Abstract
Optical tissue phantoms enable to mimic the optical properties of biological tissues for biomedical device calibration, new equipment validation, and clinical training for the detection, and treatment of diseases. Unfortunately, current methods for their development present some problems, such as a lack of repeatability in their optical properties. Where the use of three-dimensional (3D) printing or 3D bioprinting could address these issues. This paper aims to evaluate the use of this technology in the development of optical tissue phantoms. A competitive technology intelligence methodology was applied by analyzing Scopus, Web of Science, and patents from January 1, 2000, to July 31, 2018. The main trends regarding methods, materials, and uses, as well as predominant countries, institutions, and journals, were determined. The results revealed that, while 3D printing is already employed (in total, 108 scientific papers and 18 patent families were identified), 3D bioprinting is not yet applied for optical tissue phantoms. Nevertheless, it is expected to have significant growth. This research gives biomedical scientists a new window of opportunity for exploring the use of 3D bioprinting in a new area that may support testing of new equipment and development of techniques for the diagnosis and treatment of diseases.
Keywords: competitive technology intelligence, additive manufacturing, biophotonics, scientometrics, patentometrics
1. Introduction
Three-dimensional (3D) printing, also known as additive manufacturing[1], was introduced >30 years ago, in 1984, when Charles Hull invented 3D lithography[2]. 3D printing is defined by the American Society for Testing and Materials (ASTM) as a “process of joining materials to make parts from 3D model data, usually layer upon layer, as opposed to subtractive manufacturing and formative manufacturing methodologies”[3]. The process starts with the creation of a 3D model on a computer, after which, it is sent to a 3D printer[4].
The competitive dynamics of 3D printing may alter the modus operandi of multiple industries, and its effects will be observed in the nature of the design of new products, placing special emphasis on how they will be conceived, manufactured, tested, and applied in many areas, such as the military, automotive industry, electrical device engineering, and medicine[5]. One of the current technologies employing 3D printing for health care is biofabrication. This term refers to a relatively new process that focuses on the concept of personalized medicine. According to Jürgen et al.[6], it can be defined as “the automated generation of biologically functional products with the structural organization from living cells, bioactive molecules, biomaterials, and cell aggregates such as microtissues or hybrid cell-material constructs, through bioprinting, and subsequent tissue maturation processes.” Under this approach, according to Mandrycky et al.[7], 3D bioprinting is considered a “3D fabrication technology used to precisely dispense cell-laden biomaterials for the construction of complex 3D functional living tissues or artificial organs,” using an additive manufacturing strategy by depositing substrates such as living cells, nucleic acids, drug particles, proteins, and other biological components[8].
Recently, 3D bioprinting has gained significant attention[9], having wide utility in various areas of medicine, it enables the fabrication of living tissue with precise digital control[10]. In addition, the interest of academia and industry in 3D bioprinting is growing[11]. The applications of 3D bioprinting have spread from the development of tissue models for research, drug discovery, and toxicology[12] to the possibility of developing functional tissues and organs for transplantation[4]. Specifically, bioprinted models give a better understanding of physiological processes, including the mechanisms that can produce diseases, as well as those that are part of the prevention, detection, and treatment of those illnesses[13-16]. Some future challenges for 3D bioprinting are related to technical factors, such as improving its resolution and printing speed, in addition to increasing the number of available materials[12] to better mimic the biological, structural, mechanical, and even optical characteristics of organs and biological tissues[17].
Biophotonics concerns the study of the interaction of visible light and biological systems[18]. It is commonly used in medicine to study biological tissue to detect, diagnose, and treat diseases in a minimally invasive or non-invasive way[19,20]. Biophotonics techniques have been shown to be less harmful than are other biomedical techniques that use ionizing radiation, such as X-rays[21]. The light-biological tissue interaction is mediated through the optical characteristics of the biological medium. These characteristics are related to how the light travels and propagates through the biological medium (refractive index and scattering coefficient) and how it is absorbed by the medium (absorption coefficient), factors that determine the biological tissues’ unique behavior in response to light. To mimic these properties, objects known as optical tissue phantoms have been used in biophotonics research and development (R&D)[22]. A typical phantom consists of a base material, scattering, and absorber materials, and sometimes contrast enhancement agents, such as fluorophores[23,24]. Optical tissue phantoms have been used as calibrators aiming to establish global standards for the measurement of biomedical techniques like imaging[24]. Moreover, they have been utilized for the development of new techniques and prototypes for the diagnosis and treatment of diseases; furthermore, they are suitable for clinical trials, as well as for the improvement, and routine quality control of prototypes [14-16,23,24,26]. Also, it is important to implement standardized protocols for the development of phantoms because they must present minimum variation over time in their properties[27], as any change in the phantom could be interpreted as an alteration in the performance[25].
Most phantoms that have been used in laboratories are based on materials that do not entirely reflect the optical properties, heterogeneities, or complex, multilayered structures of biological tissues[27,28]. In addition, the methodologies that have been proposed for their development generally consume a great amount of time, and the optical properties of the produced phantoms present significant variations[28]. In general terms, phantoms have been developed in a single-layer way, with homogeneous optical properties that do not entirely reflect the complex behavior of biological tissue. Researchers’ goal is to build multilayered phantoms with heterogeneities that better mimic the structure of biological tissues[22]. The most commonly used methods of producing multilayer and heterogeneous phantoms are mold multilayer curing[30], integration after mold casting, and spin coating[31].
Not only the methodologies used to create optical tissue phantoms are important but also the materials play a key role. Phantom matrices represent >95% of the total volume of the phantom, and consequently, they have the most significant effect on its applications. Liquid, gelatinous, or solid substances can be used as phantom matrices[22,32]. These materials are selected according to their properties, including their stability over time[22]. Gelatinous materials have been shown to be the most viable option for the development of phantoms because they have thermal and mechanical properties that closely match those of biological tissue[26,29] and their lifetime is longer than that of phantoms composed with liquid substances[22]. They also allow the integration of a wide variety of substances to simulate both optical and biochemical properties [22,26,33], making gelatinous materials ideal for biophysical studies and generating complex structures. Gelatins and agarose are commonly used materials that have been adopted in many laboratories since the mid-1990s[22]. Thereafter, methods for the elaboration of phantoms have usually been carried out by hand.
