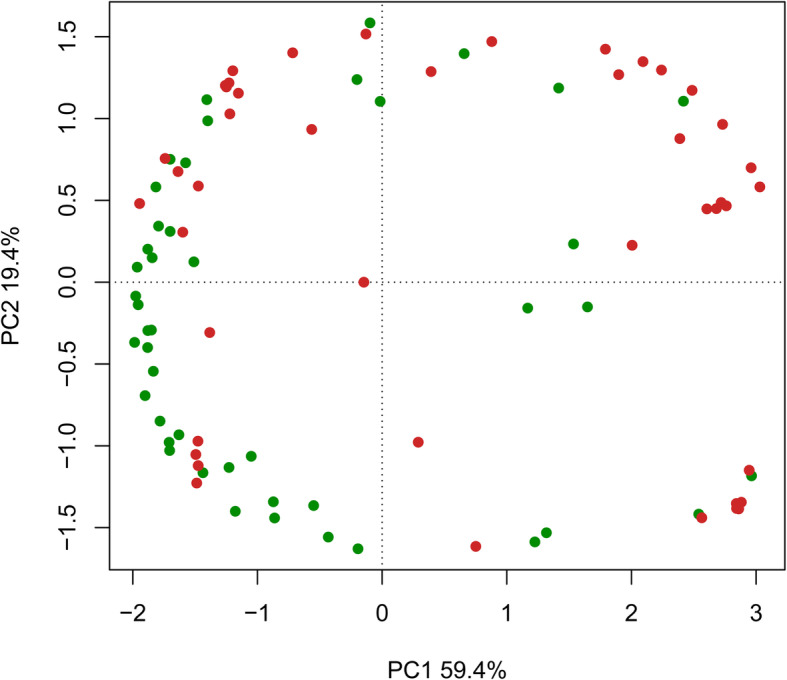
PCA of AE patients (orange) and healthy controls (green) based on the Bray-Curtis distance. The percentages of variance explained by PC1 and PC2 are 59.4 and 19.4%, respectively
Fig. 2.
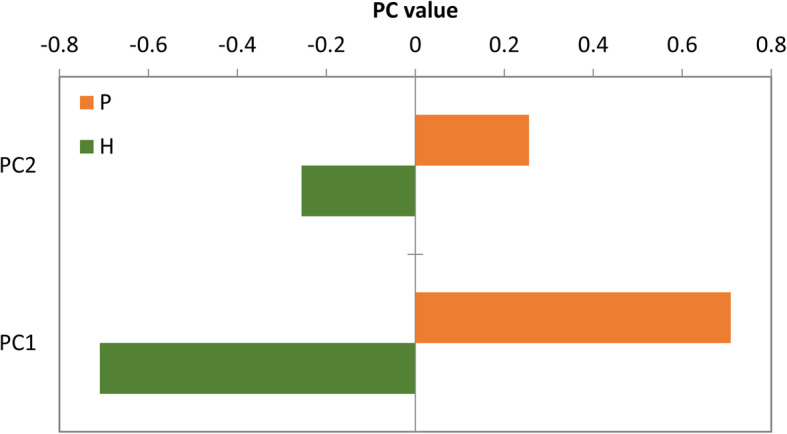
Comparison of PC1 and PC2 values between AE patients and healthy controls
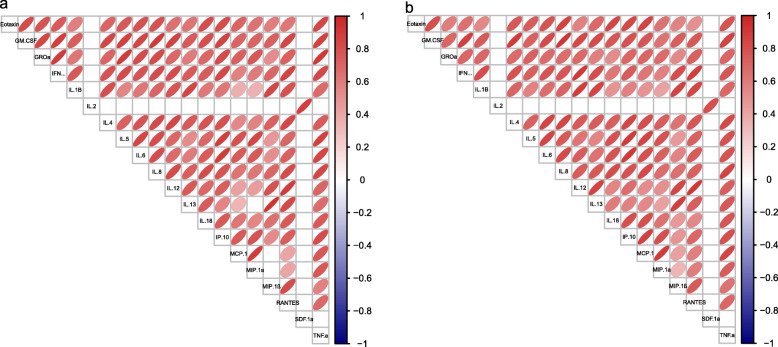
Correlations between the cytokines
The correlations among some cytokines were obviously different between the two groups. In the healthy control group, there was no correlation between MIP-1α and IL-13 or among MCP-1, MCP-1α and MIP-1β (Fig. 3a). These cytokines had weak correlations in the AE patient group (Fig. 3b).
