Abstract
A novel series of macrocyclic pyrazolo[1,5-a]pyrimidine derivatives as respiratory syncytial virus (RSV) fusion glycoprotein (F protein) inhibitors were designed and synthesized based on docking studies of acyclic inhibitors. This effort resulted in the discovery of several macrocyclic compounds, such as 12b, 12f, and 12h, with low nanomolar to subnanomolar activities against the wild-type RSV F protein A2. In addition, 12h showed a single-digit nanomolar potency against the previously reported drug-resistant mutant D486N. Molecular modeling and computational analyses suggested that 12h binds to the D486N mutant while maintaining a rigid bioactive conformation via macrocyclization and that it interacts with a hydrophobic cavity of the mutant using a new interaction surface of 12h. This report describes the rational design of macrocyclic compounds with dual inhibitory activities against wild-type and mutant RSV F proteins.
Keywords: Respiratory syncytial virus; RSV; fusion glycoprotein inhibitor; macrocyclization; pyrazolo[1,5-a]pyrimidine
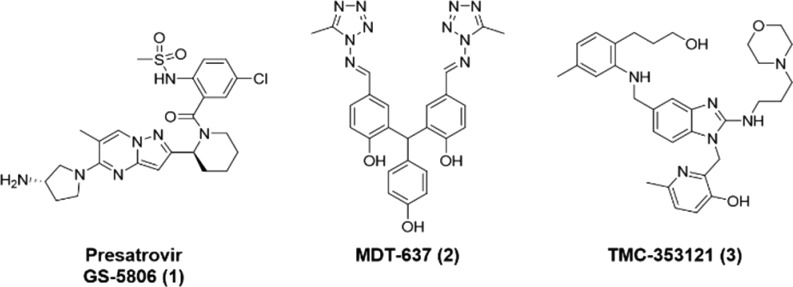
Respiratory syncytial virus (RSV) is a major cause of lower respiratory tract infections, such as atopic asthma, pneumonia, and severe bronchiolitis,1−7 particularly in infants and young children,8 in adults and elderly people with chronic respiratory conditions and heart disease, and in immunocompromised patients.9,10 RSV has spread throughout the world and repeatedly infects humans from early in life. A global systematic review estimated that 33.1 million RSV-related acute lower respiratory tract infection (RSV-ALRI) episodes resulted in about 3.2 million hospitalizations and 59,600 in-hospital deaths in 2015.11 The mortality rate associated with RSV infection is second to that of measles and higher than that of influenza.12 However, no effective treatment for RSV infection is currently available, aside from supportive care.13−16 Two drugs have been approved for the treatment of RSV: palivizumab (Synagis), which is a humanized monoclonal antibody that binds to fusion glycoprotein (F protein) and inhibits infection by blocking virus binding to or fusion with host cells, and ribavirin (Virazole), which is a synthetic guanosine nucleoside analogue and an antiviral agent with a broad range of antiviral and immunomodulatory effects on DNA or RNA viruses. However, the use of palivizumab is restricted to high-risk infants because of its high cost, and the use of ribavirin is limited because of concerns regarding efficacy and toxicity.13,14,17−27 Inhibitors of RSV F protein have been developed as a class of anti-RSV agents. RSV F protein inhibitors include several classes of compounds, such as pyrazolo[1,5-a]pyrimidines,28−31 benzimidazoles,32−34 and piperazinyl-quinolines.35 Among these compounds, presatovir (GS-5806, 1) showed potent anti-RSV effects in clinical trials and achieved proof-of-concept in human RSV challenge studies.28 (Figure 1). In clinical trials, several presatovir-resistant mutants of RSV F protein have been isolated.36 Major drug-resistant mutations have been detected in three regions: (1) a fusion peptide [amino acid (aa) residues 140–144]; (2) a cysteine-rich region (aa residues 392–401); and (3) heptad repeat B (aa residues 486–489).37 Among the known mutations, the D486N mutant has been isolated in several clinical trials of RSV F protein inhibitors including GS-5806 (1), and the D486N mutant has shown cross-resistance to compounds with various chemical structures such as MDT-637 (2) and TMC-353121 (3) (Figure 1).37 The emergence of drug- and cross-resistant mutants such as D486N is a potential concern to human health38 based on the prevalence of another RNA virus, the influenza virus (A/H1N1), 98% of which became resistant and active 12 years after the launch of the anti-influenza drug Tamiflu.39,40 Therefore, the development of effective RSV F protein inhibitors against the D486N mutant is urgently required before the global spread of mutant strains.
Figure 1.
RSV F protein inhibitors investigated in early stage clinical studies.