Further technological advances have occurred, especially in the development of hydrogels, which began to be studied in the late 1990s for their use as phantom matrices[26]. Hydrogels are biocompatible materials; they resemble tissue extracellular matrix[34] and replicate some physicochemical properties of biological tissues[35], and they can be used for drug delivery. This application is extremely attractive for enhancing phototherapies, such as photodynamic therapy[36-38]. These phototherapies require the application of a substance known as a photosensitizer before irradiation with light for treating various diseases[39]. Hydrogels can carry diverse types of photosensitizers to the target cells more effectively than the methods that are commonly used at present can[36]. Among the hydrogels, polyvinyl alcohol (PVA) gel shows the best performance since its optical scattering properties can be easily modified by adding microspheres or nanoparticles or through freeze-thaw cycles [33,40,41], as well as for its ease in mimicking heterogeneities that can represent illness or a damaged tissue[26,40].
Recently, 3D printing and 3D bioprinting have been proposed as methods for the generation of tissue phantoms[42,43]. In comparison with conventional manufacturing processes, these technologies have multiple advantages, such as a short production cycle and freeform fabrication of objects with complex geometric characteristics and internal structures. Moreover, 3D bioprinting has been used for freeform fabrication of non-homogeneous, multilayered complex structures, such as skin tissue[43]. It represents a very attractive solution to biomedicine since it allows the creation of layered structures that are similar to biological tissues[14]. These features make it suitable for fabricating optical tissue phantoms for clinical applications. A window of opportunity can be opened in biophotonics where 3D bioprinting has not been adopted as in other fields[44]. Here, 3D bioprinting would allow the development of more realistic phantoms.
This paper presents a competitive technology intelligence study of the presence of 3D printing and 3D bioprinting to develop optical tissue phantoms, using a methodology created by Rodríguez-Salvador et al.[11], for identifying the scientific and technological trends regarding methods, materials, and uses, as well as determining the most prolific countries, organizations, and journals in this domain.
2. Methodology
Systematic research on the state-of-the-art and determination of trends and time horizons of 3D printing and 3D bioprinting applied to the manufacturing of optical tissue phantoms were carried out, based on the approach of Rodríguez-Salvador et al.[11]. It consists of iterative processing of information that includes a planning stage, source determination, data gathering, and analysis; every step was supported by expert feedback, as explained below.
2.1 Planning Stage
It consists of an organization process to define the objectives, participants, timing, and specific activities of the study. In this case, the objective was to identify the presence of 3D printing or 3D bioprinting to produce optical tissue phantoms for biophotonics; determining methods, materials, and uses, as well as the most prolific countries, organizations, and journals in this field.
2.2 Data Source Determination
It starts with the identification of primary and secondary sources. The first one is based on the opinion of experts in the area; in this case, it involves the development of optical tissue phantoms and 3D printing or bioprinting while secondary sources include scientific and technological documents. This research involved scientific papers, conference proceedings, and patents. Web of Science (WoS) and Scopus were analyzed using scientometric tools. Patents were examined using a patentometric platform, PatSeer. This software was designed for research, analysis, and project management and allows access to nearly 120 million records from the main patent offices worldwide[45].
2.3 Data Gathering
This phase begins with the determination of a search strategy, first by identifying the appropriate terms and keywords, which will ensure that the most relevant information is collected. In this study, the keywords were identified from a state-of-the-art review and with the support of experts in the field. It is important to note that the main query terminology was obtained from the research study of Rodríguez-Salvador et al.[11], where the principal technologies and applications to the health sector of 3D bioprinting were researched; for completing the query, experts were also consulted. Data collection in this study focused on the terms that are applied to the use of 3D printing for developing optical tissue phantoms. After the literature review and expert validation, three main categories were identified, as follows: (i) Terms referring to 3D printing and 3D bioprinting; (ii) those exclusively concerning optical tissue phantoms; and (iii) those involving the applications of biophotonics, which, according to the experts consulted, should be subdivided into diagnosis and phototherapy. The obtained terms were utilized for the development of different search queries, adapted according to the consulted databases. Boolean operators and exclusion terms were used. In addition, the study considered a time interval from 2000 (since 3D printing applications in health care emerged by that time[46,47]) to 2018 (specifically, until July 31, 2018, as the collection activity ended on that date).
2.4 Analysis
In this research, this step involves the use of scientometric and patentometric tools. First, a manual examination to rule out duplicated and non-relevant documents were carried out. Then, grouping information and a statistical analysis were performed. Expert feedback during this process was essential. Scientometric and patentometric analyses were developed using PatSeer platform and special software programmed in R language, to mine data from the papers that were found.
Using this iterative process and adapting the search query from Rodríguez-Salvador et al.[11], changing and adding suitable keywords, and using Boolean operators, We design the following query: ((((3d) OR (3d)) OR ((three dimensional) OR (threedimensional))) AND ((*print* OR manufactur* OR fabricat*) OR (rapid prototyp*) OR ((layer by layer) OR (layerbylayer))) AND ((optic*) AND (mimic* OR bio* OR simulat* OR tissue) AND (phantom*)) AND (((tissue OR bio* OR diagnos*) AND (diffus* OR reflectance OR fluorescence OR imaging) AND spectroscopy) OR ((phototherapy) OR (photodynamic AND (therapy OR treatment))))) AND NOT (surgic* OR nuclear OR ultrasound OR radiotherapy OR (xray)). The global query was adapted according to each of the databases consulted.
3 Results and Discussion
3.1 Scientometric Analysis
After a detailed examination to rule out non-relevant documents, 81 papers were found in the Scopus database and 58 papers in the WoS database from January 1, 2000, to July 31, 2018. The previously mentioned designed query was adapted for each database. Subsequently, a de-duplication process was performed to find any possible repeated documents between the two databases. In the end, a total of 108 documents were identified.
A research relating to a creation of a 3D optical tissue phantom to analyze epithelial cancer was also found. While it does not entail the formal definition of 3D bioprinting[4,6,7], it represents an interesting effort to build biologically functional 3D structures, from the early 2000 s. This paper was authored by Sokolov et al.[48], and its process involves cervical cells embedded in a collagen matrix where blood cells are added later. Furthermore, a layer of epithelial cells is placed on top of the phantom to completely simulate the cervical tissue, even in different stages of cancer.
