Abstract
As microfluidic devices are designed to tackle more intricate tasks, the architecture of microfluidic devices becomes more complex, and more sophisticated fabrication techniques are in demand. Therefore, it is sensible to fabricate microfluidic devices by three-dimensional (3D)-printing, which is well-recognized for its unique ability to monolithically fabricate complex structures using a near-net-shape additive manufacturing process. Many 3D-printed microfluidic platforms have been demonstrated but can 3D-printed microfluidics meet the demanding requirements in today’s context, and has microfluidics truly benefited from 3D-printing? In contrast to 3D-printed microfluidics, some go the other way around and exploit microfluidics for 3D-printing. Many innovative printing strategies have been made possible with microfluidics-enabled 3D-printing, although the limitations are also largely evident. In this perspective article, we take a look at the current development in 3D-printed microfluidics and microfluidics-enabled 3D printing with a strong focus on the limitations of the two technologies. More importantly, we attempt to identify the innovations required to overcome these limitations and to develop new high-value applications that would make a scientific and social impact in the future.
Keywords: 3D-printing, Bioprinting, Microfluidics
1. Introduction
Microfluidics is already a mature technology that is widely adopted in the bioanalytical investigation, clinical diagnostics, and chemical sensing and synthesis. Microfluidic technology has many compelling advantages over its bulk flow counterpart, such as low reagent and sample consumption, favorable thermodynamics and chemical reaction kinetics, laminar flow profile, precise handling of single bioparticles, and high degree of parallelization and multiplexing[1-4]. Many advanced analytical systems, such as next-generation sequencers and molecular diagnostic platforms, incorporate certain microfluidic components these days.
Conventional fabrication of microfluidic devices heavily relies on micromachining techniques. The earlier fabrication methods are derived from techniques used in microelectronic and mechanical systems (MEMS). Various microfluidic components are created by etching microstructures into silicon. The well-established MEMS technology is readily applied to the fabrication of microfluidic chips, giving microfluidics a Kickstart. Innovations in silicon-based microfluidic networks, actuators, pumps, mixers, and valves emerge at a rapid rate, giving rise to many novels and unique microfluidic applications such as cell sorting and trapping, biochemical sensing, genetic analysis, and drug delivery[5-9]. In spite of their great potential to revolutionize biomedical research, these silicon-based microfluidic devices experience difficulty when trying to find their way into biological laboratories, and one of the main obstacles is the complicated fabrication workflow. Although engineers may think that the fabrication of silicon-based microfluidic devices is simpler compared to MEMS devices, it is still a daunting task for biomedical researchers to take on. This issue is not resolved until the polydimethylsiloxane (PDMS)-based soft lithography, which is a simple molding-based fabrication technique, is developed[10]. Although traditional micromachining process is still involved in PDMS-based fabrication, it is limited to the making of molds. With the ready-made mold, the chip fabrication workflow is reduced to pouring PDMS, punching access ports and bonding PMDS to glass. Compared to the silicon-based microfluidic devices, PMDS-based devices find a bigger audience among biomedical researchers. The PDMS-based device is made more popular by the invention of PDMS-based multilayer pneumatic valves and pumps[11], which enables system-level integration of multifaced devices for intricate tasks such as single-cell analysis[3,12].
The PDMS-based microfluidics has its pros and cons. On the one hand, PDMS is able to precisely replicate the lithographically defined patterns with nanometer resolution. In addition, PDMS is biocompatible and well-suited for cell studies[13-15]. It also has favorable optical properties such as great transparency and low autofluorescence, which is compatible with various optical sensing modalities. The low cost of PDMS and the reusability of the mold make PDMS-based microfluidic devices reasonably affordable. On the other hand, PDMS is water vapor permeable. Samples in PDMS chips are susceptible to evaporation and bubbles in the event of a heating or prolonged incubation. PDMS is also prone to protein fouling, which would affect the accuracy of biosensing. Furthermore, the fabrication of a PDMS-based microfluidic device still heavily relies on manual assembly.
Nowadays, as microfluidic devices are designed to tackle more intricate tasks, the architecture of microfluidic devices becomes more complex, and more sophisticated fabrication techniques are in demand. Therefore, it is sensible to fabricate microfluidic devices by three-dimensional (3D)-printing, which is well-recognized for its unique ability to monolithically fabricate complex structures using a near-net-shape additive manufacturing process. As a matter of fact, a great number of 3D-printed microfluidic devices have been reported in the past few years followed by several review papers that provide a fairly comprehensive evaluation of these devices and an optimistic future outlook on 3D-printed microfluidics[16-20]. One of the reviews even touts 3D-printing as the upcoming revolution in microfluidics[21].
While majority studies employ 3D-printing for microfluidic device fabrication, a number of studies go the other way around and incorporate microfluidic components in 3D-printers for added functions and improved printing performance. These microfluidic components offer excellent fluidic control of 3D-printing inks, simplifying multi-material, and high-throughput parallel printing. The laminar flow profile of microfluidics allows concurrent printing of multiple inks through a single nozzle and time-controlled crosslinking of hydrogel inks using hydrodynamic focusing. Furthermore, additional functional components, such as surface acoustic waves, can be incorporated to modulate the distribution of chemical constituents in multiphase inks. These works point out a new direction in which 3D-printing and microfluidics could work synergistically to accomplish previously unattainable tasks.
In this perspective article, we evaluate the up-to-date development of 3D-printed microfluidics and microfluidics-enabled 3D-printing with a strong emphasis on their limitations. We would express our opinions on the future innovations required to overcome these limitations and to develop new high-value applications. We hope to answer whether 3D-printing is more well-suited for microfluidics or it is the other way around, but we will leave the discussion open.
2. 3D-printing for Microfluidics
3D-printing is an umbrella term encompassing a number of additive manufacturing technologies, but not all of them are applicable to printing microfluidic devices. Based on their suitability for microfluidics, we loosely categorize 3D-printing into extrusion-based technology (e.g., fused deposition modeling [FDM]), liquid resin-based technology (e.g., stereolithography [SLA], digital light processing, and two-photon polymerization [2PP]) which also includes inkjet-based 3D-printing (e.g., material jetting) due to the similar curing mechanism, powder-based technology (e.g., Multi Jet Fusion [MJF], selective laser sintering [SLS], selective laser melting [SLM], and electron beam melting), and other less common 3D-printing technologies. The technical aspects of these 3D-printing technologies have been discussed extensively in many reviews[16-21]; hence, we will skip it in this article. Majority of microfluidic devices are fabricated with extrusion-based technology or liquid resin-based technology.
The fabrication of microfluidic devices by 3D-printing can be either direct or indirect. Direct 3D-printing constructs the microfluidic chip by enclosing the microchannels and other microfluidic components with the ink materials. Indirect 3D-printing produces a mold using the ink materials, and the chip is fabricated by casting PMDS against the mold. The final microfluidic chip does not consist of any ink materials. In this perspective article, we will mainly focus on the direct printing approach except in a few cases in which a sacrificial mold is required for complex 3D microfluidic networks.
2.1. Current Development in 3D-printed Microfluidics
Research in 3D-printed microfluidics aims to create functional microfluidic components, realize complex microfluidic architecture, and demonstrates biomedical applications.