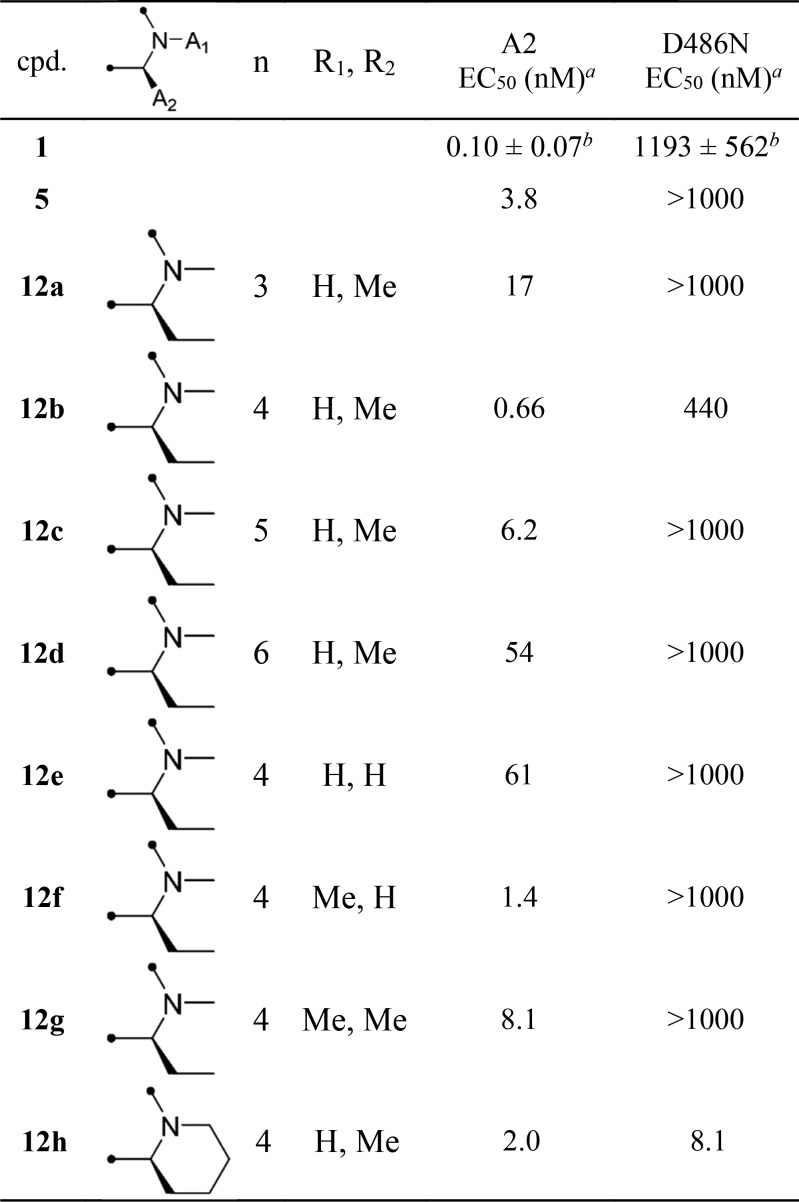
Molecular docking is a widely used computer simulation procedure for the study of protein–ligand interactions and for drug discovery and development. The structure of wild-type (A2) RSV F protein was experimentally reported in the Protein Data Bank (PDB) as PDB entry 5EA3. A molecular docking model of the A2 RSV F protein and 1 suggested the interaction of the hydrogen atom of a methylsulfonamide group and the pyrazolo[1,5-a]pyrimidine framework for the function of 1 in RSV F protein inhibition (Figure 2A).
Figure 2.
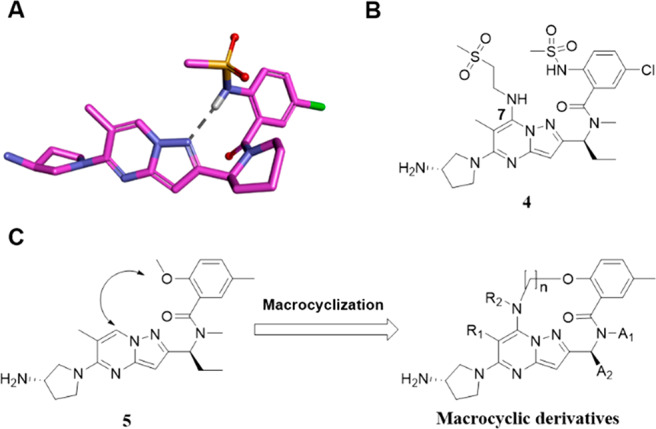
Macrocyclic strategy. (A) Conformation of 1 docked on RSV A2 F protein as predicted by a docking simulation, indicating that the 2-position of the benzoyl moiety and the 1- or 7-position of the pyrazolo[1,5-a]pyrimidine scaffold are in proximity to each other. (B) A compound with a substitution at the 7-position of pyrazolo[1,5-a]pyrimidine in our previous report.41 (C) Macrocyclic derivatives designed to mimic the binding conformation of 5 via macrocyclization.