Fig. 3.
Spearman’s correlations among cytokines in the healthy controls (a) and the AE patients (b)
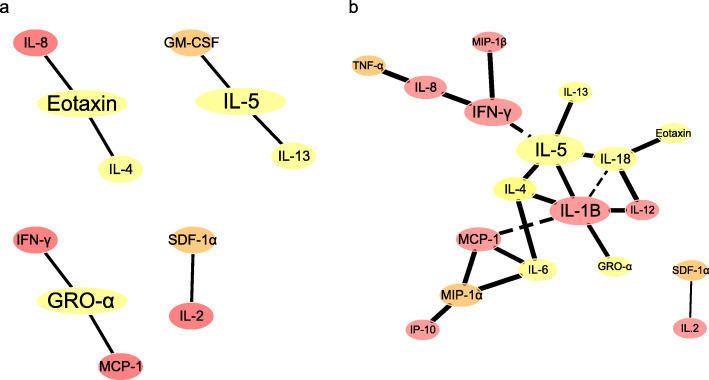
A partial correlation network analysis of the cytokines confirmed the presence of more complex cytokine interactions in the AE patient group than in the healthy control group (Fig. 4a and b). In the healthy controls, the correlations were simple, and just a few of cytokines showed correlations between each other: IL-8(Th1)_Eotaxin(Th2)_IL-4(Th2); GM-CSF (Th2)_IL-5(Th2)_IL-13(Th2); (IFN)-γ (Th1)_(GRO)-α (Th2)_MCP-1α(Th1); and SDF 1α(Th2)_IL-2(Th1)(Fig. 4a). In the AE patients group, Th1 and Th2 cytokines formed a more complicated network, and more Th1 (MCP-1α, IL-1β, IFN-γ, and IL-8) and Th2 cytokines (IL-5, IL-4, IL-18) showed close correlations (Fig. 4b).
Fig. 4.
Partial correlation networks among cytokines in the healthy controls (a) and AE patients (b). Note: The colour of the node indicates the origin of the cytokine: red, Th1; yellow, Th2; and orange, both Th1 and Th2. The size of the node indicates the betweenness centrality; a node with increased betweenness centrality has increased control over the network. The solid and dotted lines indicate positive and negative relationships, respectively
Discussion
In the present study, the expression levels of 20 cytokines were detected to determine some differences between AE patients and healthy controls, while the results were confusing, as they differed among individuals in both the AE patient and healthy control groups. We then summarized the related literature about cytokines associated with human AE, and the results showed that the kinds of ‘different cytokines’ were not always consistent (Table 3), indicating that it is seemingly difficult to discover biomarkers for human AE at the cytokine expression level due to the complex factors in AE affecting the process.
Table 3.