The results obtained showed the early incursion of 3D printing into developing optical tissue phantoms; however, the presence of 3D bioprinting to create these phantoms was not detected. Tables 1-3 present the most representative articles that were found, ordered by techniques or methods, materials, and applications.
Table 1.
Methods used for 3D printed optical tissue phantoms
| Paper | Institution/country | Description |
|---|---|---|
| Wang et al.[13] “3D printing method for freeform fabrication of optical phantoms simulating heterogeneous biological tissue” |
Center for Biomedical Engineering, University of science and technology of China/China | A 3D printing method was developed for the fabrication of tissue-simulating phantoms with a multilayer structure that consists in selectively depositing the phantom materials layer by layer using spin coating. The goal was to develop a skin tissue phantom as a standard for testing biomedical optical devices |
| Lurie et al.[49] “Three-dimensional, distendable bladder phantom for optical coherence tomography and white light cystoscopy” |
Department of Electrical Engineering, Stanford University/United States | A new spin coating protocol was developed to mitigate the nonuniformity of 3D model topology. The 3D printed phantom mimics the size, structure, microscale surface topology, and optical properties of a cancerous bladder for performing optical coherence tomography tests |
3D: Three-dimensional
Table 3.
Uses of 3D printed optical tissue phantoms
| Paper | Institution/country | Description |
|---|---|---|
| Ghassemi et al.[52] “Rapid prototyping of biomimetic vascular phantoms for hyperspectral reflectance imaging” | FDA/United States | The 3D printing of a human retinal vasculature phantom filled with hemoglobin solution was developed. The purpose was applying the phantom in tests of a near-infrared hyperspectral reflectance imaging system for the analysis of the human retina |
| Bentz et al.[53] “3D printed optical phantoms and deep tissue imaging for in vivo applications including oral surgery” | School of Electrical and Computer Engineering, Purdue University/United States | In the study, 3D printed mouse phantoms were created for use in deep fluorescence imaging tests. These phantoms represent an alternative to animal experimentation in these types of tests |
| Lv et al.[54] “Design of a portable phantom device to simulate tissue oxygenation and blood perfusion” | School of Engineering Science, University of Science and Technology of China/China | An optical tissue phantom was fabricated using 3D printing to mimic blood vessels. The phantom was used to calibrate and validate medical optical devices |
FDA: Food and drug administration, 3D: Three-dimensional
Table 2.
Materials used for 3D printed optical tissue phantoms
| Paper | Institution/country | Description |
|---|---|---|
| Zhao et al.[16] “3D printing of tissue-simulating phantoms for calibration of biomedical optical devices” | Department of Precision Machinery and Precision Instrumentation, University of Science and Technology of China/China | An optical tissue phantom with mechanical and optical heterogeneities was created using 3D printing. The process uses gel wax polydimethylsiloxane and colorless light-curable ink as matrix materials, titanium dioxide (TiO2) powder as the scatterer, and graphite powder and black carbon as absorbers |
| Kim et al.[50] “3D printing-assisted fabrication of double-layered optical tissue phantoms for laser tattoo treatments” | Electrical and Mechanical Engineering, Pukyong National University/Korea | A double-layered phantom made of gelatin and agar as matrix materials and a mixture of TiO2 powder as the scatterer and coffee as the absorber was developed. Then, 3D printing for precise control of the thickness of each layer was used |
| Sangha et al.[51] “Adjustable photoacoustic tomography probe improves light delivery and image quality” | Weldon School of Biomedical Engineering, Purdue University/United States | A depth-profiling 3D-printed phantom was created using PVA and polyethylene tubes. The PVA was treated with a freeze-thaw cycle to modify its optical scattering properties. This phantom can be used for acoustic and optical analysis |
PVA: Polyvinyl alcohol, 3D: Three-dimensional
The global results of the scientometric analysis are presented in Figure 1A-1D.
Figure 1.
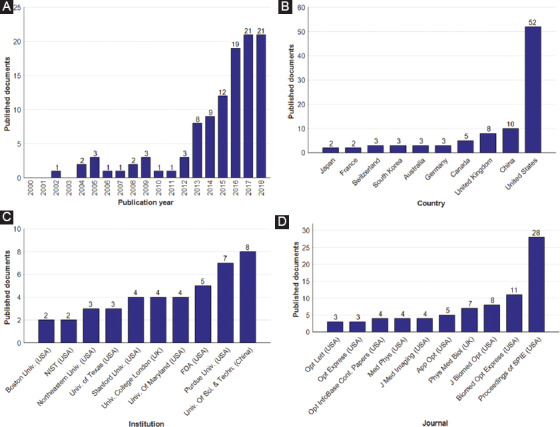
Summary of published papers on 3D printed optical tissue phantoms, which are indexed in both Scopus and Web of Science databases, grouped by (A) publication year, (B) affiliation country, (C) institution of principal author, and (D) journal in which the paper is published. In B-D, only the 10 most frequent papers are shown. Note: National Institute of Standards and Technology (NIST), Food and Drug Administration (FDA).
Curve fitting allowed us to find the behavior and trends of the number of publications per year on 3D printed optical tissue phantoms. An exponential regression was performed excluding the data from the year 2018. The obtained equation that describes the data growth is shown in Equation 1, with a value of the coefficient of determination, R2 = 0.9527:
y =(2.097×10-268)e0.3071x
If the growth rate continues with the same behavior, then the total number of papers will be 29 for 2018, and 54 will be published in 2020. As shown in Figure 1A, the number of articles referring to the fabrication of optical tissue phantoms using 3D printing technology has increased since 2013.
Insights exhibit that the United States (52 documents) is the top country for publications on 3D printed optical tissue phantoms, representing 48.15% of all publications, followed by China (10 documents) and the United Kingdom (8 documents). As shown in Figure 1C, while eight of the leading institutions in terms of publications in this field come from the United States, the institution with the highest number of papers in the area is the University of Science and Technology of China (8 documents), followed by Purdue University of the United States (7 documents). It is interesting to note that, of the top 10 publishing institutions, two belong to the United States government, namely the Food and Drug Administration (FDA), which is responsible for the control and supervision of products related to food and health[55], and the National Institute of Standards and Technology, which “promotes innovation and competitiveness by advancing measurement science, standards, and technology”[56]. Since optical tissue phantoms are intended to become standards for the measurement, calibration, and approval of biomedical devices, both institutions are interested in carrying out research in the area. Finally, the results reveal that most publications, 25.93% of all documents, were issued in the Proceedings of the SPIE, a conference record of the Society of Photo-Optical Instrumentation Engineers. It is also important to note that, of the 108 documents found, 35 are conference proceedings and 73 are journal papers.