Earlier work in this field primarily focused on the monolithic fabrication of conventional microfluidic devices to bypass the traditional microfabrication. These microfluidic devices fabricated by 3D-printing were limited to those with only basic passive microfluidic components, such as microchannels and microchambers. Donvito et al. printed a monolithic microfluidic device with a T-junction using inkjet-based 3D-printing for microdroplet generation[22]. Chen et al. fabricated a microplate reader-compatible microfluidic device using an inkjet-based technique and demonstrated quantitative blood testing on this device[23]. Kitson et al. developed several types of 3D-printed chemical reactionware using FDM for both organic and inorganic synthesis[24]. Bishop et al. also printed a single-channel microfluidic device with standard interface connectors using FDM for nanoparticle preparation[25]. Takenaga et al. developed an SLA-printed biocompatible microfluidic device with integrated biosensor for the study of cell culture conditions[26].
The most notable revolution that 3D-printing brings to microfluidics is the ability to freely design and fabricate in the third dimension. 3D-printing transforms the conventional planar microfluidic features into convoluted 3D microfluidic networks packed into a small footprint. It enables monolithic fabrication of overlapping microfluidic components stacked in the vertical direction, bypassing the multi-layer bonding process required in traditional microfluidic fabrication. The true 3D microfluidic architecture offers an additional degree of freedom for fluidic manipulation. Several groups explore 3D-printing’s unique ability to monolithically create 3D structures to realize true 3D microfluidic architectures that were unattainable by the traditional microfabrication techniques. Lee et al. fabricated a helical channel using SLA for inertia-based bacteria separation (Figure 1). The helical channel spiraled up in the z-direction and formed a true 3D microchannel with a trapezoid cross-section[27]. The 3D helical design significantly reduced the device footprint compared to the planer spiral design. Shallan et al. used a liquid resin-based 3D printer to fabricate 3D microchannels for more efficient passive mixing[28]. Monaghan et al. developed a 3D microfluidic device coupled with optical fibers to monitor chemical synthesis[29]. The group used the same approach to fabricate a 3D tree-like chemical gradient generator with reduced footprint and high portability[28]. Cabot et al. used a similar 3D-printed microfluidic passive mixer to improve sample mixing in a capillary electrophoresis assay that measured the pKa[30]. A highly complex interconnected 3D microfluidic network was fabricated by casting epoxy or agarose against a 3D-printed sacrificial mold[31]. After casting, the mold made of isomalt was dissolved to clear space for microfluidic channels. 3D-printing also enabled easy integration of chip-user interface that coupled the external fluid into the microfluidic chip. A good example was demonstrated by Anderson et al. who fabricated a microfluidic drug screening platform that incorporated standard membrane devices for the cell culture and standard thread fitting for the coupling of tubing[32]. Another example was demonstrated by Au et al. who printed a Luer lock fitting on the microfluidic device as a standard fluid connector[33].
Figure 1.
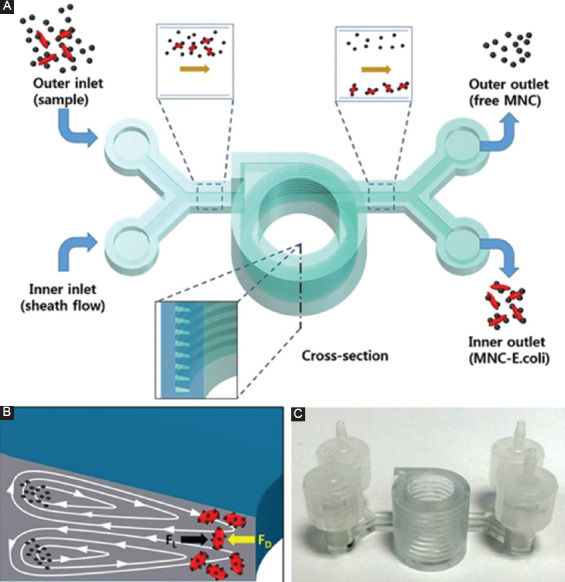
A three-dimensional (3D)-printed true 3D microfluidic device with standard fluidic coupling. (A) The schematic illustration of the 3D-printed device showing the cross-section of the 3D helical channel. (B) The cross-section of the channel is trapezoid in shape. (C) The actual 3D helical microfluidic device. Reproduced from Ref. Lee et al.[27] with the permission granted under the creative common license.
One of the reasons for PDMS being so popular in microfluidics is due to its high flexibility that enables the fabrication of multilayer pneumatic valves and pumps. Each multilayer pneumatic valve consists of two overlapping crisscross microchannels separated by a thin PDMS membrane at the intersection. One of the microchannels carries the sample fluid, and the other one carries the control fluid (sometimes just air). When the control channel is pressurized, the thin PDMS membrane deflects, creating a bulge that blocks the fluidic channel. The enabling factor of the multilayer pneumatic valve is the low Young’s modulus of PDMS, which allows the thin membrane to deflect easily. In contrast, most 3D-printed plastic materials have Young’s modulus hundreds or thousands of times larger than PDMS, which makes it difficult to pneumatically deflect the 3D-printed membrane. Nevertheless, using relatively flexible plastic, active valving has been demonstrated in a 3D-printed monolithic microfluidic device (Figure 2). In this work, Au et al. printed a multilayer membrane valve using watershed (a biocompatible resin) with Young’s modulus of 2.7 GPa[20]. The diameter and thickness of the circular membrane were 5 mm and 100 µm, respectively. The membrane would deflect by ~200 µm under 2.9 psi pressure. Due to the large Young’s modulus, the size of the membrane was considerably larger than the PDMS-based valve to achieve the required deflection for valve closure. A similar circular membrane valve was demonstrated by Gong et al.[34] By pushing the thickness of the membrane down to ~20 µm, they were able to reduce the diameter of the membrane to ~1 mm and pack the valves into a dense array. The required diameter of the membrane in the valve at various membrane thickness was studied by Rogers et al.[35] The same design was also used as an active Micropump in 3D-printed microfluidics[34]. A 3D-printed Quake valve was demonstrated by Keating et al. using an inkjet-based technique that is capable of printing multiple materials[36]. Tangoplus, a rubber-like flexible material was used to print the membrane while other parts of the microfluidic device were printed with rigid plastic material. Nonetheless, Tangoplus was less flexible than PDMS, and the dimension of the control channel was in the millimeter range. In addition to active valves, passive valves were also created in 3D-printed microfluidic devices. These were usually one-way check valves similar to those in silicon-based MEMS device. Sochol et al. printed microfluidic circuitry components, such as fluidic diodes and transistors, by incorporating these designs[37]. Chen et al. incorporated these passive valves to prevent backflow in a 3D-printed microfluidic multi-chamber cell culture device that modeled the circulatory system[38].
Figure 2.