The expression levels of cytokines in E. multilocularis-infected humans
| Cytokine type | Cytokine | Experimental type | Methods and results | Specimens | Citation |
|---|---|---|---|---|---|
| TH2 | IL-10 | in vitro | IL-10 levels in CD8+ lymphocytes from progressive AE patients (N = 12) were definitely increased after in vitro culture with crude E. multilocularis antigen using a protocol for intracellular staining of cytokines followed by fluorescence-activated cell sorting (FACS) analysis. | CD8+ lymphocytes cultured in vitro | Kilwinski et al. [17] |
| TH2 | IL-10 | in vitro | Peripheral blood mononuclear cells (PBMCs) isolated from progressive AE patients (N = 9) secreted significantly higher amounts of IL-10 than those isolated from abortive AE patients (N = 3); IL-10 was detected by using real-time PCR. | PBMCs cultured with Emf stimulation. | Godot et al. [18] |
| TH2 | IL-10 | clinical test | Serum IL-10 levels were significantly higher in AE patients (N = 40) than in healthy controls (N = 20), with a tendency to higher concentrations in progressive cases; IL-10 was determined by ELISA. | Serum | Wellinghausen et al. [19] |
| TH2 | IL-5 | in vitro | IL-5 production was particularly increased in PBMCs from patients with advanced AE (n = 14) after stimulation with crude E. multilocularis antigenic preparations; IL-5 was detected by RT-PCR . | PBMCs | Jenne et al. [20] |
| TH2 |
IL-5, IL-6, IL-10 |
clinical test | Plasma concentration levels of IL-5, IL-6, and IL-10 were slightly increased in consecutive AE patients (N = 28), and IL-23 concentration levels were significantly higher in AE patients; the cytokines were detected by ELISA. | Plasma | Tuxun et al. [21] |
| TH2 | TGF-β | clinical test | Serum TGF-β levels were high, and TGF-β was expressed by most of the infiltrating lymphocytes in progressive AE patients (N = 18) by means oOf immunochemical staining of liver sections. | Surgical biopsy specimens | Zhang et al. [22] |
| TH2 | TGF-β | in vitro | Higher levels of TGF-β were observed in PBMC supernatant after exposure to Em vesicular fluid (VF) than that from healthy blood donors; TGF-β was detected using flow cytometry and ELISA, respectively. | PBMCs supernatant | Bellanger et al. [23] |
| TH2 | TGF-β | in vitro | A significant increase in TGF-β production was induced in PBMCs from healthy blood donors after exposure to Em-VF and Toll-like receptor agonists by using Multiplex Luminex® bead technology. | PBMCs exposure to Em-VF and Toll-like receptor agonists | Bellanger et al. [24] |
| TH1 | IL-8, MCP-1 | in vitro | Peripheral blood cells isolated from AE patients (N = 30) induced significant IL-8 and MCP-1 production when cultured with viable proliferating E. multilocularis metacestode (Em) vesicles; IL-8 and MCP-1 were detected by ELISA. | PBMCs cultured with Em vesicles | Dreweck et al. [25] |
| TH1 | IFN-γ | clinical test | The mean concentration of IFN-γ in serum from AE patients (N = 23) was higher than that in control group; IFN-γ was detected by double antibody sandwich. | Serum | Shi et al. [26] |
| TH9 | IL-9 | in vitro | Th9-related cytokine IL-9 mRNA levels were both elevated in PBMCs and in hepatic lesion and paralesion tissues in AE patients (N = 14); IL-9 mRNA levels were detected by real-time PCR. | PBMCs and liver tissues | Tuxun et al. [10] |
| Th17 | IL-17 | clinical test | The plasma levels of the proinflammatory cytokine IL-17B and its soluble receptor sIL-17RB were significantly elevated in AE patients (N = 93); IL-17B was detected by ELISA. | Plasma | Lechner et al. [27] |
| Th17 | IL-23 | in vitro | IL-17A and IL-23 mRNAs levels were significantly elevated in the PBMCs isolated from AE patients (N = 30), and the levels were detected by real-time PCR. | PBMCs | Tuxun, et al. [28] |
| TH2 and TH1 |
IL-10, IFN-γ |
in vitro | Emf-stimulated mononuclear cells from the central part of the granulomatous lesions secreted more IL-10 (TH2) and less IFN-γ (TH1) than cells from the periphery of the granuloma in progressive AE patients (N = 1); the cytokines were detected by ELISA. | Emf-stimulated mononuclear cells | Harraga et al. [29] |
| TH2 and TH1 |
IL-31, IL-33, IL-27, SDF-1, eotaxin |