The constrained conformation of 1 obtained by the docking simulation agreed well with the X-ray structure of 1 reported by Gilead.28 These data indicated that the conformation of 1 was critical for facilitating the interaction with RSV F protein resulting in the potent anti-RSV activity. The constrained conformation of 1 led to the idea of further stabilizing the active conformation of 1 to improve anti-RSV activity. Constraining a molecule in its active conformation by macrocyclization represents a powerful strategy for the rational design of functionally active compounds.
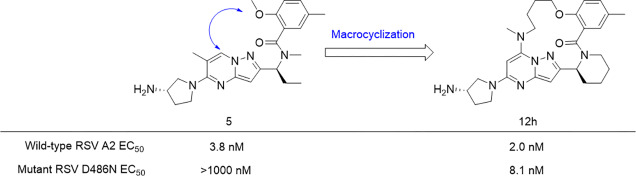
The structural framework of 1 calculated by the molecular docking study showed that the 2-position of the benzoyl moiety and the 1- or 7-position of the pyrazolo[1,5-a]pyrimidine scaffold were in proximity to each other. Previous studies revealed the possibility of a substitution at the 7-position of the pyrazolo[1,5-a]pyrimidine scaffold, as relatively large substituents were found to be well tolerated at the 7-position (Figure 2B).41,42 Therefore, a series of 14-, 15-, 16-, and 17-membered macrocyclic compounds were designed and synthesized by connecting the 2-position of the benzoyl moiety and the 7-position of the pyrazolo[1,5-a]pyrimidine scaffold (Figure 2C). Since the docking simulation of nonmacrocyclic pyrazolo[1,5-a]pyrimidine derivatives 1 and 5 (methylsulfonamido and methoxy at the 2-position of the benzoyl moiety, respectively) with RSV A2 F protein showed that the binding conformations of 1 and 5 were similar to each other, 5 was chosen as the lead compound for macrocyclization (Figure S1).
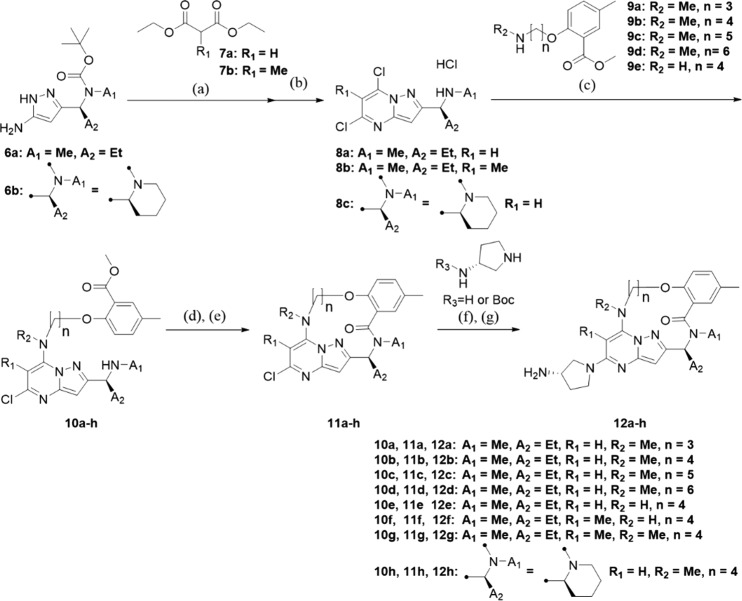
The general synthetic route of macrocyclic derivatives is shown in Scheme 1. The dihydropyrazolopyrimidine skeleton was synthesized by the reaction of aminopyrazole 6a–b with the appropriate diethylmalonate 7a–b under basic conditions. Deprotection of the Boc group and dichlorination were simultaneously accomplished by treatment with phosphorus oxychloride to obtain 8a–c. Intermediates 8a–c were aminated, and the resulting compounds 10a–h were then hydrolyzed and underwent intramolecular condensation reactions to yield 11a–h. Finally, the desired products 12a–h were synthesized by introducing an aminopyrrolidine moiety under microwave irradiation and basic conditions. The Boc group was deprotected under an acidic condition to afford 12a–h. The detailed procedures are described in the Supporting Information.
Scheme 1. General Synthetic Procedure for Macrocyclic Compounds.
Reagents and conditions: (a) NaOEt (2.94 M in EtOH), EtOH, 90 °C, 68% to quant.; (b) POCl3, 90-110 °C, 42% to quant.; (c) Et3N, EtOH, 70 °C, 42% to quant.; (d) 1 M NaOH aq, THF, iPrOH; (e) HATU, Et3N, DMF, rt, 2 steps, 16% to quant.; (f) Et3N, NMP, microwave, 150 °C, 27–98%; (g) 4 N HCl in dioxane, 1,4-dioxane, rt.