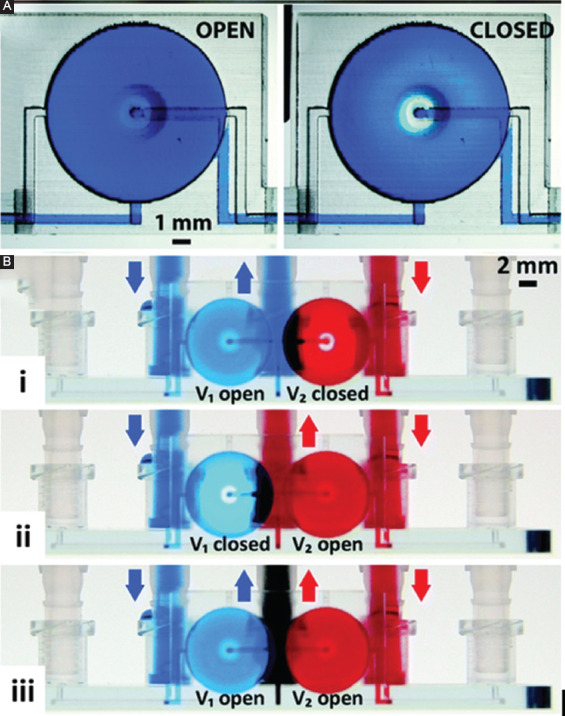
Three-dimensional (3D)-printed active microfluidic membrane valve. (A) The valve is open and closed configuration. (B) Fluidic control with the valve. (i) Valve 1 (V1, left) is open and valve 2 (V2, right) is closed. Only blue liquid flows in the central channel. (ii) V1 is closed and V2 is open. Only red liquid flows in the central channel. (iii) Both valves are open. A mixture of blue and red liquids flow in the central channel. Reproduced from Ref. Au et al.[20] with permission from Royal Chemical Society.
Another enhancement brought to microfluidics by 3D-printing is device modulation. With 3D-printing technology, it is straightforward to fabricate individual modules, each of which contains a single microfluidic component and to incorporate standard connectors on the individual modules for easy assembly. Bhargava et al. 3D-printed cubes with a female port and a male connector (Figure 3)[39]. These cubes, which functioned as microfluidic modules, created elastic reversible liquid-tight seals when coupled together. Microfluidic components, such as straight channels, helical channels, and reaction chambers, were embedded in these modules. Non-fluidic components, such as optical components, were also introduced into individual modules. A fully functional 3D microfluidic network was constructed by plug-and-play. Lee et al. developed a 3D-printed modular microfluidic system assembled together with horseshoe-shaped pins that functioned somewhat like a stapler bullet[40]. To prevent leakage, O-rings were used at the fluidic interface between the modules. Nie et al. designed lego-like microfluidic modules with press-fit connectors along the edge of each modular block. Due to the poor sealing, this system was only designed for capillary-driven flow and could not operate under high pressure. Vittayarukskul and Lee built a truly Lego-like modular microfluidic platform with 3D-printed parts[41]. The microfluidic modules were embedded in the Lego-patented building block[42]. In this design, the motherboard was fabricated by direct 3D-printing, whereas the individual modules were fabricated by casting PDMS against 3D-printed molds. A 3D microfluidic network was constructed by stacking the modules through the press-fit Lego interface. The PDMS acted as a rubber seal to prevent the leakage. Another reconfigurable microfluidic system was reported by Po, which used magnets to couple of individual modules[43]. Two ring magnets were embedded at the two ends of each modular block. The magnetic force pulled two adjacent modules together tightly enough to prevent fluid leakage. The center hole in the ring magnet provided access for fluids at the interface.
Figure 3.
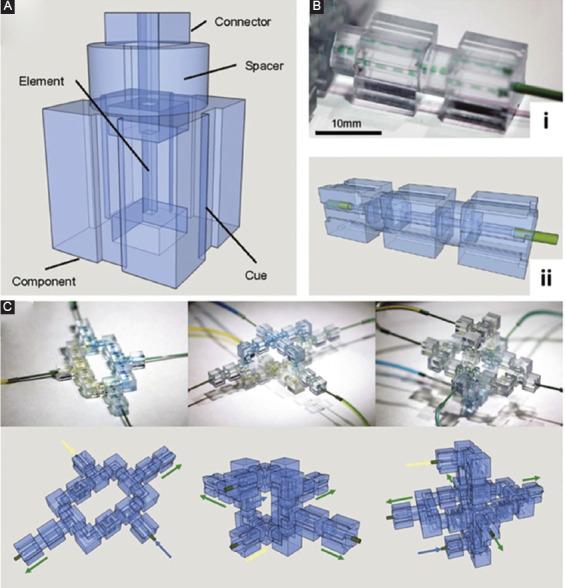
Three-dimensional (3D)-printed modular microfluidics. (A) Individual microfluidic module. (B) A microfluidic droplet generator is constructed by cascading three 3D-printed modules. (C) Several complex 3D microfluidic configurations constructed from 3D-printed microfluidic modules. Reproduced from Ref. Bhargava et al.[39] with permission from the National Academy of Science (US). Copyright (2015) National Academy of Sciences.
2.2. 3D-Printed Microfluidics, Are We There Yet?
It seems 3D-printing technology has brought many innovations to microfluidics. Many complex microfluidic architectures and novel fabrication approaches have only been made possible through the use of 3D-printing. 3D-printing also shortens the time required from design to fabrication, providing a valuable rapid prototyping tool for microfluidic devices. But has microfluidics truly benefited from these innovations? Since the first demonstration of the microfluidics-based “lab on a chip” in the 1990s, microfluidics has made a significant process. Nowadays, microfluidics is already a mature technology that enjoys its prosperity in biomedical fields. The research emphasis on microfluidics has gradually shifted from the device fabrication techniques, the fundamental physics of fluidic behaviors, and fluidic actuation and sensing mechanisms, to high-value applications, such as large-scale single-cell/molecule analysis for genomic and proteomic studies as well as sample-to-answer total analysis for point-of-care diagnostics[1]. Therefore, any present and future development in microfluidics ought to be oriented toward specific applications for high scientific and social impact. That is not to say new fabrication technologies, such as 3D-printing, are not in demand, but these new technologies must bring new values and fulfill requirements dictated by the applications.
In spite of its many compelling advantages, 3D-printing faces unique problems that may hinder its applicability in microfluidics for high-value applications.
One of such problems is the limited printer resolution, particularly in the lateral direction. The lateral resolution of a 3D-printer is determined by the minimal line width generated in a single pass, and the vertical resolution is determined by the minimal thickness of each layer. The extrusion-based printers, such as FDM, have a lateral resolution of hundreds of microns and a vertical resolution of tens of microns. The resolution of liquid resin-based printers, such as SLA and inkjet, has a lateral resolution of tens of microns and a vertical resolution of down to single-digital microns[44]. (Although 2PP is also a liquid resin-based 3D-printing technology, it is a breed of its own, which we will discuss separately in later sections.) Although many 3D-printing systems claim a printing resolution in the true microscale (<100 µm), the actual size of 3D-printed microfluidic features mostly fall in the millimeter to the submillimeter range, because each feature is constructed by several 3D-printed lines. In a rough estimate, the minimal size of a microfluidic feature is one order of magnitude larger than the printer resolution[45]. Many current high-value applications of microfluidics require a high degree of parallelization and the ability to handle micro-objects such as single cells, both of which demand microfluidic features with a size ranging from several microns to tens of microns. For example, the microfluidic feature in the microdroplet generator chip, which is used to create microscale droplets to encapsulate single cells, usually contains features in the range of tens of microns[3,46]. To achieve a microfluidic feature in this size range, the printer resolution has to reach single-digital microns or even lower. Although some of the latest liquid resin-based 3D printers have a submicron vertical resolution, their lateral resolution is still not high enough[47]. So far, the only 3D-printing technology that is capable of true microscale fabrication is 2PP. 2PP pushes the printer resolution to the diffraction limit of the optics, reaching a submicron printer resolution in both lateral and vertical directions. Many amazing 3D microstructures have been fabricated using 2PP. However, the problem of 2PP is its slow printing speed. In addition to the microscale features, the microfluidic devices also consist of other macroscale features. These large features would take too long for 2PP to print, which defeats the whole purpose of rapid prototyping using 3D-printing.
