Abstract
Biofabrication is an emerging multidisciplinary field that makes a revolutionary impact on the researches on life science, biomedical engineering, and both basic and clinical medicine, has progressed tremendously over the past few years. Recently, there has been a big boom in three-dimensional (3D) printing or additive manufacturing (AM) research worldwide, and there is a significant increase not only in the number of researchers turning their attention to AM but also publications demonstrating the potential applications of 3D printing techniques in multiple fields. Biofabrication and bioprinting hold great promise for the innovation of engineering-based organ replacing medicine. In this mini review, various challenges in the field of tissue engineering are focused from the point of view of the biofabrication - strategies to bridge the gap between organ shortage and mission of medical innovation research seek to achieve organ-specific treatments or regenerative therapies. Four major challenges are discussed including (i) challenge of producing organs by AM, (ii) digitalization of tissue engineering and regenerative medicine, (iii) rapid production of organs beyond the biological natural course, and (iv) extracorporeal organ engineering.
Keywords: Biofabrication, bioprinting, tissue engineering, regenerative medicine
1. Introduction and Backgrounds of Biofabrication (Bioprinting)
Organ failure is a critical issue in the health-care sector and there are currently millions of people waiting for organ transplants throughout the world[1-7]. The demand for organ transplantation has increased over time. Although organ donation can save the lives of many needy patients, the problem is, there simply are not enough organ donors to meet demand[8,9]. Hospitals and health centers with organ transplant facilities have been facing critical shortage of donor organs and the scale of the problem is getting worse every year all over the world. There has been a tremendous increase in the number of patients on organ transplant waiting list as well as a sharp rise in the number of patients dying while awaiting lifesaving organ transplant operations for years. As a result, one in 10 on transplant waiting list dies before organ is found and millions of people die due to the lack of available organs for transplant[10-13].
The fundamental concepts and the recorded use of the term tissue engineering, as it is applied today in biomedical field, was originally published by Langer and Vacanti (1993) in Science[14]. Since then, tissue engineering research has grown exponentially due to the recognition that tissue engineering base strategies have the potential to replace, repair, and regenerate tissue/organs for a variety of biomedical applications including transplantation, therapeutic investigation, bioassay, disease modeling, drug development, and delivery. The rapidly evolving cross-disciplinary field of tissue engineering along with its intimately intertwined field of regenerative medicine continues to develop and advance.
The most intrinsic purpose, mission, and goal of tissue engineering and regenerative medicine (TERM) is to provide alternate solutions for restoring, replacing, and maintaining of organ functions of the problematic tissue/organ of interest using applied science and engineering approaches[14,15]. The crucial difference between TERM and general science is that TERM is not a traditional way of doing science or to simply elucidate the origins and mechanisms of natural phenomenon based on intellectual curiosity but has a practical purposes of producing bioartificial organs that make possible medical treatments for patients with serious tissue/organ diseases/injuries and offers new hope to many patients who are suffering from end-stage organ failure.
During recent years, a large number of scientists, researchers, clinicians, and biomedical engineering companies have been actively engaged in tissue engineering and regenerative medicine research. Simple tissues (e.g., skin and engineered cartilage) have already been developed and being used clinically[16-20]. Several other less complex tissues manufactured from a variety of biomaterials using a plethora of engineering approaches are at different stages of development. Despite scientific progress in tissue engineering, there are still several big obstacles in producing complex, functional, and large-sized threedimensional (3D) tissues/organs, especially the tissues of the vital organs that are urgently required for experimental and clinical transplantation applications.
2. Final Mission of Biofabrication - Bioprinting
The challenging fields of biofabrication-bioprinting have emerged as revolutionary approaches to break the limitations of conventional TERM methods by offering potential technological solution. In brief, bioprinting can be defined as the manufacturing process by employing computer-aided two-dimensional (2D)/3D printing techniques to create 2D/3D patterns and to construct complex 3D structures with living and non-living biological raw materials to produce 2D or 3D tissues and organs of interest[21,22]. The official definition of the term “biofabrication” for TERM was recently proposed by the International Society for Biofabrication (ISBF)[23]. According to ISBF, biofabrication can be defined as “the automated generation of biologically functional products with structural organization from living cells, bioactive molecules, biomaterials, cell aggregates such as microtissues, or hybrid cell material constructs, through bioprinting or bioassembly and subsequent tissue maturation processes.” In a more narrow sense, emerging field of biofabrication basically enables the researchers to use or to combine advanced fabrication technologies including 2D/3D printing, biomanufacturing, and bioassembly of living 3D functional biological products using smart and cytocompatible biomaterials.
Recently, there has been a big boom in 3D printing or additive manufacturing (AM) research, and various 3D printers have been developed, commercialized, and distributed worldwide. Today, many researchers from different backgrounds (science, engineering, and medical) have joined the interdisciplinary research field of bioprinting and biofabrication to open the doors to previously unimaginable possibilities in medicine or to search possible application of 3D printing in the biomedical field. 3D fabricated plastic organ models have become very popular for medical education. In TERM research field, biological 3D tissue models are one of the most attractive topics of application of the bioprinting and biofabrication, such as 3D tissue models for drug screening, disease models, tissue or organ on a chip, medical sensors, and biological actuators[24-26]. Such research studies are certainly very useful and effective for drug discovery, drug development, and pharmaceutical industry.
Although drug administration is the first choice for treating patients with diseased/injured organs or organ failure, drugs are effective only in the early stages of the disease or minor injuries. Moreover, for the patients who require organ replacement or artificial organs for transplantation, generally, no significant effect can be expected for drug treatment.
Thus, the biomanufacturing of complex tissue/organ substitutes that fully mimic the natural physiological conditions of particular tissues/organs could help to alleviate organ failure/replacement issue. In the recent past, there has been a substantial and commendable progress in the field of TERM[27-35]. Although successful fabrication of various tissue models has been reported, taking the engineered complex tissues/organ constructs from the bench to the bedside still needs focused efforts on scientific as well as potential technological fronts[2,31,36-50].
3. Biofabrication Bioprinting Solving Various Challenges
As mentioned above, the main focus of bioprinting and biofabrication research is to overcome various challenges of tissue engineering and future studies could prove “whether human beings can produce organs using printing and manufacturing techniques.” In this mini review paper, we have focused on various challenges which need to be overcome by bioprinting and biofabrication to produce organ or organ substitutes.
3.1. Challenge of Producing Organs by AM
In bioprinting and biofabrication, 3D tissues and organs are constructed by aligning living cells and biomaterials and by stacking or laminating them in 3D[51,52]. Such bottom-up fabrication method of building 3D structures is called AM. Most of the conventional manufacturing techniques used to build 3D objects are based on molding and subtractive methods. By traditional manufacturing, 3D shapes can be made by starting from an object having an initial size or shape, materials are often molded, carved out, or removed by a sharp cutting tool until product of the desired shape is formed. However, there are still challenging issues associated with these methods, especially the inability to create or control the internal structure of the 3D objects. When it comes to manufacturing of 3D structures that specifically mimic human tissue- and organspecific microarchitecture, these conventional approaches certainly are not useful because all vital organs are highly complex in nature, and each individual organ possesses its own specialized microsized histological, anatomical, and morphological structures which are very essential to perform all organ-specific physiological activities[53].