An association map among authors, keywords, and journals contained in the documents analyzed was also developed. For this task, a software programmed in R language was created. The map is shown in Figure 2.
Figure 2.
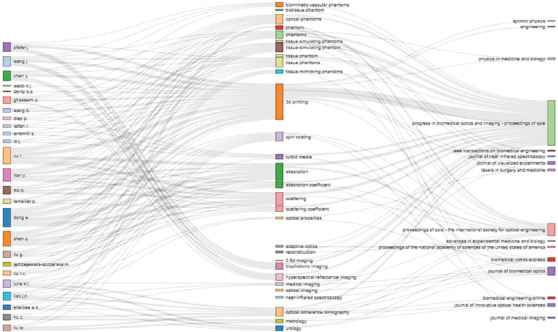
Association map showing the top authors, keywords, and journals or conference proceedings. Keywords are grouped relating to phantoms, methods, optical properties, and applications.
Each link between authors, keywords, and journals is represented by a single line. When the number of interactions increases, the lines become thicker. The frequency of each author, keyword, and journal title is also shown, represented by the height of the rectangle placed next to each word. It is observed that the top author is Erbao Dong from the University of Science and Technology of China, with 6 papers published, followed by Shuwei Shen from the same university, Jianting Wang from the FDA (USA) and Brian Z. Bentz from School of Electrical and Computer Engineering of Purdue University (USA) who published 5 papers each. Keywords comprised four categories. The first one refers to phantoms proper, where the following terminology was detected: Phantoms, biomimetic tissue phantoms, tissue phantoms, optical phantoms, tissue-simulating phantoms, tissue-mimicking phantoms, and a special type of phantom, the biomimetic vascular phantom . The second category covers methods applied to produce optical tissue phantoms; in this case, the results show that the spin coating is the most frequent process. The third classification focuses on determining optical properties, illustrating that the coefficients related to absorption and scattering and turbid media (a term that describes a material with strong optical scattering and absorption characteristics like the biological tissues[57]) predominate. The fourth group covers keywords related to applications of 3D printed optical tissue phantoms. In this regard, it can be observed that phantoms are mainly used in techniques for detecting illnesses, such as biophotonic imaging, hyperspectral reflectance imaging, near-infrared spectroscopy, and optical coherence tomography. In terms of journals, the map shows that the highest number of publications on this topic comes from the proceedings of the SPIE.
Specifically, the use of 3D bioprinting for optical tissue phantoms development was not detected in the documents obtained. Conversely, the use of 3D bioprinting to produce phantoms to be applied in other areas (not for optical applications) is present, especially for ultrasound imaging[58-63]. Since 2010, these phantoms have been referred to as “biophantoms,” and they have begun to be commonly used for ultrasound research[58].
Conventionally, phantoms have been made by hand using materials that can mimic optical properties, such as intralipid, TiO2, inks, and resins, among other materials[22], instead of using living cells. Unique advantages of using 3D printing for the development of optical tissue phantoms were detected in this research, including easy production, complex multilayer fabrication, ability to add substances to mimic heterogeneities of biological tissues, low cost, and lifecycle environmental friendliness, including reusability of phantoms’ parts and materials, and avoiding toxic resources.
3.2 Patentometric Analysis
A total of 34 patients were obtained in the search period established from January 1, 2000, to July 31, 2018. The query presented in previous sections was applied and adapted to be used on PatSeer software. After reviewing each patent in detail and carrying out a de-duplication process, the total number of documents was reduced to 23. Finally, the patents were grouped by family, resulting in only 18 patent families. As in scientometric analysis, there are no documents related to using 3D bioprinting to produce optical tissue phantoms, but there are for 3D printing. Figure 3 shows the number of patent families published during the time interval analyzed and their priority countries.
Figure 3.
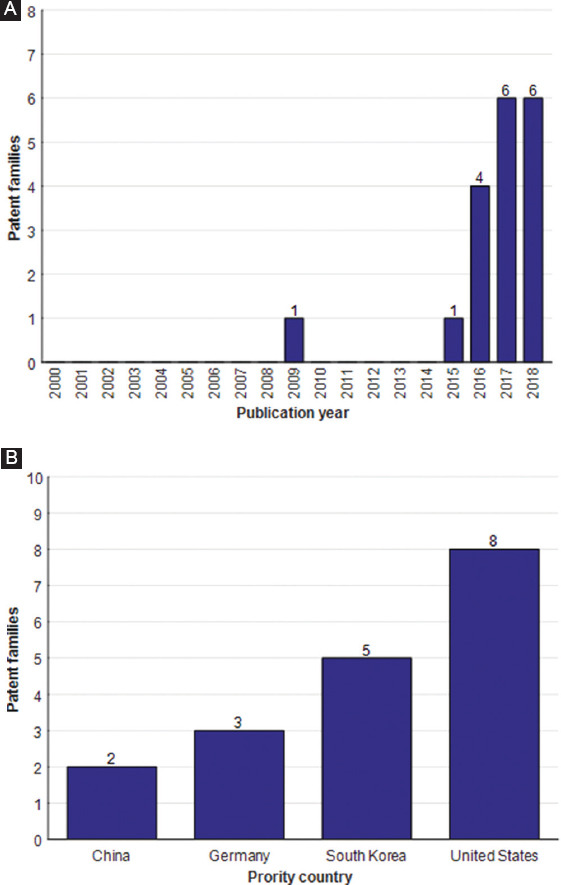
Summary of published patents on 3D printed optical tissue phantoms from January 1, 2000, to July 31, 2018, grouped by (A) the number of patent families published and (B) priority countries.
From the documents analyzed, it was found that the early patent involving the use of 3D printing to create optical tissue phantoms was published in 2009. Following this, there is a time gap until the publication of a patent in 2015. Four patients were published in 2016, and six patients each were published in 2017, and the first half of 2018. Although there is a growing trend in the number of patents, there are not enough data for estimating a mathematical function that describes the growth behavior. The main reason for the small number of patents could be that 3D printing technology is not yet fully developed for optical tissue phantoms and it takes longer to publish a patent than it does to put out a scientific paper.