in vitro | The spontaneous cellular release of TH2-type cytokines IL-31 and IL-33 was clearly depressed in patients with cured, stable, and progressive AE (N = 57), whereas the levels of the TH1-type cytokine IL-27, anti-inflammatory cytokine SDF-1, and eotaxin increased with disease progression; the cytokines were detected by ELISA. | PBMC culture supernatants | Huang et al. [30] |
| Both TH1 and TH2 |
MIP-1α, MIP-1β, RANTES, GRO-α |
in vitro | The production of CC and CXC chemokines, which associate with inflammation (MIP-1α/CCL3, MIP-1β/CCL4, RANTES/CCL5 and GRO-α/CXCL1) was constitutively higher in PBMCs when cultured with E. multilocularis antigen in patients with progressive, stable or cured AE (N = 75) than in controls; the chemokines were detected by ELISA. | PBMCs cultured with Em antigen | Kocherscheidt et al. [31] |
To further understand the differences in cytokines between the two groups, we used omics methods, and the results of PCA analysis indicated that cytokine compositions were obviously different between the two groups and that the networks of cytokine correlations were more complicated in the AE patient group than that in the healthy control group. According to the correlation network results, the weak or strong correlations between Th1 and Th2 cytokines could explain the essential differences between the AE patients and the healthy controls. E. multilocularis metacestodes may modulate the secretion of Th1 and Th2 cytokines by Th lymphocytes in AE patients [32], and the cytokine orientation depends on the host immune response induced by E. multilocularis antigens [33]. Th1 cell activation induces considerable protective immunity, which involves the initiating cytokines IFN-α and IL-12 and the effector cytokines IFN-γ and tumour necrosis factor (TNF)-α, to defend against intracellular parasitic infections [34, 35]. Th2 cytokines allow parasites to proliferate at low rates by producing high levels of IL-4, IL-5 and IL-10 [13, 14]. E. multilocularis antigenic preparations have been reported to induce increased IL-5 production due to the activation of CD4+ T lymphocytes in patients with progressive AE [20]. In the present study, the correlation between the Th2-type cytokine IL-5 and the Th1-type cytokines IFN-γ and IL-1β in AE patients may be associated with the enhanced immunological response induced by parasite infection, which indicates that an inflammatory reaction is induced in AE patients. In addition, the Th2 cytokines GRO-α and eotaxin were well controlled by the Th1 cytokines MCP-1, IFN-γ and IL-8 in the healthy control group, whereas GRO-α and eotaxin levels were poorly controlled by Th1 cytokines in the AE patient group, confirming the presence of an inflammatory response in AE patients. The altered correlations among the cytokines may explain the essential differences between the AE patients and the healthy controls.
Furthermore, this parasite can secrete proteins to regulate the host immune response and survive in humans for long periods of time, and different secreted protein profiles at different stages of AE progression may explain the complex interactions between the parasite and the host [36]. A proteomic analysis of cyst vesicular fluids in AE patients contributed to the identification of potential molecular markers for diagnostic and follow-up tools, but the mechanism underlying the interplay between secreted proteins and cytokines requires further exploration.
Conclusions
The correlations between Th1 and Th2 cytokines were simple in the healthy control group but complex in the AE patient group. Th1 cytokines, such as IFN-γ, IL-1β and MCP-1, had high betweenness centrality in AE patients, whereas Th2 cytokines, such as GRO-α, eotaxin and IL-5, had high betweenness centrality in the healthy control group. These findings may provide a new point of view to study the significant difference between AE patients and healthy individuals. However, more studies in the future will be required to clarify the “biomarkers”, such as studies including “clinically relevant populations” (such as liver non-parasitic benign cysts, liver abscesses, and mesenteric cysts) as controls, with prospective follow-up and involving large sample sizes.
Acknowledgements
We are extremely grateful to Dr. Haining Fan for the advice on the study design.