Therefore, the technologies to construct 3D structure both internal and external structures simultaneously are highly needed for TERM research. As such, any structure we fabricate needs to exactly match that level of complex structural heterogeneity. Thus, the only hope for generating such structures is AM technology. For this reason, bioprinting and biofabrication have ever being challenged to produce organs or their spare bioparts by AM approach. As mentioned in Groll et al. (2016), bioprinting is a complimentary strategy within biofabrication. With bio-AM, this term can be used for describing a holistic approach that combines both bioprinting and biofabrication technologies for constructing engineered tissues/organs. Indeed, bio-AM has the potential to transform global health care and medicine, but bio-AM is still in its infancy and there are several obvious challenges that need to be overcome. Since such AM techniques have been only recently proposed and developed, only limited research is reported on their application for realizing truly biologically inspired new engineering solutions for clinical health care and bioindustry.
Although biological materials, especially living cells, can be regarded as the key materials for bio-AM, several other well-controlled and biocompatible biomaterials, and adequate integration of biological tissue components with their application contexts are also needed to obtain biologically active 2D/3D tissues and organs or related bioproducts, but as yet, such materials have never been utilized in usual AM purposes[54-57]. There is no doubt that all of the AM procedures required to be carried out in biologically safe environment. Although bio-AM can be helpful for the biomanufacturing, there are practically no established 3D biofabrication machines which can realize the manufacturing of arbitrary 3D structures. Therefore, it is necessary to develop advanced biofabrication machines to achieve the goal of arranging several different materials (including cells) in 3D space for engineering of multicellular constructs (tissues/organs) or bioproducts on demand. Apparently, a growing number of 3D printers have entered into the mainstream biomanufacturing technology, but only extrusion-based bioprinting (EBB) is rapidly growing[21,22,24,37,43,45]. Even though EBB is considered to be the most accepted technique in tissue engineering field to date, this technique also suffers from several limitations. For example, the resolution of extrusion type printers is still very poor (more than 500 μm)[41,44,58-78]. On the other hand, an average human cells range in size from about 10 to 30 µm, while very fine capillary vessels, which are the essential tissue components are on the order of 10 µm in diameter. Therefore, 3D bioprinters with sufficient resolution ability are needed, ultimately.
In addition, diverse bioink formulation is also one of the challenging aspects of this field. Typical adult human body consists of myriad of cell populations, tissue components, and microstructures that work together to perform particular body functions. At present, effective materials for bioink of 3D bioprinting or biofabrication are extremely limited, the reader is referred to more specialized reports[54,56,57,79]. Therefore, the development and formulation of effective bioinks are necessary for the clinical application of bioprinting technology. More importantly, novel kind of hydrogel materials exhibiting remarkably favorable properties, including compatibility with different bioprinting methods, rapid or instantaneous gelling properties, flexibility and stability in medium, cytocompatibility, or biofunctionality, are still needed for proper cell growth, differentiation, and tissue formation or regeneration during bioprinting processes (pre-bioprinting, bioprinting, and post-bioprinting). The formulation of wide range of the biomaterials to design a variety of bioinks exhibiting the abovementioned properties remains an important research direction for biofabrication research. Nowadays, bioink is one of the emerging and hot topics in bioprinting and biofabrication[80,81]. Expanding biofabrication technology and fostering the invention of new biomanufacturing machines and development of novel bioink materials could advance the reliability of bio-AM, and only bio-AM can potentially deal with the increasing demand for replacing/regenerating tissues/organs. In the end, such developments could be promising in on-demand tissue engineering or bioindustrial applications using bio-AM technologies.
3.2. Challenge of the Digitalization of TERM
The rate at which biofabrication research is being carried our worldwide reflects remarkable technological achievements. Printers (including 3D printers) are considered as excellent output devices[21,23,61,77,80-82]. Recent trends in implementation of bioprinters in biofabrication technology within the field of TERM have shown that the number of research papers has significantly grown, while bioprinting and biofabrication have become more popular research areas[23,81].
Broadly speaking, biofabrication is complex technology comprised several techniques including computer-aided design (CAD), computer-aided engineering (CAE), and biological processes and subprocesses. CAD, CAM, and CAE terminologies for biofabrication have been proposed to parallel similar strategies in other engineering fields. However, there are several big and important distinctions between the disciplines. To avoid confusion, we propose that for biofabrication (bioengineering of complex tissues/organs), these terms should be used as bio-CAD, bio-CAM, and bio-CAE (Figure 1).
Figure 1.
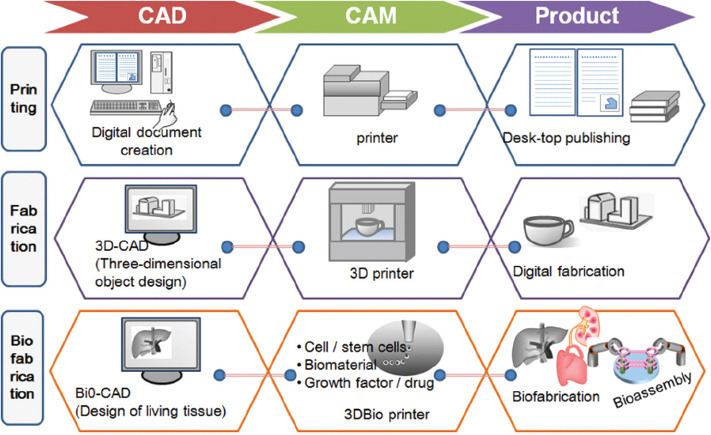
The sketch shows a schematic drawing of the potential strategy for digitalization of tissue engineering and regenerative medicine.
Bio-CAD, bio-CAM, and bio-CAE approaches are generally regarded as promising future technology for the biomanufacturing of complex and heterogeneous bioconstructs. Bio-CAD process can be used to design tissue and organ blueprints, bio-CAM process can be to manufacture biological products, and bio-CAE process can be used to create complex bioarchitectures, and validate and optimize biomanufacturing tools and bioproducts. Effective implementation of bio-CAD, bio-CAM, and bio-CAE processes (biofabrication) depends on the combination of several interrelated components/parameters, such as biomaterials, biomolecules, cells, and tissues, integrated with computational approaches (e.g., digital designing, information mapping, sophisticated virtual product modeling, 3D simulation, and data mining).
Digitization of bio-CAD, bio-CAM, and bio-CAE processes are the key issues of the day for biofabrication scientists and researchers. Indeed, the current biofabrication initiatives are possible, but computer programmers and technologists will undoubtedly have to invent highly inclusive digital technologies that involve integrating imaging, database, and computer numerically controlled bioprinting machines and artificial intelligence[83-91]. If all these parameters will function in a coordinated way, then we can achieve the final objective of digital fabrication in TERM or automated production of biologically functional human tissues and organs for transplantation and commercialization purposes[92-105].