Similarly to the scientometric analysis, the most prolific country in the area is the United States, having eight published patent families, followed by South Korea with five, Germany with three, and finally, China with two. In terms of assignees, the Korea Photonics Technological Institute and Pukyong National University (both in South Korea) have the highest number of patent families; they coauthored three patents, followed by Siemens (Germany) with two patents. Table 4 shows the patent families’ assignees ordered by country position.
Table 4.
Patent assignees by country. The number of patent families for each assignee is in parentheses
| Country | Assignee |
|---|---|
| United states | Board of Trustees of Leland Stanford (1) Erica Burgett, Rebecca Howell (1) Georgia Tech Research Institution (1) Mayo Foundation (1) Purdue University Research Foundation (1) Siemens Corp. U.S. (1) University of Massachusetts (1) University of Indiana Res. Tech. Corp. (1) |
| South Korea | Korea Photonic Technological Institute (3) Pukyong National University (3) Samsung Life Public Welfare Foundation (1) University of Ulsan (1) |
| Germany | Max Planck Gesellschaft (1) Siemens AG (2) |
| China | Shenzhen Institute of Advanced Tech. (1) Taishan Medical University (1) |
Finally, only three institutions were detected that had published scientific papers and patents on 3D printed optical tissue phantoms, which are the following: Purdue University (seven scientific papers and one family patent published) and Georgia Institute of Technology and Pukyong National University (one scientific paper and one family patent each).
3.3 Global Trends in 3D Printed Optical Tissue Phantoms
The main focus on the development of optical tissue phantoms through 3D printing was determined after a detailed analysis of the scientific papers and patent families. The results are categorized in Table 5 according to methods, materials, and uses.
Table 5.
Global trends for optical tissue phantoms development using 3D printing
| Category | Trend |
|---|---|
| Methods | Spin coating process predominates because this technique allows thin layers to be generated (30–60 μm in thickness), which better mimic the layer structure of biological tissues |
| Materials | Polydopamine, polydimethylsiloxane, and the hydrogel, PVA, are present to elaborate matrix tissue phantoms. To mimic scattering properties, TiO2 powders, intralipids, and PVA (involving a freeze-thaw treatment) are used. Finally, those applied to mimic absorption properties include India ink, dyes, graphite powder, hemoglobin solutions, and coffee |
| Uses | Applications are divided into the following: Diagnosis techniques, specifically: (1) Medical imaging procedures: confocal microscopy, optical coherence tomography, optical diffusion tomography, hyperspectral reflectance imaging, fluorescence imaging and photoacoustic; and (2) spectroscopy (diffuse reflectance and near-infrared spectroscopy); and Phototherapy techniques, especially in tests for tattoo removal using laser |
PVA: Polyvinyl alcohol, 3D: Three-dimensional
As can be observed in Table 5, the most used method for developing optical tissue phantoms by 3D printing is spin coating, mainly because this process enables the creation of multilayered structures with micron-thick layers, such as biological tissues[49]. In contrast, the predominant materials for the matrix of 3D printed optical tissue phantoms include both polymers and hydrogels since they facilitate the addition of other substances to improve the optical properties of the phantom[31] and can be used easily for 3D printing[16,51]. Materials of non-biological origin (TiO2, India ink, dyes, graphite powder, and PVA) are preferred over biological materials (such as intralipid and hemoglobin solutions). Finally, two categories of phantom applications were identified, namely diagnosis and phototherapy, with the first predominating; the dearth of research on optical tissue phantoms for phototherapy may be because phantoms do not yet possess thermal properties and dynamics like blood flow in tissue, which is crucial for phototherapies[64]. However, efforts are being made to develop phantoms that include vascular tissue filled with blood or hemoglobin solutions to mimic the tissue oxygenation and blood perfusion[31,54,65].
4 Conclusions
In this study, scientometric and patentometric analyses were carried out to identify trends in the use of 3D printing or 3D bioprinting to develop optical tissue phantoms, focusing on the following key elements: Methods, materials, and uses, as well as predominant countries, institutions, and journals. The results revealed that 3D printing is already used for the development of optical tissue phantoms, where the spin coating is the most frequently employed method. Materials such as polymers and hydrogels prevail as the phantom matrix; meanwhile, to mimic optical properties, the use of synthetic materials such as TiO2, India ink, or dyes outweighs that of biological materials (intralipids, hemoglobin, etc.). Finally, it was identified that 3D printed optical tissue phantoms are mainly focused on diagnostic purposes rather than phototherapy.
The insights obtained in this study illustrate that the more active countries in R&D on optical tissue phantoms using 3D printing are the United States and China. The main institutions that publish scientific papers in this area are located in the United States; meanwhile, in terms of patents, the leading institutions come from South Korea. Only three institutions were detected that published both scientific papers and patents in the field - Purdue University and Georgia Institute of Technology, both in the United States and Pukyong National University in South Korea. Specifically, a quarter of the total scientific papers identified was published in the proceedings of the SPIE.
Regarding limitations, this research was restricted by a lack of information, due to the novelty of applying 3D printing in the development of optical tissue phantoms. This issue led to obtaining few scientific papers and especially patents, and it was not possible to identify a clear trend of the behavior of patents published in this field. In terms of 3D bioprinting, no documents using this technique to produce optical tissue phantoms were detected, which limited the study to analyzing publications on 3D-printed phantoms.
Since the applications of both 3D printing and 3D bioprinting are growing, future analysis will be based on a higher number of publications, especially for the latter. Indeed, 3D bioprinting is already utilized to produce phantoms, but in other areas, such as ultrasound imaging, where they are known as “biophantoms”[63]. Ultimately, 3D printing technology has a disruptive potential for the development of optical tissue phantoms; this technology has distinct advantages over traditional methods, such as the development of complex multilayered structures, easy production, and low cost. Unique biophotonic prototypes can be built using 3D bioprinting, thereby facilitating diagnosis tests, equipment validation, and so on[66]. The 3D bioprinting domain is still in development, and its uses and applications in many areas have not been completely studied, so this research reveals a new window of opportunity to explore.
Conflicts of Interest
No conflicts of interest are reported by the authors.