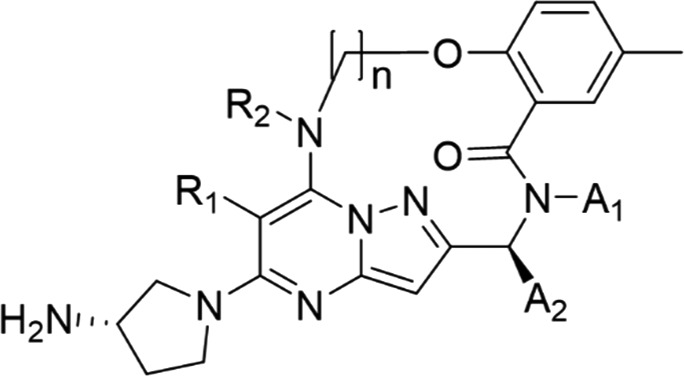
The inhibition of the cytopathic effect (CPE) induced by RSV infection was used to evaluate the antiviral activities of the synthesized compounds. Table 1 shows the anti-RSV activities of nonmacrocyclic compounds (1, 5) and macrocyclic compounds (12a–h) in wild-type (A2) and a resistant mutant (D486N). Macrocyclic compounds (12a–d) with a ring size of from 14 to 17 were synthesized to investigate the optimal ring size mimicking the constrained conformation obtained by the docking simulation of 1 and RSV F protein. An alkanolamine linker was selected for macrocyclization to simplify the synthetic route of the macrocyclic compounds. Among 12a–d, 12b with a ring size of 15 had the most potent anti-RSV activity, which was 5.8-fold more potent than that of the nonmacrocyclic compound 5.
Table 1. Structures and Antiviral Activities of Pyrazolo[1,5-a]pyrimidine Derivatives.
EC50 values of CPE inhibitory activities of all compounds were evaluated in HEp-2 cells infected with RSV A2 or D486N. The values represent the geometric means of three independent experiments (n = 3).
EC50 values reported by Gilead.37
In addition, 12b showed an increased activity against the D486N mutant by more than twice the activity levels observed for 1 and 5. Compounds 12b and 12c with a ring size of 15 and 16, respectively, showed moderate activities, but the activities of 12a and 12d with ring sizes of 14 and 17, respectively, were markedly attenuated. In the docking simulation of 12b with the A2 protein to determine its binding conformation, 12b bound to the protein in the same binding mode as 1 when superposed at the active site (Figure 3). The amino group of the aminopyrrolidine moiety in 12b formed a hydrogen bond with the carboxyl group of Asp486 in the A2 protein. The benzene ring and the pyrazolo[1,5-a]pyrimidine scaffold of 12b were restrained to be in the same plane as those of 1. These results suggested that 12b formed a three-dimensional structure when binding to the A2 protein, similar to that of 1, with a constrained conformation with an intramolecular hydrogen bonding motif. The macrocyclic ring in 12b appeared to stabilize the conformation responsible for the potent anti-RSV activity that was comparable to that of 1. Subsequently, substituents at the 6- and 7-positions (R1, R2) of the pyrazolo[1,5-a]pyrimidine scaffold were investigated using 12b with the optimal macrocycle ring size. Among the macrocyclic compounds with or without a methyl group at the 6- and 7-positions (12b, 12e–g), the combination of substituents in 12b (R1 = H, R2 = Me) had the most potent anti-RSV activity, while the opposite combination in 12f (R1 = Me, R2 = H) resulted in an attenuated activity [EC50 values: 0.66 nM (12b) and 1.4 nM (12f)]. A dimethylated analogue 12g (R1 = Me, R2 = Me) exhibited a single-digit nanomolar activity, which was moderately less potent than those of the monomethylated analogues (12b, 12f); however, a nonsubstituted analogue 12e (R1 = H, R2 = H) showed a markedly reduced activity [EC50 values: 8.1 nM (12g) and 61 nM (12e)]. The A2 protein has a hydrophobic pocket accommodating the R1 and R2 substituents of the pyrazolo[1,5-a]pyrimidine derivatives. Therefore, 12b and 12f with a monomethyl group appear to exhibit potent activity by efficiently occupying the hydrophobic pocket. In contrast, 12e with no methyl group showed decreased activity by the loss of the hydrophobic interaction. Compound 12g with a dimethyl group, which occupies the hydrophobic pocket, showed moderate activity, possibly due to the distorted macrocyclic conformation caused by steric repulsion between the two methyl groups.
Figure 3.
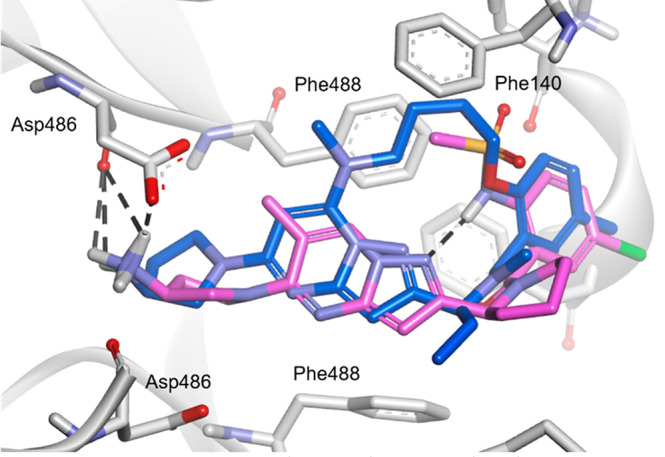
Docking model of 1 (magenta) and 12b (blue) binding to a 3-fold symmetric cavity in prefusion RSV A2 F protein (gray). Hydrogen bonds are depicted as dashed black lines.