4. Challenge to Rapid Production of Organs beyond the Biological Natural Course
The human body with its interconnected organ systems is the most advanced and complex living structure in the known universe. In the natural process inside our bodies, organ developmental phenomena are strictly controlled by natural laws of embryology and anatomy. All of organs arise through a process that begins with the fertilized egg which undergoes through cell divisions, differentiation processes resulting in the formation of organs and ends with a new individual living entity. From a pragmatic point of view, there are several factors which directly or indirectly influence the continued growth and development of organs (differentiation of the cells into two or more histologically specialized structures that organize to form specific organs and organs systems) to the time of delivery. The rate of organ growth and development varies individually and is dependent on multiple sequential and reciprocal interactive influences mediated by genetic structure, genetic information and genetic diversity traits, maternal traits, and internal environmental control mechanisms of the body.
Morphology, histology, and functional anatomy are largely limited to the hierarchical level of organs. Thus, the anatomical and histomorphologic features build up continually according to the growth of the organ and whole body through many complicated processes across respective assembly locations in the body. For example, if you see the complex embryology of heart, it forms initially in the embryonic disc as a simple paired tube inside the forming pericardial cavity. However, as it develops four chambers with four valves, the complexity creates even more complex dynamics of the physiological systems[106-109].
In the natural process inside our bodies, tissue and structures originate and mature as an individual grows. Therefore, it is not so easy to establish feasible organs substitute through natural processes because it takes many years for an individual and its organs to grow, develop, and generate sufficient functions. In contrasts, patients who require organ transplants cannot wait for organs for such a long time. For this reason, organs or organ substitutes for transplantation should be provided as soon as possible, ideally within a few months. There are many excellent scientific researches on generation of organs based on the embryological phenomena[110-113]; however, it seems difficult to provide enough organs for patients with organ failure/disease during the waiting period. For these reasons, natural process is generally considered as unsuitable option for complex tissue and organ regeneration (Figure 2).
Figure 2.
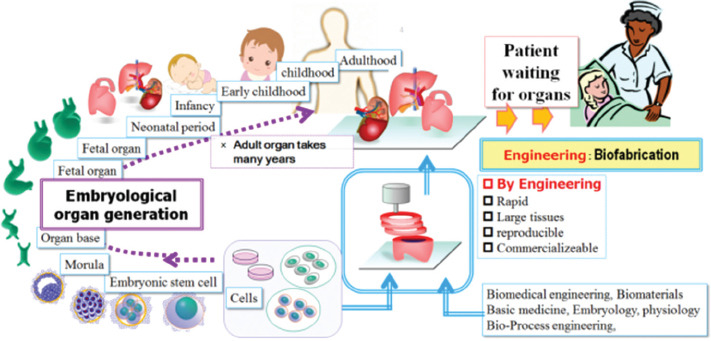
The sketch shows a schematic drawing of the strategy of rapid production of the organs beyond the biological natural course.
Although several tissue engineering approaches have been implemented for various biomedical research strategies, differences in the natural formation of living human tissues/organs with their unique shapes and material properties, hierarchical, morphological, anatomical, and physiological biochemical features limit the ability of conventional tissue engineering approaches to regenerate complex metabolic organs. In other words, tissues/organs require more sophisticated biomimetic 3D microenvironments capable of providing multilayer information to differentiating cells. Novel biomanufacturing methodologies and biomimetic materials still need to be developed and be used to assemble microscale building blocks capable of accurately mimicking/replacing 3D complex Humanscale living vascularized tissue/organ analogs with optimal physiological activity.
Therefore, bio-AM is the only hope to overcome these challenges and to simultaneously construct fully functional organ analogs with great complexity (including histologically essential structures) by applying 2D/3D printing and related AM strategies. Although available biofabrication methods have several features and capabilities, still there are limitations to exploit the exact structure/function of the native tissue organ. More complex material distribution and construction of anatomically and physiologically relevant tissues/organs may not be easily achieved without advanced biofabrication approaches. To produce a substitute for natural organ or autologous tissue graft that is ready to be transplanted, improvements in biofabrication technologies for facilitating the precise programmable designing, computer-controlled efficient biofabrication, and post-fabrication incubation are urgently needed. There is still a long way to go in fabricating bioartificial organs on demand, but these technologies are achievable. If biofabrication approaches will work out, it will become possible to fabricate not only Humanscale patientspecific tissue grafts/organs at a rapid pace (irrespective of organ size) but it will also shorten the time required of growth performance and development of organs. Then, no patient will ever have to wait on lengthy transplant lists for donor organs, and no patient will ever have to take powerful, debilitating and potentially fatal drugs to treat chronic pains and to prevent their immune systems from rejecting new body parts or from attacking the transplanted organ when the organ is not closely matched.
1.5. Challenge to Extracorporeal Organ Engineering
In conventional TERM methods including cell transplantation and the scaffold-based tissue repair strategies, morphogenesis of tissue architectures and regulation of essential biochemical and physiological processes all depends on the regenerative ability of cells and the recipient in vivo. While modern tissue engineering approaches are good at making tissues in the laboratory, we still cannot control the important processes of complex tissue and organ after implantation. Here comes the role of bio-AM filed to make extracorporeal systems having the capability to repair/return organ function permanently or for implantation. Broadly speaking, bio-AM is aiming at challenges of producing extracorporeal organ reconstruction systems, in which the important tissue structures can be printed, fabricated, assembled, cultured, and matured before implantation. In addition, ideally, the functions of engineered organs should also be established before implantation.
It is often thought that the in vivo approaches are physiologically better and look realistic enough. However, this is not necessarily true and in vivo approaches are not always better for organ engineering. One of the main limitations of in vivo approaches for tissue engineering research is the regeneration of only fibrous tissues. It has been demonstrated that fibroblasts and inflammatory cells are the most strongly proliferating cells in the living body consuming large amount of nutrients and oxygen to provide energy for proliferation of metabolically active cells[114-116]. Therefore, the transplanted cells cannot survive without a blood supply, sufficient supply of essential nutrients, or adequate means for waste product removal. Instead, outside the body, the desired tissues or organs can be grown without pro-fibrogenic environment. Therefore, better in vitro strategies are really needed to support, instruct, and maintain cells inside immuneprivileged 3D microenvironments, where cultured cells do not lose their unique intrinsic characteristics (Figure 3).
Figure 3.