Abbreviations
- AE
Alveolar echinococcosis
- ELISA
Enzyme-linked immunosorbent assay
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- GRO-α
Growth-regulated oncogene-alpha
- IP-10
Interferon-gamma (IFN-γ)-inducible protein 10
- IL
Interleukin
- MIP-1α
Macrophage inflammatory protein-1α
- MIP-1β
Macrophage inflammatory protein-1β
- MCP-1α
Monocyte chemoattractant protein
- RANTES
Regulated on activation, normal T cell expressed and secreted
- PCA
Principal component analysis
- SDF-1α
Stromal cell-derived factor-1α
- Th cells
T helper cells
- TNF-α
Tumour necrosis factor α
- WHO
World Health Organization
Authors’ contributions
X M, HX C, HN F and JY Z conceived of and designed the study, and interpreted the results. X M, XF Z and J L carried out the screening and epidemiological data collection in epidemic areas. YF L, CZ Z, W L, and JY M conducted clinical assessments. N L, JX Z and YS W carried out the blood collection, serum separation and data collection of part epidemic areas. W W and PZ Z performed the experiments of Elisa. X M, XY Z and Q Z performed the statistical analyses and wrote the manuscript. KM S and PY L did the work of literature review. All authors read and approved the final manuscript.
Funding
This study was supported by the Science and Technology Major Project of Qinghai Province (No. 2016-SF-A5).
Availability of data and materials
Data supporting the conclusions are included within the article.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Qinghai Institute of Endemic Disease Control and Prevention, and all participants signed informed consent forms.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Huixia Cai, Email: huixia_1107@163.com.
Haining Fan, Email: fanhaining@medmail.com.cn.
References
- 1.Gencheva DG, Menchev DN, Penchev DK, Tokmakova MP. An incidental finding of heart echinococcosis in a patient with infective endocarditis: a case report. Folia Med (Plovdiv) 2017;59:110–113. doi: 10.1515/folmed-2017-0017. [DOI] [PubMed] [Google Scholar]
- 2.Kern P, Silva AMD, Akhan O, Mullhaupt B, Vizcaychipi KA, Budke C, et al. The echinococcoses: diagnosis, clinical management and burden of disease. Adv Parasitol. 2017;96:259–369. doi: 10.1016/bs.apar.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Tamarozzi F, Mariconti M, Neumayr A, Brunetti E. The intermediate host immune response in cystic echinococcosis. Parasite Immunol. 2016;38:170–181. doi: 10.1111/pim.12301. [DOI] [PubMed] [Google Scholar]
- 4.Wen H, Vuitton L, Tuxun T, Li J, Vuitton DA, Zhang W, et al. Echinococcosis: advances in the 21st century. Clin Microbiol Rev. 2019;32:e00075–e00018. doi: 10.1128/CMR.00075-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng X, Qi X, Yang L, Duan X, Fang B, Gongsang Q, et al. Human cystic and alveolar echinococcosis in the Tibet autonomous region (TAR), China. J Helminthol. 2015;89:671–679. doi: 10.1017/S0022149X15000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumann S, Shi R, Liu W, Bao H, Schmidberger J, Kratzer W, et al. Worldwide literature on epidemiology of human alveolar echinococcosis: a systematic review of research published in the twenty-first century. Infection. 2019;47:703–727. doi: 10.1007/s15010-019-01325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li T, Chen X, Zhen R, Qiu J, Qiu D, Xiao N, et al. Widespread co-endemicity of human cystic and alveolar echinococcosis on the eastern Tibetan plateau, Northwest Sichuan/Southeast Qinghai. China Acta Trop. 2010;113:248–256. doi: 10.1016/j.actatropica.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nunnari G, Pinzone MR, Gruttadauria S, Celesia BM, Madeddu G, Malaguarnera G, et al. Hepatic echinococcosis: clinical and therapeutic aspects. World J Gastroenterol. 2012;18:1448–1458. doi: 10.3748/wjg.v18.i13.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Jebbawi F, Bellanger AP, Beldi G, Millon L, Gottstein B. Immunotherapy of alveolar echinococcosis via PD-1/PD-L1 immune checkpoint blockade in mice. Parasite Immunol. 