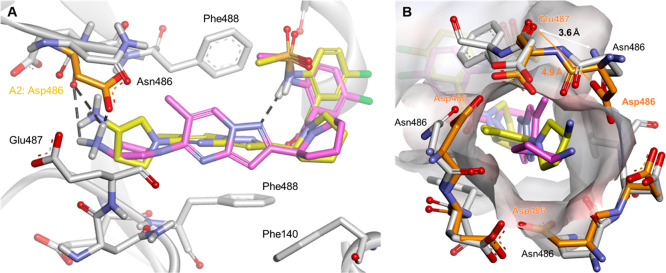
To investigate the effect of further restriction of the conformational variability of the ring structure on anti-RSV activity, an analogue with a piperidine ring (12h) in place of the 1-methyaminopropyl moiety (12b) was synthesized. The anti-RSV activity of 12h against the D486N mutant protein was dramatically improved by a factor of more that 100-fold, compared with the activities of nonmacrocyclic compounds (1, 5), while the activity of 12h against the A2 protein was slightly attenuated compared with the activity of 12b. The promising anti-RSV activity of 12h against the D486N mutant protein led us to perform a molecular docking simulation to predict the conformation of 12h when it interacts with the D486N mutant. The structure of the D486N mutant protein used for the simulation was created by manually correcting the coordinates from the structure of wild-type RSV A2 F protein (PDB entry 5EA3). When comparing the binding conformation of 1, which showed different anti-RSV activities against the RSV A2 F protein (EC50: 0.10 nM) and the D486N mutant (EC50: 1193 nM), the two docking models indicated that the aminopyrrolidine moiety of 1 interacts with different amino acid residues of the two proteins. The amino group of aminopyrrolidine is likely protonated and forms a Coulombic interaction with the carboxyl group of Asp486 in the A2 protein (Figure 4A, magenta). Conversely, in the D486N mutant, the amino group of the aminopyrrolidine moiety does not interact with the Asn486 residue but rather possesses a weak electrostatic interaction with the carboxyl group of the Glu487 residue, which is located at a deeper position (Figure 4A, yellow). Therefore, one of the likely reasons for the loss of the activity of 1 against the D486N mutant is that the hydrogen bond between the ionized aminopyrrolidine group and the Asp486 residue was broken and the binding mode of 1 was changed by the interaction of the aminopyrrolidine group with Glu487. With regard to the shape of the binding pocket for the aminopyrrolidine moiety of 1, the pocket in the D486N mutant was slightly narrower than that in the A2 protein, with a space created by three Asp486 residues of the F protein trimer. The distance between the main chain carbonyl group of Glu487 and the carboxyl group of Asp486 was 4.9 Å in the A2 protein (Figure 4B, orange), while the distance between the main chain carbonyl group of Glu487 and the carbamoyl group of Asn486 was 3.6 Å in the D486N mutant (Figure 4B, gray). The structure models of A2 and the D486N mutant demonstrated that the D486N mutation likely reduces electrostatic repulsion between the two amino acid residues at positions 486 and 487. Compound 1 interacts weakly with the D486N mutant via a different binding mode from that used to bind with the A2 protein, presumably because of steric hindrance at the binding site in the D486N mutant. In particular, the pyrazolo[1,5-a]pyrimidine moiety of 1 is highly twisted, thereby preventing interference between the methyl group at the 6-position of pyrazolo[1,5-a]pyrimidine and the binding pocket of the D486N mutant. Furthermore, the conformational flexibility of 1 increases because of the loss of intramolecular hydrogen-bonding. Therefore, the reason for the weak activity of 1 against the D486N mutant can be explained well by the distinct conformational preferences of 1 when interacting with the proteins.
Figure 4.
(A) Docking model of 1 (yellow) binding to the RSV D486N F protein (gray) superposed with 1 (magenta) binding to the RSV A2 (orange). Hydrogen bonds are depicted as dashed black lines. (B) Aminopyrrolidine moiety docking site and surrounding residues of the D486N (gray) superposed with those of the A2 protein (orange). The surface of the D486N protein is shown in transparent gray.