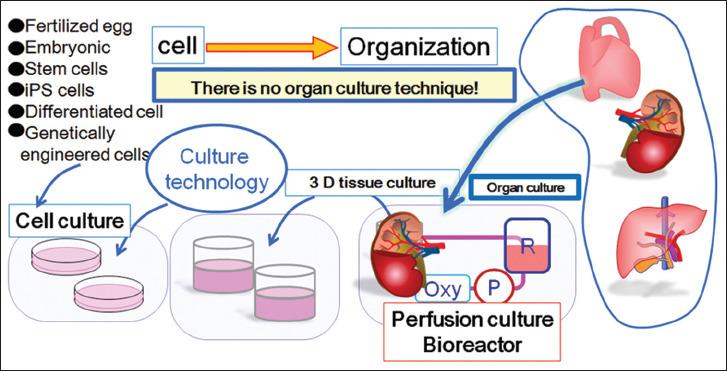
The sketch shows a schematic drawing of the general culture strategy and organ perfusion bioreactor technology.
As mentioned above, bioprinting is the only hope for fabricating appropriate environments for cells to be able to effectively produce spare parts of the human tissue/organs beyond the limits attained in vivo. For instance, biofabricated organs can be incubated in the environment under the high or low oxygen tension, high or low growth factors, and exposure of drugs which have high pharmacological activity. Control of such incubating parameters is impossible in vivo situations due to harmful effects on the recipients. Therefore, the concept of extracorporeal system is clinically appealing and advanced extracorporeal systems are necessary for biofabrication research. Such systems may enable precise spatial and temporal control arbitrarily without affecting the recipients. Building on this momentum, the next regenerative medicine frontier lies in how biofabrication researchers can better develop novel extracorporeal systems and bioprocess engineering aims. However, no one has ever succeeded to produce functional engineered organs. One of the reasons is because we cannot do anything with natural process at all after implantation. Thus, biofabrication is aiming at extracorporeal organ engineering, in which tissue formation can be controlled and facilitated under human control.
2.6. Conclusion
In this mini review, four major challenges by bioprinting and biofabrication are focused and explained from the views from the final mission. These challenges were started just from the beginning of this research field but are still important issues urgently needed to be addressed. Based on our more than 15 years of research endeavors in this field, we anticipate that biofabrication (bioprinting) holds great promise for the development of artificial tissue/organs. There are indeed still many technological issues; however, such problems are also essential to better understand potential limitations of conventional methods, overcome challenges, and produce organs or organ substitutes. Even though we cannot foresee the use of available biofabrication approaches for fabricating fully functional organs in the near future, there is great potential and promise for the applications of biofabrication approaches in the research area of TERM. We conclude with the quote of Theodor von Karman who once said “Scientists study the world as it is; engineers create the world that has never been.” Tissue engineers’ worldwide have been doing amazing research and putting their efforts on the development and implementation of advance biofabrication technologies (e.g., bioprinting) to create the world of biomedicine that has never been.
References
- 1.Robert J M, Christopher M C. Pathophysiology and clinical evaluation of acute heart failure. Nat Rev Cardiol. 2016;13(1):28–35. doi: 10.1038/nrcardio.2015.134. https://doi.org/10.1038/nrcardio.2015.134. [DOI] [PubMed] [Google Scholar]
- 2.Wouters O J, O'Donoghue D J, Ritchie J. Early chronic kidney disease:Diagnosis, management and models of care. Nat Rev Nephrol. 2015;11(8):491–502. doi: 10.1038/nrneph.2015.85. https://doi.org/10.1038/nrneph.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiv K S, Ashok C. Acute–on–chronic liver failure:Terminology, mechanisms and management. Nat Rev Gastroenterol Hepatol. 2016;13(3):131–149. doi: 10.1038/nrgastro.2015.219. https://doi.org/10.1038/nrgastro.2015.219. [DOI] [PubMed] [Google Scholar]
- 4.Pfeifer R, Teuben M, Andruszkow H, et al. Mortality patterns in patients with multiple trauma:A systematic review of autopsy studies. PLoS One. 2016;11(2):e0148844. doi: 10.1371/journal.pone.0148844. https://doi.org/10.1371/journal.pone.0148844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lelubre C, Vincent J L. Mechanisms and treatment of organ failure in sepsis. Nat Rev Nephrol. 2018;14(7):417–427. doi: 10.1038/s41581-018-0005-7. https://doi.org/10.1038/s41581-018-0005-7. [DOI] [PubMed] [Google Scholar]
- 6.Gyöngyösi M, Haller P M, Blake D J. Meta–analysis of cell therapy studies in heart failure and acute myocardial infarction. Circ Res. 2018;123(2):301–308. doi: 10.1161/CIRCRESAHA.117.311302. https://doi.org/10.1161/CIRCRESAHA.117.311302. [DOI] [PubMed] [Google Scholar]
- 7.Katrina R. KMO inhibitor for multi–organ failure in experimental acute pancreatitis. Nat Rev Gastroenterol Hepatol. 2016;13(2):61. doi: 10.1038/nrgastro.2016.6. https://doi.org/10.1038/nrgastro.2016.6. [DOI] [PubMed] [Google Scholar]
- 8.Kumaran S, Vincenzo V, Maria L L M. Current progress in public health models addressing the critical organ shortage. Int J Surg. 2014;12:1363–1368. doi: 10.1016/j.ijsu.2014.11.011. https://doi.org/10.1016/j.ijsu.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Shruti G, Jason T, Marie B. Overview of lung transplantation, heart–lung transplantation, liver–lung transplantation, and combined hematopoietic stem cell transplantation and lung transplantation. Clin Chest Med. 2017;38(4):623–640. doi: 10.1016/j.ccm.2017.07.004. https://doi.org/10.1016/j.ccm.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Wil L S, Jacob J S, Paul S M. The organ transplant imperative. Mayo Clin Proc. 2017;92(6):940–946. doi: 10.1016/j.mayocp.2017.03.005. https://doi.org/10.1016/j.mayocp.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Abouna G M. Organ shortage crisis:Problems and possible solutions. Transplant Proc. 2008;40(1):34–38. doi: 10.1016/j.transproceed.2007.11.067. https://doi.org/10.1016/j.transproceed.2007.11.067. [DOI] [PubMed] [Google Scholar]
- 12.Seetapun D, Ross J J. Eliminating the organ transplant waiting list:The future with perfusion decellularized organs. Surgery. 2017;161(6):1474–1478. doi: 10.1016/j.surg.2016.09.041. https://doi.org/10.1016/j.surg.2016.09.041. [DOI] [PubMed] [Google Scholar]
- 13.Douville F, Godin G, Vezina–Im LA. Organ and tissue donation in clinical settings:A systematic review of the impact of interventions aimed at health professionals. Transplant Res. 2014;3(1):8. doi: 10.1186/2047-1440-3-8. https://doi.org/10.1186/2047-1440-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langer R, Vacanti J P. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. https://doi.org/10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 15.Atala A, Lanza R P. Preface. In: Atala A, Lanza R P, editors. Methods of Tissue Engineering. San Diego: Academic Press; 2001. [Google Scholar]