The quality of the 3D-printed microfluidic devices is another big concern. First, the dimension fidelity of 3D-printing is poor at the microscale. In one study, the measured dimension of millimeter and submillimeter features is <6% off nominal[29]. However, when attempting to print true microscale features (<100 µm), the measured dimension is more than 60% off-nominal (Table 1). In addition, the side wall of the 3D-printed microfluidic channel may be leaning, resulting in an undesired trapezoid cross section. Second, the surface of 3D-printed parts is known to be rough with evident welding lines between layers. In bulk parts, the external surface can be easily polished. In 3D-printed microfluidic devices, it is almost impossible to polish the internal surface of the microfluidic components. Besides the welding lines, the surface of 3D-printed microfluidic components is often speckled with particulates and microcavities[48]. The desire to achieve a smooth surface in microfluidics is beyond esthetics but rather a practical concern. The imperfections may substantially alter the flow behavior and the way the surface interacts with biomolecules, leading to unexpected analytical outcomes. To improve the surface quality of 3D-printed microfluidic features, one could either improve the printer resolution or develop micro polishing techniques to smoothen the surface post printing.
Table 1.
Dimension fidelity of microfluidic channels printed with SLA. Reproduced from Ref. Monaghan et al.[29] with permission from the Royal Chemical Society.
| Channel width (μm) | Distance to the next channel in CAD model (μm) | Measured width on test piece (μm) |
|---|---|---|
| 500 | 1000 | 949±2.64 |
| 500 | 500 | 494±2.10 |
| 500 | 250 | 258±1.94 |
| 500 | 125 | 130±4.49 |
| 500 | 62.5 | 104±0.84 |
SLA: Stereolithography; CAD: Computer-aided design
Several other issues of 3D-printed microfluidic devices, such as biocompatibility and optical transparency, have also been noted[16,18,21]. However, these problems have been mitigated with the development of new materials. Nevertheless, a more systematic investigation of these materials would greatly benefit the community.
2.3. Future of 3D-printed Microfluidics
To answer the question set in the previous section, I do not believe 3D-printed microfluidics is quite there. The upcoming 3D-printing revolution in microfluidics might be on its way, but definitely not in sight yet. Thus far, 3D-printing has only been used as an alternative fabrication technique for microfluidics, hence does not add much new value to the field. The functions of most reported 3D-printed microfluidic devices can be easily realized by conventional microfluidic devices fabricated using 2D lithography techniques. Compared to the flattened 2D microfluidic network, the 3D microfluidic network does not show significant advantages.
3D-printing adds value by offering monolithic near-net-shape fabrication of complex structures. To truly revolutionize microfluidics, in addition to improving the printer resolution, 3D-printing must also improve its capability of multi-material, multiprocess, and multiscale printing to monolithically create highly integrated and multifunctional microfluidic devices for high-value biomedical applications.
Besides the microfluidic architecture, a fully functional bioanalytical microfluidic platform also includes sensing elements, solid substrate for molecule and cell adsorption, active actuators, and other components. In traditional lithography-based microfabrication, these components are usually fabricated separately and assembled manually at the chip bonding stage. Solid substrate and surface modification are usually introduced post-fabrication by packing additional materials (e.g., particles or gel matrix) in the microfluidic network or through in situ chemical reactions. At present, several inkjet-based 3D printers are able to concurrently print multiple materials. A rigid microfluidic device with flexible membranes as the pneumatic valve has been demonstrated using this approach[36]. Nevertheless, the number of materials that can be printed concurrently is small, and they can only be printed using the same process. The ultimate goal is to be able to print complex microfluidic devices with many types of materials, such as a rigid plastic microfluidic chip with flexible membranes, metal electrodes, hydrogel matrix, nanoparticle-packed beds, and magnetic composite actuators, all in one go.
To accomplish multi-material printing, multiple printing processes must be integrated to cope with different bonding mechanisms. While plastic materials can be printed by extruding molten plastic or crosslinking photopolymer resins with a low-energy light source, metal powders require a high-energy laser or electron beam to bond together. Furthermore, the material feeding mechanisms are also drastically different for different 3D-printing processes. In FDM, material is fed to the extruder in the form of filaments; in SLA, liquid-resin is kept in a reservoir and reflows after each layer is printed; in inkjet printers, liquid resins are feed to the printhead through a tubing; and in SLS and SLM, precursor materials in the form powders are loaded into a powder bed and spread by a roller after each layer is printed. These material bonding and feeding mechanisms are incompatible. To realize multiprocess printing, the partially printed parts need to be transferred between platforms, and the printing processes must have the ability to resume from the breakpoint. A technique known as the print-pause-print (PPP) is able to suspend the printing process for users to add prefabricated components (e.g., electrodes) to the partially printed parts and resume the printing from the breakpoint to embed these added parts within the 3D-printed microfluidic device[49]. This technique points out a possible direction for multiprocess 3D-printing. However, it does not address the challenges associated with the cross-platform transfer of the partially printed parts.
The quality of 3D-printed microfluidic devices can be significantly improved using ultrahigh-resolution 3D-printing technologies such as 2PP. However, it would be impractical to print the entire microfluidic device solely using 2PP due to the extremely slow printing speed. In many microfluidic devices, a large portion of the device body plays a structural rather than functional role, which means a big part of the device body can be printed with a fast and low-resolution process. Only the parts that form the microfluidic architectures need to be printed with a high-resolution process. These parts contain microscale structures that directly interact with the fluids; hence, their surface quality is more critical. Therefore, multiscale 3D-printing that provides both high-speed and high-resolution fabrication of microfluidic devices is highly coveted. The multiscale 3D-printing must be able to adjust the printing resolution and printing speed according to the required specifications.
3. Microfluidics for 3D-printing
The relationship between 3D-printing and microfluidics could go the other way around. Microfluidics could also serve as the enabler of 3D-printing technologies. Extrusion-based 3D-printing is one of the most popular technologies, especially in bioprinting. As the scope of bioprinting expands, the type of materials to be printed becomes more and more intricate. New applications often require printing multiphase and multicomponent materials that cannot be handled by the conventional extrusion printhead. Microfluidics, with its exceptional ability to manipulate a small amount of fluids, has been incorporated into the printhead to add a layer of fluidic control for sophisticated bioprinting.