When comparing the binding conformation of 12h, which showed potent anti-RSV activities against the A2 protein (EC50: 2.0 nM) and the D486N mutant (EC50: 8.1 nM), the two docking models indicated that the docking poses of 12h with the A2 protein and D486N were similar to each other, unlike in the case of 1 (Figures 4 and 5). The overall conformation of 12h does not change markedly upon binding to the D486N mutant, probably because of the effect of macrocyclization, which facilitates the accessibility of the aminopyrrolidine moiety to the narrow binding pocket of the D486N mutant protein. In particular, the pyrazolo[1,5-a]pyrimidine moiety of 12h is located at almost the same position in the binding pockets of both proteins. These results suggest that the active conformation of 12h is maintained well by macrocyclization, enabling the potent anti-RSV activities against the A2 and D486N mutant proteins. The improved activity of 12h against the D486N mutant can be attributed to the introduction of a linker moiety for interaction with the protein. The hydrocarbon chain of the linker appears to promote the hydrophobic interaction with Phe140 and Phe488 in the D486N mutant (Figure 5, blue). The binding poses of the linker moiety of 12h in the A2 and D486N mutant proteins were slightly different. In the binding pose of 12h to the A2, the carbon atoms in the linker moiety are slightly distant from the Phe140 residue for the CH−π interaction of 12h with Phe140 (Figure 5, light blue); therefore, it is presumed that the activity of 12h against the A2 was not improved. Macrocyclization appears to provide an adequate geometrical fit of 12h in the binding pocket of the D486N mutant to allow a potent anti-RSV activity in terms of molecular conformation and hydrophobic interaction. The effect of the piperidine ring on the markedly increased activity of 12h for the D486N mutant compared to that of 12b with an acyclic amine has not been well explained by the docking models in this study. One possible explanation is that the replacement of the acyclic amine in 12b with the piperidine ring further increases the conformational constraint of the molecule and results in an active conformation. Another possible explanation is that the substituent at the 5-position on the benzene ring of 12h is oriented more appropriately for the interaction with the D486N mutant compared to that of 12b. The mobility of the benzoyl group of 12h seems to be decreased compared to that of 12b, which enables the substituent at the 5-position on the benzene ring to adopt a preferred orientation. Although a crystal of the complex of 12h and the D486N mutant suitable for X-ray analysis has not been obtained to investigate the role of the 5-position substituent, it has been found that substituents at the 5-position considerably affected the activity against the D486N mutant (data not shown). In future studies, the cocrystal structures of macrocyclic derivatives complexed with the A2 and D486N proteins could help to elucidate the structural and functional relationships further.
Figure 5.
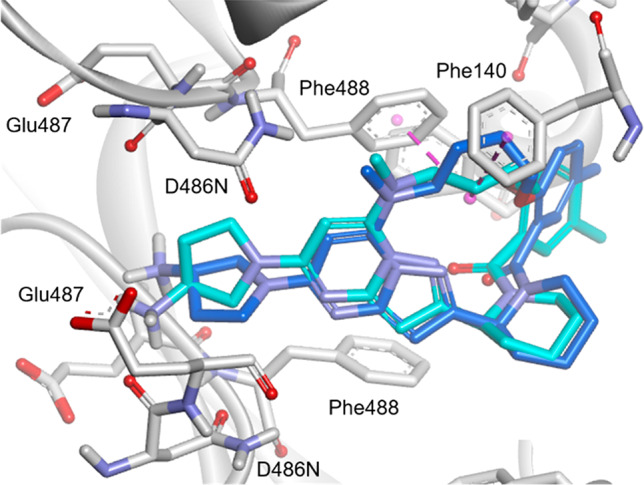
Docking model of 12h (blue) binding to the RSV D486N F protein (gray) superposed with 12h (light blue) binding to the A2 protein. CH−π interactions are depicted as dashed pink lines.
In summary, we designed and synthesized a new series of macrocyclic pyrazolo[1,5-a]pyrimidine derivatives as RSV F protein inhibitors based on a molecular docking study. Among the macrocyclic derivatives in which the pyrazolo[1,5-a]pyrimidine moiety was covalently linked to the aryl moiety via a linker, 12b with a 15-membered macrocyclic ring showed the most potent activity against the wild-type RSV A2, with a 5.8-fold more potent activity than that of 5, which has no macrocyclic constraints. Furthermore, 12h also exhibited a potent effect against the clinically reported drug-resistant mutant D486N. To our knowledge, 12h is the first small molecule RSV F protein inhibitor that has been shown to be effective against the D486N mutant. A docking study of 12h with the D486N mutant suggested that macrocyclization constrains the compound structure, locking it in a bioactive conformation, and creates a new interaction surface that promotes the hydrophobic interaction of the linker moiety. The rational design and facile synthesis of macrocyclic pyrazolo[1,5-a]pyrimidine derivatives are of great value in developing new drugs with potent dual anti-RSV activities against not only wild-type but also F protein mutants. Currently, further optimization study is in progress.
Acknowledgments
The authors thank Taiji Asami for carefully reading the manuscript and for making useful suggestions for its improvement; Takashi Yoshizumi and Susumu Yamanobe for helpful discussions; and Akira Higuchi for analytical and spectral studies.