- 16.Guangdong Z, Haiyue J, Zongqi Y. 2018In vitroregeneration of patient–specific ear–shaped cartilage and its first clinical application for auricular reconstruction. E-Biomed. 28:287–302. doi: 10.1016/j.ebiom.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atala A, Bauer S B, Soker S. Tissue–engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–1246. doi: 10.1016/S0140-6736(06)68438-9. https://doi.org/10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 18.Fulco I, Miot S, Haug M D. Engineered autologous cartilage tissue for nasal reconstruction after tumour resection:An observational first–in–human trial. Lancet. 2014;384:337–346. doi: 10.1016/S0140-6736(14)60544-4. https://doi.org/10.1016/S0140-6736(14)60544-4. [DOI] [PubMed] [Google Scholar]
- 19.Muhart M, McFalls S, Kirsner R. Bioengineered skin. Lancet. 1997;350:1142. doi: 10.1016/S0140-6736(05)63788-9. https://doi.org/10.1016/S0140-6736(05)63788-9. [DOI] [PubMed] [Google Scholar]
- 20.Olausson M, Patil P B, Kuna V K. Transplantation of an allogeneic vein bioengineered with autologous stem cells:A proof–of–concept study. italic>Lancet. 2012;380:230–237. doi: 10.1016/S0140-6736(12)60633-3. https://doi.org/10.1016/S0140-6736(12)60633-3. [DOI] [PubMed] [Google Scholar]
- 21.Mironov V, Trusk T, Kasyanov V. Biofabrication:A 21stcentury manufacturing paradigm. italic>Biofabrication. 2009;1(2):22001. doi: 10.1088/1758-5082/1/2/022001. https://doi.org/10.1088/1758-5082/1/2/022001. [DOI] [PubMed] [Google Scholar]
- 22.Guillemot F, Mironov V, Nakamura M. Bioprinting is coming of age:Report from the International conference on bioprinting and biofabrication in Bordeaux (3B'09) Biofabrication. 2010;2:10201–10207. doi: 10.1088/1758-5082/2/1/010201. https://doi.org/10.1088/1758-5082/2/1/010201. [DOI] [PubMed] [Google Scholar]
- 23.Groll J, Boland T, Blunk T. Biofabrication:Reappraising the definition of an evolving field. Biofabrication. 2016;8(1):13001. doi: 10.1088/1758-5090/8/1/013001. https://doi.org/10.1088/1758-5090/aaec52. [DOI] [PubMed] [Google Scholar]
- 24.Pati F, Ha D H, Jang J. Biomimetic 3D tissue printing for soft tissue regeneration. Biomaterials. 2015;62:164–175. doi: 10.1016/j.biomaterials.2015.05.043. https://doi.org/10.1016/j.biomaterials.2015.05.043. [DOI] [PubMed] [Google Scholar]
- 25.Lind JU, Busbee TA, Valentine AD. Instrumented cardiac microphysiological devices via multimaterial three–dimensional printing. Nat Mater. 2017;16:303–308. doi: 10.1038/nmat4782. https://doi.org/10.1038/nmat4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NIH National Center of Advancing Translational Sciences, Meet Chip. Available from: https://www.ncats.nih.gov/tissuechip/chip .
- 27.Zimmermann W H, Melnychenko I, Wasmeier G. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–458. doi: 10.1038/nm1394. https://doi.org/10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 28.Yanez M, Rincon J, Dones A. 2014In vivoassessment of printed microvasculature in a bilayer skin graft to treat full–thickness wounds. Tissue Eng Part A. 21:224–233. doi: 10.1089/ten.tea.2013.0561. https://doi.org/10.1089/ten.tea.2013.0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Homan K A, Kolesky D B, Skylar–Scott M A. Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci Rep. 2016;6:34845. doi: 10.1038/srep34845. https://doi.org/10.1038/srep34845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pourchet L J, Thepot A, Albouy M. Human skin 3D bioprinting using scaffold–free approach. Adv Healthc Mater. 2017;6:1601101. doi: 10.1002/adhm.201601101. https://doi.org/10.1002/adhm.201601101. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y S, Arneri A, Bersini S. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart–on–a–chip. Biomaterials. 2016;110:45–59. doi: 10.1016/j.biomaterials.2016.09.003. https://doi.org/10.1016/j.biomaterials.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma X, Qu X, Zhu W. Deterministically patterned biomimetic human iPSC–derived hepatic model via rapid 3D bioprinting. Proc Nat Acad Sci. 2016;113:2206–2211. doi: 10.1073/pnas.1524510113. https://doi.org/10.1073/pnas.1524510113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seol Y J, Park J Y, Jeong W. Development of hybrid scaffolds using ceramic and hydrogel for articular cartilage tissue regeneration. J Biomed Mater Res Part A. 2015;103:1404–1413. doi: 10.1002/jbm.a.35276. https://doi.org/10.1002/jbm.a.35276. [DOI] [PubMed] [Google Scholar]
- 34.Lee J S, Kim B S, Seo D. Three–dimensional cell printing of large–volume tissues:Application to ear regeneration. Tissue Eng Part C. 2017;23:136–145. doi: 10.1089/ten.TEC.2016.0362. https://doi.org/10.1089/ten.tec.2016.0362. [DOI] [PubMed] [Google Scholar]
- 35.Liu L, Wang X. Creation of a vascular system for complex organ manufacturing. Int J Bioprint. 2015;1:77–86. [Google Scholar]
- 36.Lokmic Z, Mitchell G M. Engineering the microcirculation. Tissue Eng Part B. 2008;14(1):87–103. doi: 10.1089/teb.2007.0299. https://doi.org/10.1089/teb.2007.0299. [DOI] [PubMed] [Google Scholar]
- 37.Liu F, Liu C, Chen Q H. Progress in organ 3D bioprinting. Int J Bioprint. 2018;4(1):128. doi: 10.18063/IJB.v4i1.128. https://doi.org/10.18063/ijb.v4i1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khademhosseini A, Langer R, Borenstein J. Microscale technologies for tissue engineering and biology. Proc Nat Acad Sci. 2006;103:2480–2487. doi: 10.1073/pnas.0507681102. https://doi.org/10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhuang P, Sun A X, An J. 3D neural tissue models:From spheroids to bioprinting. Biomaterials. 2018;154:113–133. doi: 10.1016/j.biomaterials.2017.10.002. https://doi.org/10.1016/j.biomaterials.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Arai K, Yoshida T, Okabe M. Fabrication of 3D culture platform with sandwich architecture for preserving liver–specific functions of hepatocytes using 3D bioprinter. J Biomed Mater Res Part A. 2017;105:1583–1592. doi: 10.1002/jbm.a.35905. https://doi.org/10.1002/jbm.a.35905. [DOI] [PubMed] [Google Scholar]