3.1. Current Development in Microfluidics-enabled 3D-printing
As a matter of fact, microfluidic components are employed in inkjet 3D printer, such as MJF, to dispense liquid in the form of droplets through microfabricated nozzles. More complex microfluidics-enabled 3D-printing arises from the need to print hydrogel fibers with controlled gelation and composition. Early solutions employ coaxial flow to extrude hydrogel microfibers with cells encapsulated in the fiber core. Ozawa et al. created a coaxial flow system by cascading tapered capillary tubing[53]. The coaxial flow focused the cell suspension in the first capillary into the core of the fiber. The gel matrix precursor was injected from the second capillary to encapsulate the core flow. The gelling agent was introduced as the sheath flow from the third capillary, crosslinking the gel matrix and forming a coaxial fiber. Pancreatic β cells encapsulated in these microfibers maintained their viability and functions. Similar approaches were demonstrated by several other groups. Instead of cascaded capillary tubing, a manifold with two orthogonal inlets and a nozzle outlet as the printhead was used to couple the gel matrix precursor and the gelling agent into a coaxial flow. The printing of alginate hydrogel microfibers was demonstrated using this setup in which the cell-laden alginate was focused by the sheath flow containing Ca2+[54-56]. The manifold could be replaced by a microfluidic chip with the same configuration and function[57,58]. Capillary tubing or needles were inserted into the microfluidic channel to generate the coaxial flow.
Microfluidics has since moved beyond the simple coaxial flow. In addition to microchannels, various types of microfluidic components have been included in the printhead to achieve more sophisticated extrusion-based 3D-printing. Microfluidics is skilled at combining multiple flows from separate inlets into a single stream. Due to the low Reynolds number, materials stay in separate laminar layers in a single microfluidic channel; hence, different materials can be printed in close proximity. Colosi et al. fabricated a simple two-inlet microfluidic chip as the printhead (Figure 4A and B)[50]. Two separate bioinks were introduced into the microfluidic chip from the two inlets and combined into a single stream. Although extruded as a single hydrogel fiber, the two bioinks stay separate. A similar two-channel design was demonstrated by Hardin et al.[59] By adjusting the flow rate at the two inlets, seamless switching between different materials during printing was accomplished. Wei et al. demonstrated a multi-inlet microfluidic printhead[60]. Cells, hydrogel precursors, sacrificial material, and water were introduced into the microfluidic chip from separate inlets. These materials were hydrodynamically focused into a single outlet channel and extruded from the nozzle for bioprinting. By adjusting the relative flow rate at the inlets, the same printhead could print cell-laden solid hydrogel fiber, cell-laden hollow hydrogel fiber, and hollow double-layered hydrogel fiber. Leng et al. took a step further and developed a programmable multi-inlet microfluidic printhead[61]. The seven inlets for bioinks were individually controlled by solenoid valves. A base biopolymer was introduced into the printhead from a separate channel and extruded continuously from a wide nozzle, forming a polymer ribbon that served as a substrate on which the bioinks were deposited. The opening and closing periods of the solenoid valves determined the extrusion length of the bioink from each of the seven nozzles thus the patterns printed with the bioinks on the base biopolymer substrate. Using this approach, authors were able to print hydrogel sheets with well-controlled pores.
Figure 4.
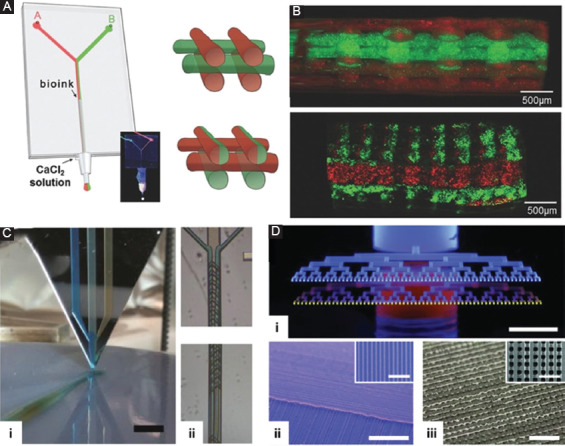
Microfluidics-enabled three-dimensional-printing. (A) Two-inlet microfluidic devices used as the printhead. Two bioinks are combined into a single micro hydrogel filament for extrusion. (B) Hydrogels printed using the microfluidic printhead shown in A. Each filament consists of two bioinks combined by the microfluidic printhead. (C) A three-inlet microfluidic printhead with passive mixer. (i) Schematic illustration of bioprinting with the microfluidic printhead. (ii) Herringbone passive mixer in the microfluidic printhead. (D) High-throughput parallel microfluidic printhead. (i) A high-degree of parallel printing with a bifurcating microfluidic network. (ii) and (iii) Microfilament arrays printed with the microfluidic printhead. A and B are reproduced from Ref. Colosi et al.[50] with permission from Wiley. C is reproduced from Ref. Serex et al.[51] with the permission granted under the creative common license. D is reproduced from Ref. Hansen et al.[52] with permission from Wiley.
Microfluidics is capable of keeping different bioinks in separate layers; even they are in close proximity. Nonetheless, in certain scenarios, it is desirable to blend multiple materials to create a multicomponent but homogeneous bioink. Fortunately, the mixing in microfluidics has been studied extensively. Numerous mixing strategies have been developed specifically for microfluidics. Designs of microfluidic mixers, both passive and active, have been incorporated into the extrusion printhead to homogenize multiple bioinks. Serex et al. developed a 3-inlet microfluidic printhead[51]. Materials from the inlets merged in the outlet channel. Various microfluidic components could be added to the outlet channel for different purposes. To promote the mixing, a herringbone structure was added to the surface of the outlet channel. As the materials traveled down the outlet channel, the herringbone induced chaotic mixing and homogenized the mixture before extruding it for printing (Figure 4C). Ober et al. studied a propeller-based active mixer for the printhead[62]. As materials from different inlets entered the mixing chamber, the propeller efficiently homogenized them in low volumes over a short timescale. Using this approach, the authors printed a structure with a fluorescent concentration gradient obtained by mixing inks at different ratios.
Microfluidics also enables a range of unique fluidic operations, which leads to unique 3D-printing strategies. Microfluidics is well known for its capability of high-degree parallelization which has been explored for high-throughput printing. Hansen et al. developed a printhead with a multi nozzle array for parallel printing (Figure 4D)[52]. Bioinks were introduced to the printhead from a single inlet which bifurcated several times, forming up to 64 outlet channels and nozzles. The bifurcating microfluidic network ensured that the extrusion rate at all nozzles was the same. This printhead could significantly improve the printing speed of tissue engineering scaffolds, which usually consisted of a large number of repetitive structures. Composite materials were often printed with multiphase inks composed a liquid-phase resin and solid-phase particles. Microfluidic components were added to the printhead to pre-condition the multiphase ink for printing. One such operation was to concentrate the particles. Serex et al. added a passive crossflow filter to the microfluidic printhead, which removed liquid from the ink as it moved toward the nozzle, leading to a high concentration of particles in the extruded ink[51]. The particle concentration could also be realized with an active concentrator. Collino et al. incorporated an acoustic wave generator to localize the particles in the microfluidic printhead[63]. When particles were localized to the center of the channel, liquid on both sides was removed by side channels, concentrating the particles to the central channel for printing. The same strategy was used to distribute particles along the print line. Particles with different morphologies would respond differently to the acoustic wave, hence were localized to different positions along the microfluidic channel. Droplet microfluidics was a special type of microfluidic system in which one of the liquids was sheared into the discrete volume by another liquid, resulting in a train of droplets in the microfluidic channel. Li et al. used a droplet microfluidic device as the printhead to print hydrogels with embedded liquid droplets[64]. The printhead used the resin to shear the aqueous solution into droplets. Authors demonstrated the printing of self-healing polymer using this approach. When damaged, the embedded droplets at the damaged surface released chemical agents to repair the fracture. Visser et al. also used droplet microfluidics for bioprinting[65]. Instead of generating droplets in a microfluidic channel, a piezo-actuated dispenser ejected droplets of hydrogel precursor in the air which later ran into a liquid stream of crosslinker that was also ejected in air. The hydrogel beads generated by the free-space droplet microfluidic system were used as the building block for bioprinting.