2018;40:e12596. doi: 10.1111/pim.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuxun T, Apaer S, Ma HZ, Zhang H, Aierken A, Lin RY, et al. The potential role of Th9 cell related cytokine and transcription factors in patients with hepatic alveolar echinococcosis. J Immunol Res. 2015;2015:895416. doi: 10.1155/2015/895416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Cardoso R, Marreros N, Muller N, Lundstrom-Stadelmann B, Siffert M, et al. Foxp3+ T regulatory cells as a potential target for immunotherapy against primary infection with echinococcus multilocularis eggs. Infect Immun. 2018;86:e00542–e00518. doi: 10.1128/IAI.00542-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mejri N, Hemphill A, Gottstein B. Triggering and modulation of the host-parasite interplay by Echinococcus multilocularis: a review. Parasitology. 2010;137:557–568. doi: 10.1017/S0031182009991533. [DOI] [PubMed] [Google Scholar]
- 13.Borish LC, Steinke JW. 2. Cytokines and chemokines. J Allergy Clin Immunol. 2003;111:S460–S475. doi: 10.1067/mai.2003.108. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Gottstein B. Immunoregulation in larval Echinococcus multilocularis infection. Parasite Immunol. 2016;38:182–192. doi: 10.1111/pim.12292. [DOI] [PubMed] [Google Scholar]
- 15.Pakala T, Molina M, Wu GY. Hepatic echinococcal cysts: a review. J Clin Transl Hepatol. 2016;4:39–46. doi: 10.14218/JCTH.2015.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Killcoyne S, Carter GW, Smith J, Boyle J. Cytoscape: a community-based framework for network modeling. Methods Mol Biol. 2009;563:219–239. doi: 10.1007/978-1-60761-175-2_12. [DOI] [PubMed] [Google Scholar]
- 17.Kilwinski J, Jenne L, Jellen-Ritter A, Radloff P, Flick W, Kern P. T lymphocyte cytokine profile at a single cell level in alveolar echinococcosis. Cytokine. 1999;11:373–381. doi: 10.1006/cyto.1998.0432. [DOI] [PubMed] [Google Scholar]
- 18.Godot V, Harraga S, Beurton I, Deschaseaux M, Sarciron E, Gottstein B, et al. Resistance/susceptibility to Echinococcus multilocularis infection and cytokine profile in humans. I. Comparison of patients with progressive and abortive lesions. Clin Exp Immunol. 2000;121:484–490. doi: 10.1046/j.1365-2249.2000.01308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wellinghausen N, Gebert P, Kern P. Interleukin (IL)-4, IL-10 and IL-12 profile in serum of patients with alveolar echinococcosis. Acta Trop. 1999;73:165–174. doi: 10.1016/S0001-706X(99)00027-3. [DOI] [PubMed] [Google Scholar]
- 20.Jenne L, Kilwinski J, Scheffold W, Kern P. IL-5 expressed by CD4+ lymphocytes from Echinococcus multilocularis-infected patients. Clin Exp Immunol. 1997;109:90–97. doi: 10.1046/j.1365-2249.1997.4031299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuxun T, Ma HZ, Apaer S, Zhang H, Aierken A, Li YP, et al. Expression of toll-like receptors 2 and 4 and related cytokines in patients with hepatic cystic and alveolar echinococcosis. Mediat Inflamm. 2015;2015:632760. doi: 10.1155/2015/632760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang S, Hue S, Sene D, Penfornis A, Bresson-Hadni S, Kantelip B, et al. Expression of major histocompatibility complex class I chain-related molecule a, NKG2D, and transforming growth factor-beta in the liver of humans with alveolar echinococcosis: new actors in the tolerance to parasites? J Infect Dis. 2008;197:1341–1349. doi: 10.1086/586709. [DOI] [PubMed] [Google Scholar]
- 23.Bellanger AP, Mougey V, Pallandre JR, Gbaguidi-Haore H, Godet Y, Millon L. Echinococcus multilocularis vesicular fluid inhibits activation and proliferation of natural killer cells. Folia Parasitol (Praha) 2017;64:2017. doi: 10.14411/fp.2017.029. [DOI] [PubMed] [Google Scholar]