- 41.Duan B, Hockaday L A, Kang K H. 3D Bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J Biomed Mater Res Part A. 2013;101:1255–1264. doi: 10.1002/jbm.a.34420. https://doi.org/10.1002/jbm.a.34420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Brien C M, Holmes B, Faucett S. Three–dimensional printing of nanomaterial scaffolds for complex tissue regeneration. Tissue Eng Part B. 2015;21:103. doi: 10.1089/ten.teb.2014.0168. https://doi.org/10.1089/ten.teb.2014.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pati F, Jang J, Ha D H. Printing three–dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Comm. 2014;5:3935. doi: 10.1038/ncomms4935. https://doi.org/10.1038/ncomms4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy S V, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32:773–785. doi: 10.1038/nbt.2958. https://doi.org/10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura M, Mir T A, Arai K. Bioprinting with pre–cultured cellular constructs to–wards tissue engineering of hierarchical tissues. Int J Bioprint. 2015;1:39–48. [Google Scholar]
- 46.Pradhan S, Hassani I, Clary J M. Polymeric biomaterials forin vitrocancer tissue engineering and drug testing applications. Tissue Eng Part B. 2016;22:470–484. doi: 10.1089/ten.TEB.2015.0567. https://doi.org/10.1089/ten.teb.2015.0567. [DOI] [PubMed] [Google Scholar]
- 47.Arai K, Tsukamoto Y, Yoshida H. The development of cell adhesive hydrogel for 3D printing. Int J Bioprint. 2016;2:153–162. https://doi.org/10.18063/IJB.2016.02.002. [Google Scholar]
- 48.Lee V K, Kim D Y, Ngo H. Creating perfused functional vascular channels using 3D bio–printing technology. Biomaterials. 2014;35:8092–8102. doi: 10.1016/j.biomaterials.2014.05.083. https://doi.org/10.1016/j.biomaterials.2014.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuo K C, Lin R Z, Tien H W. Bioengineering vascularized tissue constructs using an injectable cell–laden enzymatically cross linked collagen hydrogel derived from dermal extracellular matrix. Acta Biomater. 2015;27:151–166. doi: 10.1016/j.actbio.2015.09.002. https://doi.org/10.1016/j.actbio.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xian X, Mary C F, Xinqiao J. Three–dimensionalin vitrotumor models for cancer research and drug evaluation. Biotechnol Adv. 2014;32:1256–1268. doi: 10.1016/j.biotechadv.2014.07.009. https://doi.org/10.1016/j.biotechadv.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sundaramurthi D, Rauf S, Hauser C. 3D bioprinting technology for regenerative medicine applications. Int J Bioprint. 2016;2(2):9–26. https://doi.org/10.18063/IJB.2016.02.010. [Google Scholar]
- 52.Sears N A, Seshadri D R, Dhavalikar P S. A review of three–dimensional printing in tissue engineering. Tissue Eng Part B. 2016;22:298–310. doi: 10.1089/ten.TEB.2015.0464. https://doi.org/10.1089/ten.teb.2015.0464. [DOI] [PubMed] [Google Scholar]
- 53.Wang X, Yan Y, Zhang R. Recent trends and challenges in complex organ manufacturing. Tissue Eng Part B. 2010;16:189–197. doi: 10.1089/ten.TEB.2009.0576. https://doi.org/10.1089/ten.teb.2009.0576. [DOI] [PubMed] [Google Scholar]
- 54.Wang S, Lee J M, Yeong W Y. 2015 Smart hydrogels for 3D bioprinting. Int J Bioprint. 1:3–14. https://doi.org/10.18063/IJB.2015.01.005. [Google Scholar]
- 55.Kang H W, Lee S J, Ko I K. A 3D bioprinting system to produce human–scale tissue constructs with structural integrity. Nat Biotechnol. 2016;34:312–319. doi: 10.1038/nbt.3413. https://doi.org/10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
- 56.Kirchmajer D M, Gorkin I R, Panhuis M. 2015 An overview of the suitability of hydrogel forming polymers for extrusion–based 3D–printing. J Mater Chem B. 3:4105–4117. doi: 10.1039/c5tb00393h. https://doi.org/10.1039/C5TB00393H. [DOI] [PubMed] [Google Scholar]
- 57.Ouyang L, Yao R, Zhao Y. 2016 Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication. 8:35020. doi: 10.1088/1758-5090/8/3/035020. https://doi.org/10.1088/1758-5090/8/3/035020. [DOI] [PubMed] [Google Scholar]
- 58.Kolesky D B, Truby RL, Gladman A S. Bioprinting:3D bioprinting of vascularized, heterogeneous cell–laden tissue constructs. Adv Mater. 2014;26:3124–3130. doi: 10.1002/adma.201305506. https://doi.org/10.1002/adma.201470124. [DOI] [PubMed] [Google Scholar]
- 59.Yu J T, Xipeng T, Wai Y Y. Hybrid microscaffold–based 3D bioprinting of multi–cellular constructs with high compressive strength:A new biofabrication strategy. Sci Rep. 2016;6:39140. doi: 10.1038/srep39140. https://doi.org/10.1038/srep39140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pescosolido L, Vermonden T, Malda J. 2011In situforming IPN hydrogels of calcium alginate and dextran–HEMA for biomedical applications. Acta Biomateria. 7:1627–1633. doi: 10.1016/j.actbio.2010.11.040. https://doi.org/10.1016/j.actbio.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 61.Shim J H, Lee J S, Kim J Y. Bioprinting of a mechanically enhanced three–dimensional dual cell–laden construct for osteochondral tissue engineering using a multi–head tissue/organ building system. J Micromech Microeng. 2012;22:85014. https://doi.org/10.1088/0960-1317/22/8/085014. [Google Scholar]
- 62.Ng W L, Yeong W Y, Naing M W. Polyelectrolyte gelatin–chitosan hydrogel optimized for 3D bioprinting in skin tissue engineering. Int J Bioprint. 2016;2:53–62. https://doi.org/10.18063/IJB.2016.01.009. [Google Scholar]
- 63.Yan Y, Wang X, Pan Y. Fabrication of viable tissue–engineered constructs with 3D cell–assembly technique. Biomaterials. 2005;26:5864–5871. doi: 10.1016/j.biomaterials.2005.02.027. https://doi.org/10.1016/j.biomaterials.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 64.Yan Y, Wang X, Xiong Z. Direct construction of a three–dimensional structure with cells and hydrogel. J Bioact Compat Polym. 2005;20:259–269. https://doi.org/10.1177/0883911505053658. [Google Scholar]
- 65.Wang X, Yan Y, Pan Y. Generation of three dimensional hepatocyte/gelatin structures with rapid prototyping system. Tissue Eng. 2006;12:83–90. doi: 10.1089/ten.2006.12.83. https://doi.org/10.1089/ten.2006.12.83. [DOI] [PubMed] [Google Scholar]