References
- 1.ASTM F2792-10 2010. Standard Terminology for Additive Manufacturing Technologies. West Conshohocken, PA: ASTM International; https://www.astm.org. [Google Scholar]
- 2.Hull C W. 1984 Apparatus for Production of Three-Dimensional Objects by Stereolithography. Patent Number US4575330A. UVP Inc [Google Scholar]
- 3.Ventola C L. 2014, Medical applications for 3D printing:Current and projected uses. Pharm Ther. 39(10):704–711. [PMC free article] [PubMed] [Google Scholar]
- 4.3D Printing.com. [[Last accessed on 2018 Nov 14]];2018, What is 3D Printing? Available from: https://www.3dprinting.com/what-is-3d-printing .
- 5.Radenkovic D, Solouk A, Seifalian A. 2016, Personalized development of human organs using 3D printing technology. Med Hypotheses. 87:30–33. doi: 10.1016/j.mehy.2015.12.017. https://doi.org/10.1016/j.mehy.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 6.Jürgen G, Thomas B, Torsten B, et al. 2016, Biofabrication:Reappraising the definition of an evolving field. Biofabrication. 8(1):13001. doi: 10.1088/1758-5090/8/1/013001. https://doi.org/10.1088/1758-5090/8/1/013001. [DOI] [PubMed] [Google Scholar]
- 7.Mandrycky C, Wang Z, Kim K, et al. 2016, 3D bioprinting for engineering complex tissues. Biotechnol Adv. 34(4):422–434. doi: 10.1016/j.biotechadv.2015.12.011. https://doi.org/10.1016/j.biotechadv.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang Y, Zhang X F, Gao G, et al. 2017, 3D bioprinting and the current applications in tissue engineering. Biotechnol J. 12(8):1600734. doi: 10.1002/biot.201600734. https://doi.org/10.1002/biot.201600734. [DOI] [PubMed] [Google Scholar]
- 9.Lee J M, Sing S L, Zhou M, et al. 2018, 3D bioprinting processes:A perspective on classification and terminology. Int J Bioprint. 4(2):1–10. doi: 10.18063/IJB.v4i2.151. http://dx.doi.org/10.18063/ijb.v4i2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozbolat I T, Peng W, Ozbolat V. 2016, Application areas of 3D bioprinting. Drug Disc Today. 21(8):1257–1271. doi: 10.1016/j.drudis.2016.04.006. https://doi.org/10.1016/j.drudis.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Rodríguez-Salvador M, Rio-Belver R M, Garechana-Anacabe G. 2017, Scientometric and patentometric analyses to determine the knowledge landscape in innovative technologies:The case of 3D bioprinting. PLoS One. (e0180375) 12(6) doi: 10.1371/journal.pone.0180375. https://doi.org/10.1371/journal.pone.0180375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy S V, Atala A. 2014, 3D bioprinting of tissues and organs. Nat Biotechnol. 32:773–785. doi: 10.1038/nbt.2958. https://doi.org/10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 13.Wang M, Shen S, Yang J, et al. 2014, 3D Printing Method For Freeform Fabrication Of Optical Phantoms Simulating Heterogeneous Biological Tissue. SPIE BiOS. 8945:8945091–8945099. https://doi.org/10.1117/12.2041137. [Google Scholar]
- 14.Zhao S, Gu Y, Xue P, et al. 2010, Imaging port wine stains by fiber optical coherence tomography. J Biomed Opt. 15(3):36020. doi: 10.1117/1.3445712. https://doi.org/10.1117/1.3445712. [DOI] [PubMed] [Google Scholar]
- 15.Dong E, Wang M, Shen S, et al. 2016, 3D Printing of Tissue-Simulating Phantoms as a Traceable Standard for Biomedical Optical Measurement. Proc. SPIE. 9903:9903021–99030213. Seventh International Symposium on Precision Mechanical Measurements 990302. https://doi.org/10.1117/12.2218698. [Google Scholar]
- 16.Zhao Z, Zhou X, Shen S, et al. 2016, 3D Printing Of Tissue-Simulating Phantoms For Calibration Of Biomedical Optical Devices. SPIE/COS Photonics Asia. 10024:10024N1–10024N10. https://doi.org/10.1117/12.2246273. [Google Scholar]
- 17.Diep P, Pannem S, Sweer J, et al. 2015, Three-dimensional printed optical phantoms with customized absorption and scattering properties. Biomed Opt Express. 6(11):4212–4220. doi: 10.1364/BOE.6.004212. https://doi.org/10.1364/boe.6.004212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marcu L, Boppart S A, Hutchinson M R, et al. 2017, Biophotonics:The big picture. J Biomed Opt. 23(2):21103. doi: 10.1117/1.JBO.23.2.021103. https://doi.org/10.1117/1.JBO.23.2.021103. [DOI] [PubMed] [Google Scholar]
- 19.Andreu N, Zelmer A, Wiles S. 2011, Noninvasive biophotonic imaging for studies of infectious disease. FEMS Microbiol Rev. 35:360–394. doi: 10.1111/j.1574-6976.2010.00252.x. https://doi.org/10.1111/j.1574-6976.2010.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maloth K N, Velpula N, Kodangal S, et al. 2016, Photodynamic therapy a non-invasive treatment modality for precancerous lesions. J Lasers Med Sci. 7:30–36. doi: 10.15171/jlms.2016.07. https://doi.org/10.15171/jlms.2016.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.International Commission on Non-Ionizing Radiation Protection 2017, ICNIRP statement on diagnostic devices using non-ionizing radiation:Existing regulations and potential health risks. Health Phys. 112(3):305–321. doi: 10.1097/HP.0000000000000654. https://doi.org/10.1097/hp.0000000000000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pogue B W, Patterson M S. 2006, Review of tissue simulating phantoms for optical spectroscopy, imaging and dosimetry. J Biomed Opt. 11(4):041102. doi: 10.1117/1.2335429. https://doi.org/10.1117/1.2335429. [DOI] [PubMed] [Google Scholar]
- 23.Gao F, Li J, Zhang L, et al. 2010, Simultaneous fluorescence yield and lifetime tomography from time-resolved transmittances of small-animal-sized phantom. Appl Opt. 49(16):3163–3172. doi: 10.1364/AO.49.003163. https://doi.org/10.1364/ao.49.003163. [DOI] [PubMed] [Google Scholar]
- 24.Wang L V. 2008, Prospects of photoacoustic tomography. Med Phys. 35(12):5758–5767. doi: 10.1118/1.3013698. https://doi.org/10.1118/1.3013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nordstrom R J. 2011, Phantoms as Standards in Optical Measurements Proceedings SPIE 7906, Optical Diagnostics and Sensing XI:Toward Point-of-Care Diagnostics;and Design and Performance Validation of Phantoms Used in Conjunction with Optical Measurement of Tissue III. https://doi.org/10.1117/12.∻74. [Google Scholar]
- 26.Lamouche G, Kennedy B F, Kennedy K M, et al. 2012, Review of tissue simulating phantoms with controllable optical, mechanical and structural properties for use in optical coherence tomography. Biomed Opt Express. 3(6):1381–1398. doi: 10.1364/BOE.3.001381. https://doi.org/10.1364/boe.3.001381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouchard J, Noiseux I, Veilleux I, et al. 2011, The role of optical tissue phantom in verification and validation of medical imaging devices. 2011. Int Workshop Biophotonics. :1–3. https://doi.org/10.1109/IWBP.2011.5954806. [Google Scholar]
- 28.Oregon Medical Laser Center (OMLC) [[Last accessed on 2018 Oct 17]];2017, Optical Phantoms. Available from: https://www.omlc.org/classroom/phantom .