Glossary
Abbreviations
- RSV
respiratory syncytial virus
- F protein
fusion glycoprotein
- aa
amino acid
- PDB
protein data bank
- HATU
1-[bis(dimethylamino)methyliumyl]-1H-1,2,3-triazolo[4,5-b]pyridine-3-oxide hexafluorophosphate
- CPE
cytopathic effect
- EC50
half maximal effective concentration
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.0c00008.
Synthetic procedure, computational details, and biological assay protocols (PDF)
Author Contributions
The manuscript was written through contributions from all the authors. All the authors approved the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Sigurs N.; Bjarnason R.; Sigurbergsson F.; Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am. J. Respir. Crit. Care Med. 2000, 161, 1501–1507. 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- Sigurs N.; Aljassim F.; Kjellman B.; Robinson P. D.; Sigurbergsson F.; Bjarnason R.; Gustafsson P. M. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax 2010, 65, 1045–1052. 10.1136/thx.2009.121582. [DOI] [PubMed] [Google Scholar]
- Sigurs N.; Gustafsson P. M.; Bjarnason R.; Lundberg F.; Schmidt S.; Sigurbergsson F.; Kjellman B. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am. J. Respir. Crit. Care Med. 2005, 171, 137–141. 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- Stein R. T.; Sherrill D.; Morgan W. J.; Holberg C. J.; Halonen M.; Taussig L. M.; Wright A. L.; Martinez F. D. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 1999, 354, 541–545. 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- Bont L.; Steijn M.; van Aalderen W. M.; Kimpen J. L. Impact of wheezing after respiratory syncytial virus infection on health-related quality of life. Pediatr. Infect. Dis. J. 2004, 23, 414–417. 10.1097/01.inf.0000122604.32137.29. [DOI] [PubMed] [Google Scholar]
- Henderson J.; Hilliard T. N.; Sherriff A.; Stalker D.; Al Shammari N.; Thomas H. M. Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: a longitudinal birth cohort study. Pediatr. Allergy Immunol. 2005, 16, 386–392. 10.1111/j.1399-3038.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- Nair H.; Nokes D. J.; Gessner B. D.; Dherani M.; Madhi S. A.; Singleton R. J.; O’Brien K. L.; Roca A.; Wright P. F.; Bruce N.; et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010, 375, 1545–1555. 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano R.; Naghavi M.; Foreman K.; Lim S.; Shibuya K.; Aboyans V.; Abraham J.; Adair T.; Aggarwal R.; Ahn S. Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resch B.; Manzoni P.; Lanari M. Severe respiratory syncytial virus (RSV) infection in infants with neuromuscular diseases and immune deficiency syndromes. Paediatr. Respir. Rev. 2009, 10, 148–153. 10.1016/j.prrv.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Hall C. B.; Powell K. R.; MacDonald N. E.; Gala C. L.; Menegus M. E.; Suffin S. C.; Cohen H. J. Respiratory syncytial viral infection in children with compromised immune function. N. Engl. J. Med. 1986, 315, 77–81. 10.1056/NEJM198607103150201. [DOI] [PubMed] [Google Scholar]
- Shi T.; McAllister D. A.; O’Brien K. L.; Simoes E. A. F.; Madhi S. A.; Gessner B. D.; Polack F. P.; Balsells E.; Acacio S.; Aguayo C.; et al. RSV Global Epidemiology Network, Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017, 390, 946–958. 10.1016/S0140-6736(17)30938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. A.; McMichael A. J. Social and environmental risk factors in the emergence of infectious diseases. Nat. Med. 2004, 10, S70–76. 10.1038/nm1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. K.; Seales S.; Budzik C. Respiratory syncytial virus bronchiolitis in children. Am. Fam. Physician. 2017, 95, 94–99. [PubMed] [Google Scholar]
- Kou M.; Hwang V.; Ramkellawan N. Bronchiolitis: from practice guideline to clinical practice. Emerg. Med. Clin. North. Am. 2018, 36, 275–286. 10.1016/j.emc.2017.12.006. [DOI] [PubMed] [Google Scholar]
- Offer I.; Ashkenazi S.; Livni G.; Shalit I. The diagnostic and therapeutic approach to acute bronchiolitis in hospitalized children in Israel: a nationwide survey. Isr. Med. Assoc. J. 2000, 2, 108–110. [PubMed] [Google Scholar]
- Livni G.; Rachmel A.; Marom D.; Yaari A.; Tirosh N.; Ashkenazi S. A randomized, double-blind study examining the comparative efficacies and safety of inhaled epinephrine and nasal decongestant in hospitalized infants with acute bronchiolitis. Pediatr. Infect. Dis. J. 2010, 29, 71–73. 10.1097/INF.0b013e3181b0602e. [DOI] [PubMed] [Google Scholar]