- 66.Zhang T, Yan Y, Wang X. Three–dimensional gelatin and gelatin/hyaluronan hydrogel structures for traumatic brain injury. J Bioact Compat Polym. 2007;22:19–29. https://doi.org/10.1177/0883911506074025. [Google Scholar]
- 67.Zhao X, Wang X. Preparation of an adipose–derived stem cell (ADSC)/fibrin–PLGA construct based on a rapid prototyping technique. J Bioact Compat Polym. 2013;28(3):191–203. https://doi.org/10.1177/0883911513481892. [Google Scholar]
- 68.Zhao X, Liu L, Wang J. 2014In vitrovascularization of a combined system based on a 3D printing technique. J Tissue Eng Regen Med. 10:833–842. doi: 10.1002/term.1863. https://doi.org/10.1002/term.1863. [DOI] [PubMed] [Google Scholar]
- 69.Yao R, Zhang R, Yan Y. 2009In vitroangiogenesis of 3D tissue engineered adipose tissue. J Bioact Compat Polym. 24:5–24. https://doi.org/10.1177/0883911508099367. [Google Scholar]
- 70.Xu M, Wang X, Yan Y. A cell–assembly derived physiological 3D model of the metabolic syndrome, based on adipose–derived stromal cells and a gelatin/alginate/fibrinogen matrix. Biomaterials. 2010;31(14):3868–3877. doi: 10.1016/j.biomaterials.2010.01.111. https://doi.org/10.1016/j.biomaterials.2010.01.111. [DOI] [PubMed] [Google Scholar]
- 71.Xu M, Yan Y, Liu H. Control adipose–derived stromal cells differentiation into adipose and endothelial cells in a 3–D structure established by cell–assembly technique. Adv Obstetr Gynecol. 2009;57:279–283. [Google Scholar]
- 72.Li S, Yan Y, Xiong Z. Gradient hydrogel construct based on an improved cell assembling system. J Bioact Compat Polym. 2009;24:84–99. https://doi.org/10.1177/0883911509103357. [Google Scholar]
- 73.Xu Y, Wang X. Fluid and cell behaviors along a 3D printed alginate/gelatin/fibrin channel. Biotechnol Bioeng. https://doi.org/10.1002/bit.25579. 2015;112(8):1683–1695. doi: 10.1002/bit.25579. [DOI] [PubMed] [Google Scholar]
- 74.Ghazanfari A, Li W, Leu M C. 2017 A novel freeform extrusion fabrication process for producing solid ceramic components with uniform layered radiation drying. Addit Manuf. 15:102–112. https://doi.org/10.1016/j.addma.2017.04.001. [Google Scholar]
- 75.Lee J S, Hong J M, Jung J W. 3D printing of composite tissue with complex shape applied to ear regeneration. Biofabrication. 2014;6(2):24103. doi: 10.1088/1758-5082/6/2/024103. https://doi.org/10.1088/1758-5082/6/2/024103. [DOI] [PubMed] [Google Scholar]
- 76.Duan B, Kapetanovic E, Hockaday L A. Three–dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells. Acta Biomateria. 2014;10:1836–1846. doi: 10.1016/j.actbio.2013.12.005. https://doi.org/10.1016/j.actbio.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kolesky D B, Homan K A, Skylar–Scott M A. Three–dimensional bioprinting of thick vascularized tissues. italic>Proc Nat Acad Sci. 2016;113:3179–3184. doi: 10.1073/pnas.1521342113. https://doi.org/10.1073/pnas.1521342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Campos D F, Blaeser A, Korsten A. The stiffness and structure of three–dimensional printed hydrogels direct the differentiation of mesenchymal stromal cells toward adipogenic and osteogenic lineage. italic>Tissue Eng Part A. 2014;21:740–756. doi: 10.1089/ten.TEA.2014.0231. https://doi.org/10.1089/ten.tea.2014.0231. [DOI] [PubMed] [Google Scholar]
- 79.Nakamura M, Iwanaga S, Henmi C. Biomatrices and biomaterials for future developments of bioprinting and biofabrication. Biofabrication. 2010;2:14110–14116. doi: 10.1088/1758-5082/2/1/014110. https://doi.org/10.1088/1758-5082/2/1/014110. [DOI] [PubMed] [Google Scholar]
- 80.Moroni L, Boland T, Burdick J A. Biofabrication:A guide to technology and terminology. Trends Biotechnol. 2017;36:384–402. doi: 10.1016/j.tibtech.2017.10.015. https://doi.org/10.1016/j.tibtech.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 81.Mir T A, Nakamura M. 3D–bio printing:Towards the era of manufacturing human organs as spare parts for healthcare and medicine. Tissue Eng Part B. 2017;23:245–256. doi: 10.1089/ten.TEB.2016.0398. https://doi.org/10.1089/ten.teb.2016.0398. https://doi.org/10.1042/BA20030108. [DOI] [PubMed] [Google Scholar]
- 82.Sun W, Lal P. Recent development on computer aided tissue engineering:Overview, scope and challenges. Biotechnol Appl Biochem. 2004;39:29–47. doi: 10.1042/BA20030108. https://doi.org/10.1042/BA20030108. [DOI] [PubMed] [Google Scholar]
- 83.Sanjairaj V. 3D bioprinting of skin:A state–of–the–art review on modeling, materials, and processes. Biofabrication. 2016;8(3):32001. doi: 10.1088/1758-5090/8/3/032001. https://doi.org/10.1088/1758-5090/8/3/032001. [DOI] [PubMed] [Google Scholar]
- 84.Whitford W, Hoying J B. Digital biomanufacturing supporting vascularization in 3D bioprinting. Int J Bioprint. 2017;3:18–26. doi: 10.18063/IJB.2017.01.002. https://doi.org/10.18063/IJB.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brown F, Hahn M. Informatics technologies in an evolving R and D landscape. Bioprocess Int. 2012;10:64–69. [Google Scholar]
- 86.Hiller J, Lipson H. Design and analysis of digital materials for physical 3D voxel printing. Rapid Prototyp J. 2009;15:137–149. https://doi.org/10.1108/13552540910943441. [Google Scholar]
- 87.da Silva J V, Martins T A, Noritomi P Y. Scaffold informatics and biomimetic design:Three–dimensional medical reconstruction. Methods Mol Biol. 2012;868:91–109. doi: 10.1007/978-1-61779-764-4_6. https://doi.org/10.1007/978-1-61779-764-4_6. [DOI] [PubMed] [Google Scholar]
- 88.Fan H, Scott C. From chips to CHO cells:IT advances in upstream bioprocessing. Bioprocess Int. 2015;13:14–29. [Google Scholar]
- 89.John G T. Using optical sensors for bioprocess monitoring:A measurement technique for bioprocessors. Bioprocess Int. 2016;14(3):S45–S48. [Google Scholar]
- 90.Schmitt S. Information instead of data:User–friendly HMI concept increases process control efficiency. Bioprocess Int. 2015;13:42–46. [Google Scholar]
- 91.Moore C. Harnessing the power of big data to improve drug R and D. Bioprocess Int. 2016;14(2016):64. [Google Scholar]
- 92.Unadkat H V, Hulsman M, Cornelissen K. An algorithm–based topographical biomaterials library to instruct cell fate. Proc Nat Acad Sci. 2011;108:16565–16570. doi: 10.1073/pnas.1109861108. https://doi.org/10.1073/pnas.1109↣08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dalby M J, Gadegaard N, Oreffo R O. Harnessing nanotopography and integrin–matrix interactions to influence stem cell fate. Nat Mater. 2014;13:558. doi: 10.1038/nmat3980. https://doi.org/10.1038/nmat3980. [DOI] [PubMed] [Google Scholar]