- 24.Bellanger AP, Pallandre JR, Gbaguidi-Haore H, Knapp J, Malezieux N, Lignon T, et al. Investigating the impact of Echinococcus multilocularis vesicular fluid on human cells from healthy blood donors. J Immunol Methods. 2015;417:52–59. doi: 10.1016/j.jim.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Dreweck CM, Soboslay PT, Schulz-Key H, Gottstein B, Kern P. Cytokine and chemokine secretion by human peripheral blood cells in response to viable Echinococcus multilocularis metacestode vesicles. Parasite Immunol. 1999;21:433–438. doi: 10.1046/j.1365-3024.1999.00243.x. [DOI] [PubMed] [Google Scholar]
- 26.Shi DZ, Li FR, Bartholomot B, Vuitton DA, Craig PS. Serum sIL-2R, TNF-alpha and IFN-gamma in alveolar echinococcosis. World J Gastroenterol. 2004;10:3674–3676. doi: 10.3748/wjg.v10.i24.3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lechner CJ, Gruner B, Huang X, Hoffmann WH, Kern P, Soboslay PT. Parasite-specific IL-17-type cytokine responses and soluble IL-17 receptor levels in alveolar echinococcosis patients. Clin Dev Immunol. 2012;2012:735342. doi: 10.1155/2012/735342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuxun T, Apaer S, Ma HZ, Zhao JM, Lin RY, Aji T, et al. Plasma IL-23 and IL-5 as surrogate markers of lesion metabolic activity in patients with hepatic alveolar echinococcosis. Sci Rep. 2018;8:4417. doi: 10.1038/s41598-018-20301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harraga S, Godot V, Bresson-Hadni S, Mantion G, Vuitton DA. Profile of cytokine production within the periparasitic granuloma in human alveolar echinococcosis. Acta Trop. 2003;85:231–236. doi: 10.1016/S0001-706X(02)00218-8. [DOI] [PubMed] [Google Scholar]
- 30.Huang X, Gruner B, Lechner CJ, Kern P, Soboslay PT. Distinctive cytokine, chemokine, and antibody responses in Echinococcus multilocularis-infected patients with cured, stable, or progressive disease. Med Microbiol Immunol. 2014;203:185–193. doi: 10.1007/s00430-014-0331-8. [DOI] [PubMed] [Google Scholar]
- 31.Kocherscheidt L, Flakowski AK, Gruner B, Hamm DM, Dietz K, Kern P, et al. Echinococcus multilocularis: inflammatory and regulatory chemokine responses in patients with progressive, stable and cured alveolar echinococcosis. Exp Parasitol. 2008;119:467–474. doi: 10.1016/j.exppara.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Hubner MP, Manfras BJ, Margos MC, Eiffler D, Hoffmann WH, Schulz-Key H, et al. Echinococcus multilocularis metacestodes modulate cellular cytokine and chemokine release by peripheral blood mononuclear cells in alveolar echinococcosis patients. Clin Exp Immunol. 2006;145:243–251. doi: 10.1111/j.1365-2249.2006.03142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siracusano A, Delunardo F, Teggi A, Elena O. Cystic Echinococcosis: aspects of immune response, Immunopathogenesis and immune evasion from the human host. Endocr Metab Immune Disord Drug Targets. 2012;12:16–23. doi: 10.2174/187153012799279117. [DOI] [PubMed] [Google Scholar]
- 34.Liance M, Ricard-Blum S, Emery I, Houin R, Vuitton DA. Echinococcus multilocularis infection in mice: in vivo treatment with a low dose of IFN-gamma decreases metacestode growth and liver fibrogenesis. Parasite. 1998;5:231–237. doi: 10.1051/parasite/1998053231. [DOI] [PubMed] [Google Scholar]
- 35.Emery I, Leclerc C, Sengphommachanh K, Vuitton DA, Liance M. In vivo treatment with recombinant IL-12 protects C57BL/6J mice against secondary alveolar echinococcosis. Parasite Immunol. 1998;20:81–91. doi: 10.1046/j.1365-3024.1998.00131.x. [DOI] [PubMed] [Google Scholar]
- 36.Valot B, Rognon B, Prenel A, Baraquin A, Knapp J, Anelli M, et al. Screening of antigenic vesicular fluid proteins of Echinococcus multilocularis as potential viability biomarkers to monitor drug response in alveolar echinococcosis patients. Proteomics Clin Appl. 2017;11:1700010. doi: 10.1002/prca.201700010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the conclusions are included within the article.