3.3. What is Next for Microfluidics-enabled 3D-printing?
The incorporation of microfluidic technology in 3D printing could potentially disrupt the current norm. Fluidic operations, such as mixing, sorting, and hydrodynamic focusing, can be further explored to promote the development of new 3D printers or even hybrid 3D-printing process.
Advances in microfluidics, particularly the development of new microfluidic modalities, would also bring new opportunities to 3D-printing. There are many types of microfluidic systems in addition to the conventional closed-channel microfluidics. In a way, 3D-printing is analogous to building construction. The current extrusion-based bioprinting is equivalent to pouring concrete on site. However, buildings could also be constructed with precast modular blocks so is 3D-printing. Instead of curing the ink in situ, inks can be pre-shaped into standard modular blocks, and the 3D construction is accomplished by moving these modular blocks to designated locations. Take digital microfluidics, for example, digital microfluidics manipulates discrete droplets on an open surface with a large degree of freedom. It provides an excellent tool to prefabricate discrete building blocks as well as a means to remotely actuate these building blocks. For example, magnetic digital microfluidics manipulates droplets by a magnet through the magnetic particles added to the droplet. It has the ability to move droplets across platforms in 3D with the assistance of surface modifications. Magnetic digital microfluidics could be applied to the manipulation of precast hydrogel blocks for 3D construction.
4. Conclusion and Future Perspective
In this work, we take a critical look at both 3D-printed microfluidics and microfluidics-enabled 3D-printing technology. Certain opinions might be a bit harsh, but our conclusion that neither field is established well enough to make an impact would stand. The capability of 3D-printed microfluidics has not surpassed its conventional counterpart. An alternative fabrication method is unlikely to make a disruptive advancement to a well-established field of microfluidics. Microfluidics-enabled 3D-printing brings more possibility to multimaterial and multiphase printing, but it is currently limited to extrusion-based 3D-printing and faces difficulty in extending to other 3D-printing modalities.
For 3D-printed microfluidics to make a real impact, new multimaterial, multiprocess and multiscale 3D-printing technologies must be developed to address the issues such as surface quality, fabrication speed, and multifunctionality. 3D-printed microfluidics will only be recognized as a field of its own if a multicomponent 3D microfluidic device for high-value biomedical applications can be monolithically fabricated within a reasonably short timeframe. This goal might be achieved by applying microfluidics-enabled 3D-printing to 3D-printed microfluidics, which would provide better control for multimaterial and multiphase printing. In any case, there is still a long way to go.
Acknowledgment
The author would like to thank the funding support from Nanyang Technological University (Start Up Grant), National Additive Manufacturing Innovation Cluster (NAMIC Singapore, 2017135), Ageing Research Institute for Society and Education (ARISE Singapore, ARISE/2017/22) and Singapore Ministry of Education (Tier 1, RG49/17). This research was conducted in collaboration with HP Inc. and supported/partially supported by the Singapore Government through the Industry Alignment Fund -Industry Collaboration Projects Grant.
References
- 1.Whitesides GM. The Origins and the Future of Microfluidics. Nature. 2006;442(7101):368. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell P. Microfluidics-downsizing Large-scale Biology. Nat Biotechnol. 2001;19(8):717. doi: 10.1038/90754. [DOI] [PubMed] [Google Scholar]
- 3.Yin H, Marshall D. Microfluidics for Single Cell Analysis. Curr Opin Biotechnol. 2012;23(1):110–9. doi: 10.1016/j.copbio.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Weibel DB, Whitesides GM. Applications of Microfluidics in Chemical Biology. Curr Opin Chem Biol. 2006;10(6):584–91. doi: 10.1016/j.cbpa.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Grayson ACR, Shawgo RS, Johnson AM, et al. A bioMEMS Review:MEMS Technology for Physiologically Integrated Devices. Proc IEEE. 2004;92(1):6–21. DOI 10.1109/jproc.2003.820534. [Google Scholar]
- 6.Ziaie B, Baldi A, Lei M, et al. Hard and Soft Micromachining for BioMEMS:Review of Techniques and Examples of Applications in Microfluidics and Drug Delivery. Adv Drug Deliv Rev. 2004;56(2):145–72. doi: 10.1016/j.addr.2003.09.001. DOI 10.1016/j.addr.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Shawgo RS, Grayson ACR, Li Y, et al. BioMEMS for Drug Delivery. Curr Opin Solid State Mater Sci. 2002;6(4):329–34. [Google Scholar]
- 8.Tay FE. Microfluidics and BioMEMS Applications. Berlin, Germany: Springer; 2002. [Google Scholar]
- 9.Bashir R. BioMEMS:State-of-the-art in Detection, Opportunities and Prospects. Adv Drug Deliv Rev. 2004;56(11):1565–86. doi: 10.1016/j.addr.2004.03.002. DOI 10.1016/j.addr.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Xia Y, Whitesides GM. Soft Lithography. Angew Chem Int Ed. 1998;37(5):550–75. doi: 10.1002/(SICI)1521-3773(19980316)37:5<550::AID-ANIE550>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 11.Unger MA, Chou HP, Thorsen T, et al. Monolithic Microfabricated Valves and Pumps by Multilayer Soft Lithography. Science. 2000;288(5463):113–6. doi: 10.1126/science.288.5463.113. DOI 10.1126/science.288.5463.113. [DOI] [PubMed] [Google Scholar]
- 12.Lecault V, White AK, Singhal A, et al. Microfluidic Single Cell Analysis:From Promise to Practice. Curr Opin Chem Biol. 2012;16(3-4):381–90. doi: 10.1016/j.cbpa.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 13.Kim P, Kwon KW, Park MC, et al. Soft Lithography for Microfluidics:A Review. Biochip J. 2008;2:1–11. [Google Scholar]
- 14.Beebe DJ, Mensing GA, Walker GM. Physics and Applications of Microfluidics in Biology. Ann Rev Biomed Eng. 2002;4(1):261–86. doi: 10.1146/annurev.bioeng.4.112601.125916. [DOI] [PubMed] [Google Scholar]