- 94.Guyot Y, Papantoniou I, Chai YC. A computational model for cell/ECM growth on 3D surfaces using the level set method:a bone tissue engineering case study. Biomech Model Mechanobiol. 2014;13:1361–1371. doi: 10.1007/s10237-014-0577-5. https://doi.org/10.1007/s10237-014-0577-5. [DOI] [PubMed] [Google Scholar]
- 95.Papantoniou I, Sonnaert M, Geris L. Three–dimensional characterization of tissue engineered constructs by contrast–enhanced nanofocus computed tomography. Tissue Eng Part C Methods. 2014;20:177–187. doi: 10.1089/ten.tec.2013.0041. https://doi.org/10.1089/ten.tec.2013.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guyot Y, Luyten F P, Schrooten J. A three–dimensional computational fluid dynamics model of shear stress distribution during neotissue growth in a perfusion bioreactor. Biotechnol Bioeng. 2015;112:2591–2600. doi: 10.1002/bit.25672. https://doi.org/10.1002/bit.25672. [DOI] [PubMed] [Google Scholar]
- 97.Maes F, Claessens T, Moesen M. Computational models for wall shear stress estimation in scaffolds:A comparative study of two complete geometries. J Biomech. 2012;45:1586–1592. doi: 10.1016/j.jbiomech.2012.04.015. https://doi.org/10.1016/j.jbiomech.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 98.Lesman A, Blinder Y, Levenberg S. Modeling of flow–induced shear stress applied on 3D cellular scaffolds:Implications for vascular tissue engineering. Biotechnol Bioeng. 2010;105:645–654. doi: 10.1002/bit.22555. https://doi.org/10.1002/bit.22555. [DOI] [PubMed] [Google Scholar]
- 99.Shakhawath H, Bergstrosm D J, Chen X B. Modelling and simulation of the chondrocyte cell growth, glucose consumption and lactate production within a porous tissue scaffold inside a perfusion bioreactor. Biotechnol Rep. 2015;5:55–62. doi: 10.1016/j.btre.2014.12.002. https://doi.org/10.1016/j.btre.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guyot Y, Papantoniou I, Luyten F P. Coupling curvature dependent and shear stress–stimulated neotissue growth in dynamic bioreactor cultures:A 3D computational model of a complete scaffold. Biomech Model Mechanobiol. 2016;15:169–180. doi: 10.1007/s10237-015-0753-2. https://doi.org/10.1007/s10237-015-0753-2. [DOI] [PubMed] [Google Scholar]
- 101.Kadlec P, Gabrys B, Strandt S. Data–driven soft sensors in the process industry. Comput Chem Eng. 2009;33:795–814. https://doi.org/10.1016/j.compchemeng.2008.12.012. [Google Scholar]
- 102.de Assis A J, Filho R M. Soft sensors development for on–line bioreactor state estimation. Comput Chem Eng. 2000;24:1099–1103. https://doi.org/10.1016/S0098-1354(00)00489-0. [Google Scholar]
- 103.Viazzi S, Lambrechts T, Papantoniou I. Real–time characterization of harvesting process for adherent cell culture based on on–line imaging and model–based monitoring. Biosyst Eng. 2015;138:104–113. https://doi.org/10.1016/j.biosystemseng.2015.06.006. [Google Scholar]
- 104.Lambrechts T, Papantoniou I, Sonnaert M. Model–based cell number quantification using online single oxygen sensor data for tissue engineering perfusion bioreactors. Biotechnol Bioeng. 2014;111:1982–1992. doi: 10.1002/bit.25274. https://doi.org/10.1002/bit.25274. [DOI] [PubMed] [Google Scholar]
- 105.Hebels D, Carlier A, Coonen M. cBiT:A transcriptomics database for innovative biomaterial engineering. Biomaterials. 2017;149:88–97. doi: 10.1016/j.biomaterials.2017.10.008. https://doi.org/10.1016/j.siny.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 106.Gittenberger–de G A, Bartelings M M, Poelmann R E. Embryology of the heart and its impact on understanding fetal and neonatal heart disease. Semin Fetal Neonatal Med. 2013;18:237–244. doi: 10.1016/j.siny.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 107.Baldwin D E. Heart development. Encyclopedia Cardiovasc Res Med. 20182018:380–398. [Google Scholar]
- 108.Kloesel B, DiNardo J A, Body S C. Cardiac embryology and molecular mechanisms of congenital heart disease –A primer for anesthesiologists. Anesth Analg. 2016;123:551–569. doi: 10.1213/ANE.0000000000001451. https://doi.org/10.1213/ANE.0000000000001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Heart Embryology Video. Available from: https://www.youtube.com/watch?v=5DIUk9IXUaI .
- 110.Michał S, Monika P A, Alina W. Three–dimensional growth dynamics of the liver in the human fetus. Surg Radiol Anat. 2015;37:439–448. doi: 10.1007/s00276-015-1437-4. https://doi.org/10.1007/s00276-015-1437-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Barrya J S, Anthony R V. The pregnant sheep as a model for human pregnancy. Theriogenology. 2008;69:55–67. doi: 10.1016/j.theriogenology.2007.09.021. https://doi.org/10.1016/j.theriogenology.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Toshihiro K, Akiteru M, Toshihiko S. Development and growth of organs in living whole embryo and larval grafts in zebrafish. Sci Rep. 2017;7:16508. doi: 10.1038/s41598-017-16642-5. https://doi.org/10.1038/s41598-017-16642-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gideon H, Julian N, Oded M. Venous–derived angioblasts generate organ–specific vessels during zebrafish embryonic development. Development. 2015;142:4266–4278. doi: 10.1242/dev.129247. https://doi.org/10.1242/dev.129247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wenyao Z, Xue Z L, Tong X. Inflammatory responses of stromal fibroblasts to inflammatory epithelial cells are involved in the pathogenesis of bovine mastitis. Exp Cell Res. 2016;349:45–52. doi: 10.1016/j.yexcr.2016.09.016. https://doi.org/10.1016/j.yexcr.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 115.Sophie V L, Kapka M, Carsten T. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc Res. 2014;102:258–269. doi: 10.1093/cvr/cvu062. https://doi.org/10.1093/cvr/cvu062. [DOI] [PubMed] [Google Scholar]
- 116.Justin H, Douglas L. Role of inflammatory cells in fibroblast activation. J Mol Cell Cardiol. 2016;93:143–148. doi: 10.1016/j.yjmcc.2015.11.016. https://doi.org/10.1016/j.yjmcc.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