- 29.Vernon M L, Fréchette J, Painchaud Y, et al. 1999, Fabrication and characterization of a solid polyurethane phantom for optical imaging through scattering media. Appl Opt. 38(19):4247–4251. doi: 10.1364/ao.38.004247. https://doi.org/10.1364/ao.38.004247. [DOI] [PubMed] [Google Scholar]
- 30.Rang M, Jones A C, Zhou F, et al. 2008, Optical near-field mapping of plasmonic nanoprisms. Nano Lett. 8(10):3357–3363. doi: 10.1021/nl801808b. https://doi.org/10.1021/nl801808b. [DOI] [PubMed] [Google Scholar]
- 31.Bisaillon C É, Dufour M L, Lamouche G. 2011, Artery phantoms for intravascular optical coherence tomography:Healthy arteries. Biomed Opt Express. 2(9):2599–2613. doi: 10.1364/BOE.2.002599. https://doi.org/10.1364/boe.2.002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai P, Xu X, Wang L V. 2014, Dependence of optical scattering from intralipid in gelatin-gel based tissue-mimicking phantoms on mixing temperature and time. J Biomed Opt. 19(3):35002. doi: 10.1117/1.JBO.19.3.035002. https://doi.org/10.1117/1.jbo.19.3.035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pleijhuis R, Timmermans A, De Jong J, et al. 2014, Tissue-simulating phantoms for assessing potential near-infrared fluorescence imaging applications in breast cancer surgery. J Vis Exp. 91:51776. doi: 10.3791/51776. https://doi.org/10.3791/51776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehrban N, Teoh G Z, Birchall M A. 2016, 3D bioprinting for tissue engineering:Stem cells in hydrogels. Int J Bioprint. 2(1):14. https://doi.org/10.18063/ijb.2016.01.006. [Google Scholar]
- 35.Jang T S, Jung H D, Pan H M, et al. 2018, 3D printing of hydrogel composite systems:Recent advances in technology for tissue engineering. Int J Bioprint. 4(1):126. doi: 10.18063/IJB.v4i1.126. https://doi.org/10.18063/ijb.v4i1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Mooney D J. 2016, Designing hydrogels for controlled drug delivery. Nat Rev Mater. 1. :16071. doi: 10.1038/natrevmats.2016.71. https://doi.org/10.1038/natrevmats.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X, Xia L Y, Chen X, et al. 2017, Hydrogel-based phototherapy for fighting cancer and bacterial infection. Sci China Mater. 60(6):487–503. https://doi.org/10.1007/s40843-017-9025-3. [Google Scholar]
- 38.Guo X, Qu J, Zhu C, et al. 2018, Synchronous delivery of oxygen and photosensitizer for alleviation of hypoxia tumor microenvironment and dramatically enhanced photodynamic therapy. Drug Deliv. 25(1):585–599. doi: 10.1080/10717544.2018.1435751. https://doi.org/10.1080/10717544.2018.1435751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson B C, Patterson M S. 2008, The physics, biophysics and technology of photodynamic therapy. Phys Med Biol. 53(9):R61. doi: 10.1088/0031-9155/53/9/R01. https://doi.org/10.1088/0031-y9155/53/9/R01. [DOI] [PubMed] [Google Scholar]
- 40.Chmarra M K, Hansen R, Mårvik R, et al. 2013, Multimodal phantom of liver tissue. PLoS One. 8(5):e64180. doi: 10.1371/journal.pone.0064180. https://doi.org/10.1371/annotation/b148461a-eb97-43c9-a44c-f97e7eab1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alexei K, Srirang M, Rosalyn S, et al. 2003, Poly(vinyl alcohol) gels for use as tissue phantoms in photoacoustic mammography. Phys Med Biol. 48(3):357. doi: 10.1088/0031-9155/48/3/306. https://doi.org/10.1088/0031-9155/48/3/306. [DOI] [PubMed] [Google Scholar]
- 42.Avtzi S, Zacharopoulos A, Psycharakis S, et al. 2013, Fabrication and Characterization of a 3-D Non-Homogeneous Tissue-Like Mouse Phantom for Optical Imaging 1st International Conference “Biophotonics Riga 2013” SPIE, 6. https://doi.org/10.1117/12.2044698. [Google Scholar]
- 43.Wang K, Ho C C, Zhang C, et al. 2017, A review on the 3D printing of functional structures for medical phantoms and regenerated tissue and organ applications. Engineering. 3:653–662. https://doi.org/10.1016/J.ENG.2017.05.013. [Google Scholar]
- 44.Velasquillo C, Galue E A, Rodriquez L, et al. 2013, Skin 3D bioprinting Applications in cosmetology. J Cosmet Dermatol Sci Appl. 3(1):5. https://doi.org/10.4236/jcdsa.2013.31A012. [Google Scholar]
- 45.Seer P. [[Last accessed on 2018 Sep d24]];2018, Analysis of Patents, Search and Project Collaboration. Available from: https://www.patseer.com .