- 15.Minteer SD. Microfluidic Techniques:Reviews and Protocols. New York: Springer Science and Business Media; 2006. p. 321. [Google Scholar]
- 16.Au AK, Huynh W, Horowitz LF, et al. 3D-printed Microfluidics. Angew Chem Int Ed. 2016;55(12):3862–81. doi: 10.1002/anie.201504382. DOI 10.1002/anie.201504382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yazdi AA, Popma A, Wong W, et al. 3D Printing:An Emerging Tool for Novel Microfluidics and Lab-on-a-chip Applications. Microfluid Nanofluidics. 2016;20(3):50. DOI 10.1007/s10404-016-1715-4. [Google Scholar]
- 18.Waheed S, Cabot JM, Macdonald NP, et al. 3D Printed Microfluidic Devices:Enablers and Barriers. Lab Chip. 2016;16(11):1993–2013. doi: 10.1039/c6lc00284f. DOI 10.1039/c6lc00284f. [DOI] [PubMed] [Google Scholar]
- 19.Ho CMB, Ng SH, Li KHH, et al. 3D Printed Microfluidics for Biological Applications. Lab Chip. 2015;15(18):3627–37. doi: 10.1039/c5lc00685f. DOI 10.1039/c5lc00685f. [DOI] [PubMed] [Google Scholar]
- 20.Au AK, Bhattacharjee N, Horowitz LF, et al. 3D-printed Microfluidic Automation. Lab Chip. 2015;15(8):1934–41. doi: 10.1039/c5lc00126a. DOI 10.1039/c5lc00126a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhattacharjee N, Urrios A, Kang S, et al. The Upcoming 3D-printing Revolution in Microfluidics. Lab Chip. 2016;16(10):1720–42. doi: 10.1039/c6lc00163g. DOI 10.1039/c6lc00163g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donvito L, Galluccio L, Lombardo A, et al. Experimental Validation of a Simple, Low-cost, T-junction Droplet Generator Fabricated Through 3D Printing. J Micromech Microeng. 2015;25(3):035013. DOI 10.1088/0960-1317/25/3/035013. [Google Scholar]
- 23.Chen C, Wang Y, Lockwood SY, et al. 3D-printed Fluidic Devices Enable Quantitative Evaluation of Blood Components in Modified Storage Solutions for Use in Transfusion Medicine. Analyst. 2014;139(13):3219–26. doi: 10.1039/c3an02357e. DOI 10.1039/c3an02357e. [DOI] [PubMed] [Google Scholar]
- 24.Kitson PJ, Rosnes MH, Sans V, et al. Configurable 3D-Printed Millifluidic and Microfluidic 'Lab on a Chip'reactionware Devices. Lab Chip. 2012;12(18):3267–71. doi: 10.1039/c2lc40761b. DOI 10.1039/c2lc40761b. [DOI] [PubMed] [Google Scholar]
- 25.Bishop GW, Satterwhite JE, Bhakta S, et al. 3D-printed Fluidic Devices for Nanoparticle Preparation and Flow-injection Amperometry Using Integrated Prussian Blue Nanoparticle-modified Electrodes. Anal Chem. 2015;87(10):5437–43. doi: 10.1021/acs.analchem.5b00903. DOI 10.1021/acs.analchem.5b00903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takenaga S, Schneider B, Erbay E, et al. Fabrication of Biocompatible Lab-on-chip Devices for Biomedical Applications by Means of a 3D-printing Process. Physica Status Solidi A. 2015;212(6):1347–52. DOI 10.1002/pssa.201532053. [Google Scholar]
- 27.Lee W, Kwon D, Choi W, et al. 3D-printed Microfluidic Device for the Detection of Pathogenic Bacteria using Size-based Separation in Helical Channel with Trapezoid Cross-section. Sci Rep. 2015;5:7717. doi: 10.1038/srep07717. DOI 10.1038/srep09701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shallan AI, Smejkal P, Corban M, et al. Cost-effective Three-dimensional Printing of Visibly Transparent Microchips Within Minutes. Anal Chem. 2014;86(6):3124–30. doi: 10.1021/ac4041857. DOI 10.1021/ac4041857. [DOI] [PubMed] [Google Scholar]
- 29.Monaghan T, Harding MJ, Harris RA, et al. Customisable 3D Printed Microfluidics for Integrated Analysis and Optimisation. Lab Chip. 2016;16(17):3362–73. doi: 10.1039/c6lc00562d. DOI 10.1039/c6lc00562d. [DOI] [PubMed] [Google Scholar]
- 30.Cabot JM, Fuguet E, Rosés M, et al. Novel Instrument for Automated p K a Determination by Internal Standard Capillary Electrophoresis. Anal Chem. 2015;87(12):6165–72. doi: 10.1021/acs.analchem.5b00845. DOI 10.1021/acs.analchem.5b00845. [DOI] [PubMed] [Google Scholar]
- 31.Gelber MK, Bhargava R. Monolithic Multilayer Microfluidics via Sacrificial Molding of 3D-printed Isomalt. Lab Chip. 2015;15(7):1736–41. doi: 10.1039/c4lc01392a. DOI 10.1039/c4lc01392a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson KB, Lockwood SY, Martin RS, et al. A 3D Printed Fluidic Device that Enables Integrated Features. Anal Chem. 2013;85(12):5622–6. doi: 10.1021/ac4009594. DOI 10.1021/ac4009594. [DOI] [PubMed] [Google Scholar]
- 33.Au AK, Lee W, Folch A. Mail-order Microfluidics:Evaluation of Stereolithography for the Production of Microfluidic Devices. Lab Chip. 2014;14(7):1294–301. doi: 10.1039/c3lc51360b. DOI 10.1039/c3lc51360b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong H, Woolley AT, Nordin GP. High Density 3D Printed Microfluidic Valves, Pumps, and Multiplexers. Lab Chip. 2016;16(13):2450–8. doi: 10.1039/c6lc00565a. DOI 10.1039/c6lc00565a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogers CI, Qaderi K, Woolley AT, et al. 3D Printed Microfluidic Devices with Integrated Valves. Biomicrofluidics. 2015;9(1):016501. doi: 10.1063/1.4905840. DOI 10.1063/1.4905840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keating SJ, Gariboldi MI, Patrick WG, et al. 3D Printed Multimaterial Microfluidic Valve. PLoS One. 2016;11(8):e0160624. doi: 10.1371/journal.pone.0160624. DOI 10.1371/journal.pone.0160624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sochol R, Sweet E, Glick C, et al. 3D Printed Microfluidic Circuitry via Multijet-based Additive Manufacturing. Lab Chip. 2016;16(4):668–78. doi: 10.1039/c5lc01389e. DOI 10.1039/c5lc01389e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y, Chan HN, Michael SA, et al. A Microfluidic Circulatory System Integrated with Capillary-assisted Pressure Sensors. Lab Chip. 2017;17(4):653–62. doi: 10.1039/c6lc01427e. DOI 10.1039/c6lc01427e. [DOI] [PubMed] [Google Scholar]
- 39.Bhargava KC, Thompson B, Malmstadt N. Discrete Elements for 3D Microfluidics. Proc Natl Acad Sci. 2014;111(42):15013–8. doi: 10.1073/pnas.1414764111. DOI 10.1073/pnas.1414764111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee KG, Park KJ, Seok S, et al. 3D Printed Modules for Integrated Microfluidic Devices. RSC Adv. 2014;4(62):32876–80. DOI 10.1039/c4ra05072j. [Google Scholar]
- 41.Vittayarukskul K, Lee AP. A Truly Lego®-like Modular Microfluidics Platform. J Micromech Microeng. 2017;27(3):035004. DOI 10.1088/1361-6439/aa53ed. [Google Scholar]
- 42.Kirk CG. Toy Building Brick. Google Patents 1961 [Google Scholar]
- 43.Yuen PK. A Reconfigurable Stick-n-play Modular Microfluidic System using Magnetic Interconnects. Lab Chip. 2016;16(19):3700–7. doi: 10.1039/c6lc00741d. DOI 10.1039/c6lc00741d. [DOI] [PubMed] [Google Scholar]
- 44.Tumbleston JR, Shirvanyants D, Ermoshkin N, et al. Continuous Liquid Interface Production of 3D Objects. Science. 2015;347(6228):1349–52. doi: 10.1126/science.aaa2397. DOI 10.1126/science.aaa2397. [DOI] [PubMed] [Google Scholar]
- 45.Beauchamp MJ, Nordin GP, Woolley AT. Moving from Millifluidic to Truly Microfluidic sub-100-μm Cross-section 3D Printed Devices. Anal Bioanal Chem. 2017;409(18):4311–9. doi: 10.1007/s00216-017-0398-3. DOI 10.1007/s00216-017-0398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mazutis L, Gilbert J, Ung WL, et al. Single-cell Analysis and Sorting using Droplet-based Microfluidics. Nat Protoc. 2013;8(5):870. doi: 10.1038/nprot.2013.046. DOI 10.1038/nprot.2013.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.ASIGA. Available from: https://www.asiga.com/products/printers/pico . [Last retrieved on 2019 Jun 10]
- 48.Lee JM, Zhang M, Yeong WY. Characterization and Evaluation of 3D Printed Microfluidic Chip for Cell Processing. Microfluid Nanofluidics. 2016;20(1):5. DOI 10.1007/s10404-015-1688-8. [Google Scholar]
- 49.Li F, Macdonald NP, Guijt RM, et al. Increasing the Functionalities of 3D Printed Microchemical Devices by Single Material, Multimaterial, and Print-pause-print 3D Printing. Lab Chip. 2019;19(1):35–49. doi: 10.1039/c8lc00826d. DOI 10.1039/c8lc00826d. [DOI] [PubMed] [Google Scholar]
- 50.Colosi C, Shin SR, Manoharan V, et al. Microfluidic Bioprinting of Heterogeneous 3D Tissue Constructs using Low-viscosity Bioink. Adv Mater. 2016;28(4):677–84. doi: 10.1002/adma.201503310. DOI 10.1002/adma.201503310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Serex L, Bertsch A, Renaud P. Microfluidics:A New Layer of Control for Extrusion-based 3D Printing. Micromachines. 2018;9(2):86. doi: 10.3390/mi9020086. DOI 10.3390/mi9020086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hansen CJ, Saksena R, Kolesky DB, et al. High-throughput Printing via Microvascular Multinozzle Arrays. Adv Mater. 2013;25(1):96–102. doi: 10.1002/adma.201203321. DOI 10.1002/adma.201370002. [DOI] [PubMed] [Google Scholar]
- 53.Ozawa F, Okitsu T, Takeuchi S. Improvement in the Mechanical Properties of Cell-laden Hydrogel Microfibers using Interpenetrating Polymer Networks. ACS Biomater Sci Eng. 2017;3(3):392–8. doi: 10.1021/acsbiomaterials.6b00619. DOI 10.1021/acsbiomaterials.6b00619. [DOI] [PubMed] [Google Scholar]
- 54.Gao Q, He Y, Fu JZ, et al. Coaxial Nozzle-assisted 3D Bioprinting with Built-in Microchannels for Nutrients Delivery. Biomaterials. 2015;61:203–15. doi: 10.1016/j.biomaterials.2015.05.031. DOI 10.1016/j.biomaterials.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 55.Colosi C, Costantini M, Latini R, et al. Rapid Prototyping of Chitosan-coated Alginate Scaffolds through the use of a 3D Fiber Deposition Technique. J Mater Chem B. 2014;2(39):6779–91. doi: 10.1039/c4tb00732h. DOI 10.1039/c4tb00732h. [DOI] [PubMed] [Google Scholar]
- 56.Gao Q, Liu Z, Lin Z, et al. 3D Bioprinting of Vessel-like Structures with Multilevel Fluidic Channels. ACS Biomater Sci Eng. 2017;3(3):399–408. doi: 10.1021/acsbiomaterials.6b00643. DOI 10.1021/acsbiomaterials.6b00643. [DOI] [PubMed] [Google Scholar]
- 57.Attalla R, Ling C, Selvaganapathy P. Fabrication and Characterization of Gels with Integrated Channels using 3D Printing with Microfluidic Nozzle for Tissue Engineering Applications. Biomed Microdevices. 2016;18(1):17. doi: 10.1007/s10544-016-0042-6. DOI 10.1007/s10544-016-0042-6. [DOI] [PubMed] [Google Scholar]
- 58.Ghorbanian S, Qasaimeh MA, Akbari M, et al. Microfluidic Direct Writer with Integrated Declogging Mechanism for Fabricating Cell-laden Hydrogel Constructs. Biomed Microdevices. 2014;16(3):387–95. doi: 10.1007/s10544-014-9842-8. DOI 10.1007/s10544-014-9842-8. [DOI] [PubMed] [Google Scholar]
- 59.Hardin JO, Ober TJ, Valentine AD, et al. Microfluidic Printheads for Multimaterial 3D Printing of Viscoelastic Inks. Adv Mater. 2015;27(21):3279–84. doi: 10.1002/adma.201500222. DOI 10.1002/adma.201570145. [DOI] [PubMed] [Google Scholar]
- 60.Wei D, Sun J, Bolderson J, et al. Continuous Fabrication and Assembly of Spatial Cell-laden Fibers for a Tissue-like Construct via a Photolithographic-based Microfluidic Chip. ACS Appl Mater Interfaces. 2017;9(17):14606–17. doi: 10.1021/acsami.7b00078. DOI 10.1021/acsami.7b00078. [DOI] [PubMed] [Google Scholar]
- 61.Leng L, McAllister A, Zhang B, et al. Mosaic Hydrogels:One-step Formation of Multiscale Soft Materials. Adv Mater. 2012;24(27):3650–8. doi: 10.1002/adma.201201442. DOI 10.1002/adma.201290166. [DOI] [PubMed] [Google Scholar]
- 62.Ober TJ, Foresti D, Lewis JA. Active Mixing of Complex Fluids at the Microscale. Proc Natl Acad Sci. 2015;112(40):12293–8. doi: 10.1073/pnas.1509224112. DOI 10.1073/pnas.1509224112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Collino RR, Ray TR, Fleming RC, et al. Deposition of Ordered Two-phase Materials using Microfluidic Print Nozzles with Acoustic Focusing. Extreme Mech Lett. 2016;8:96–106. DOI 10.1016/j.eml.2016.04.003. [Google Scholar]
- 64.Li X, Zhang JM, Yi X, et al. Multimaterial Microfluidic 3D Printing of Textured Composites with Liquid Inclusions. Adv Sci. 2018;6:1800730. doi: 10.1002/advs.201800730. DOI 10.1002/advs.201800730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Visser CW, Kamperman T, Karbaat LP, et al. In-air Microfluidics Enables Rapid Fabrication of Emulsions, Suspensions, and 3D Modular (bio) Materials. Sci Adv. 2018;4(1):eaao1175. doi: 10.1126/sciadv.aao1175. DOI 10.1126/sciadv.aao1175. [DOI] [PMC free article] [PubMed] [Google Scholar]