- 46.Jang J, Yi H G, Cho D W. 2016, 3D printed tissue models:Present and future. ACS Biomater Sci Eng. 2(10):1722–1731. doi: 10.1021/acsbiomaterials.6b00129. https://doi.org/10.1021/acsbiomaterials.6b00129. [DOI] [PubMed] [Google Scholar]
- 47.Bishop E S, Mostafa S, Pakvasa M, et al. 2017, 3-D bioprinting technologies in tissue engineering and regenerative medicine:Current and future trends. Genes Dis. 4(4):185–195. doi: 10.1016/j.gendis.2017.10.002. https://doi.org/10.1016/j.gendis.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sokolov K V, Galvan J, Myakov A V, et al. 2002, Realistic three-dimensional epithelial tissue phantoms for biomedical optics. J Biomed Opt. 7(1):148–156. doi: 10.1117/1.1427052. https://doi.org/10.1117/1.1427052. [DOI] [PubMed] [Google Scholar]
- 49.Lurie K L, Smith G T, Khan S A, et al. 2014, Three-dimensional, distendable bladder phantom for optical coherence tomography and white light cystoscopy. J Biomed Opt. 19(3):36009. doi: 10.1117/1.JBO.19.3.036009. https://doi.org/10.1117/1.jbo.19.3.036009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim H, Hau N T, Chae Y G, et al. 2016, 3D printing-assisted fabrication of double-layered optical tissue phantoms for laser tattoo treatments. Lasers Surg Med. 48(4):392–399. doi: 10.1002/lsm.22469. https://doi.org/10.1002/lsm.22469. [DOI] [PubMed] [Google Scholar]
- 51.Sangha G S, Hale N J, Goergen C J. 2018, Adjustable photoacoustic tomography probe improves light delivery and image quality. Photoacoustics. 12:6–13. doi: 10.1016/j.pacs.2018.08.002. https://doi.org/10.1016/j.pacs.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghassemi P, Wang J, Melchiorri A J, et al. 2015, Rapid prototyping of biomimetic vascular phantoms for hyperspectral reflectance imaging. J Biomed Opt. 20(12):121312. doi: 10.1117/1.JBO.20.12.121312. https://doi.org/10.1117/1.jbo.20.12.121312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bentz B Z, Costas A, Gaind V, et al. 2017, 3D Printed Optical Phantoms and Deep Tissue Imaging for in vivo Applications Including Oral Surgery. Proceeding SPIE 10056, Design and Quality for Biomedical Technologies X, 1005607. https://doi.org/10.1117/12.2253763. [Google Scholar]
- 54.Lv X, Chen H, Liu G, et al. 2018, Design of a portable phantom device to simulate tissue oxygenation and blood perfusion. Appl Opt. 57(14):3938–3946. doi: 10.1364/AO.57.003938. https://doi.org/10.1364/ao.57.003938. [DOI] [PubMed] [Google Scholar]
- 55.U.S Food and Drug Administration. [[Last accessed on 2018 Oct 17]];2018, About FDA. Available from: https://www.fda.gov/AboutFDA/default.htm .
- 56.National Institute of Standards and Technology. [[Last accessed on 2018 Oct d17]];2017, About NIST. Available from: https://www.nist.gov/about-nist .
- 57.Fanjul-Vélez F, Arce-Diego J L. 2011, Light Propagation in Turbid Media:Application to Biological Tissues. Proceedings of 21st International Conference Radioelektronika. 2011 https://doir.org10.1109/RADIOELEK.2011.5936483. [Google Scholar]
- 58.Teisseire M, Han A, Abuhabsah R, et al. 2010, Ultrasonic backscatter coefficient quantitative estimates from Chinese hamster ovary cell pellet biophantoms. J Acoust Soc Am. 128(5):3175–3180. doi: 10.1121/1.3483740. https://doi.org/10.1121/1.3483740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brien W D O, Han A, Auger T. 2012, Quantitative Ultrasound from Single Cells to Biophantoms to Tumors. 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society. :1118–1120. doi: 10.1109/EMBC.2012.6346131. https://doi.org/10.1109/EMBC.2012.6346131. [DOI] [PubMed] [Google Scholar]
- 60.Han A, Abuhabsah R, Miller R J, et al. 2013, The measurement of ultrasound backscattering from cell pellet biophantoms and tumors ex vivo. J Acoust Soc Am. 134(1):686–693. doi: 10.1121/1.4807576. https://doi.org/10.1121/1.4807576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Han A, O'Brien W D. 2015, Structure function for high-concentration biophantoms of polydisperse scatterer sizes. IEEE Trans Ultrason Ferroelectr Freq Control. 62(2):303–318. doi: 10.1109/TUFFC.2014.006629. https://doi.org/10.1109/tuffc.2014.006629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cristea A, Franceschini E, Lin F, et al. 2015, Quantitative characterization of concentrated cell pellet biophantoms using statistical models for the ultrasound echo envelope. Phys Procedia. 70:1091–1095. https://doi.org/10.1016/j.phpro.2015.08.233. [Google Scholar]
- 63.Azizi S, Bayat S, Rajaram A, et al. 2018, 3D Tissue Mimicking Biophantoms for Ultrasound Imaging:Bioprinting and Image Analysis. Proceeding SPIE 10576, Medical Imaging 2018:Image-Guided Procedures, Robotic Interventions, and Modeling, 105761T. https://doi.org/10.1117/12.2293930. [Google Scholar]
- 64.Wróbel M S, Jędrzejewska-Szczerska M, Galla S, et al. 2015, Use of optical skin phantoms for preclinical evaluation of laser efficiency for skin lesion therapy. J Biomed Opt. 20(8):85003. doi: 10.1117/1.JBO.20.8.085003. https://doi.org/10.1117/1.jbo.20.8.085003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Y, Ghassemi P, Depkon A, et al. 2018, Biomimetic 3D-printed neurovascular phantoms for near-infrared fluorescence imaging. Biomed Opt Express. 9(6):2810–2824. doi: 10.1364/BOE.9.002810. https://doi.org/10.1364/boe.9.002810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilson B C, Jermyn M, Leblond F. 2018, Challenges and opportunities in clinical translation of biomedical optical spectroscopy and imaging. J Biomed Opt. 23(3):30901. doi: 10.1117/1.JBO.23.3.030901. https://doi.org/10.1117/1.JBO.23.3.030901. [DOI] [PMC free article] [PubMed] [Google Scholar]