- Meissner H. C. Viral bronchiolitis in children. N. Engl. J. Med. 2016, 374, 62–72. 10.1056/NEJMra1413456. [DOI] [PubMed] [Google Scholar]
- Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The impact-RSV study group. Pediatrics 1998, 102, 531–537. 10.1542/peds.102.3.531 [DOI] [PubMed] [Google Scholar]
- Wu P.; Escobar G. J.; Gebretsadik T.; Carroll K. N.; Li S. X.; Walsh E. M.; Mitchel E. F.; Sloan C.; Dupont W. D.; Yu C.; et al. Effectiveness of respiratory syncytial virus immunoprophylaxis on bronchiolitis hospitalizations among high-risk infants. Am. J. Epidemiol. 2018, 187, 1490–1500. 10.1093/aje/kwy008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obando-Pacheco P.; Justicia-Grande A. J.; Rivero-Calle I.; Rodríguez-Tenreiro C.; Sly P.; Ramilo O.; Mejías A.; Baraldi E.; Papadopoulos N. G.; Nair H.; et al. Respiratory syncytial virus seasonality: A global overview. J. Infect. Dis. 2018, 217, 1356–1364. 10.1093/infdis/jiy056. [DOI] [PubMed] [Google Scholar]
- Revankar G. R.; Robins R. K. Synthesis and biological activity of some nucleosides resembling guanosine: imidazo(1,2-α)pyrimidine nucleosides. Ann. N. Y. Acad. Sci. 1975, 255, 166–176. 10.1111/j.1749-6632.1975.tb29221.x. [DOI] [PubMed] [Google Scholar]
- Sidwell R. W.; Huffman J. H.; Khare G. P.; Allen L. B.; Witkowski J. T.; Robins R. K. Broad-spectrum antiviral activity of virazole: 1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science 1972, 177, 705–706. 10.1126/science.177.4050.705. [DOI] [PubMed] [Google Scholar]
- Hall C. B.; Walsh E. E.; Hruska J. F.; Betts R. F.; Hall W. J. Ribavirin treatment of experimental respiratory syncytial viral infection. A controlled double-blind study in young adults. JAMA 1983, 249, 2666–2670. 10.1001/jama.1983.03330430042027. [DOI] [PubMed] [Google Scholar]
- Streeter D. G.; Witkowski J. T.; Khare G. P.; Sidwell R. W.; Bauer R. J.; Robins R. K.; Simon L. N. Mechanism of action of 1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide (Virazole), a new broad-spectrum antiviral agent. Proc. Natl. Acad. Sci. U. S. A. 1973, 70, 1174–1178. 10.1073/pnas.70.4.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmit S. E.; Moler F. W.; Monto A. S.; Khan A. S. Ribavirin utilization and clinical effectiveness in children hospitalized with respiratory syncytial virus infection. J. Clin. Epidemiol. 1996, 49, 963–967. 10.1016/0895-4356(96)00137-0. [DOI] [PubMed] [Google Scholar]
- Graci J. D.; Cameron C. E. Mechanisms of action of ribavirin against distinct viruses. Rev. Med. Virol. 2006, 16, 37–48. 10.1002/rmv.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultgren C.; Milich D. R.; Weiland O.; Sällberg M. The antiviral compound ribavirin modulates the T helper (Th) 1/Th2 subset balance in hepatitis B and C virus-specific immune responses. J. Gen. Virol. 1998, 79, 2381–2391. 10.1099/0022-1317-79-10-2381. [DOI] [PubMed] [Google Scholar]
- Mackman R. L.; Sangi M.; Sperandio D.; Parrish J. P.; Eisenberg E.; Perron M.; Hui H.; Zhang L.; Siegel D.; Yang H.; et al. Discovery of an oral respiratory syncytial virus (RSV) fusion inhibitor (GS-5806) and clinical proof of concept in a human RSV challenge study. J. Med. Chem. 2015, 58, 1630–1643. 10.1021/jm5017768. [DOI] [PubMed] [Google Scholar]
- Babaoglu K.; Eisenberg E. J.; Machkman R. L.; Sangi M.; Siegel D.; Yang H.. Pyrazolo[1,5-a]pyrimidines as antiviral agents. World Intellectual Property Organization, WO/2011/163518, 2011.
- Tahri A.; Vendeville S. M. H.; Jonckers T. H. M.; Raboisson P. J. M.; Demin S. D.; Hu L.; Cooymans L. P.. Piperidine Substituted Pyrazolo[1,5-a]pyrimidine Derivatives with Inhibitory Activity on the Replication of the Respiratory Syncytial Virus (RSV). World Intellectual Property Organization, WO/2016/091774, 2016.
- Tahri A.; Vendeville S. M. H.; Jonckers T. H. M.; Raboisson P. J. M.; Demin S. D.; Hu L.. Piperidine Substituted Tricyclic Pyrazolo[1,5-a]pyrimidine Derivatives with Inhibitory Activity on the Replication of the Respiratory Syncytial Virus (RSV). World Intellectual Property Organization, WO/2016/091791, 2016.
- Shi W.; Jiang Z.; He H.; Xiao F.; Lin F.; Sun Y.; Hou L.; Shen L.; Han L.; Zeng M.; et al. Discovery of 3,3′-spiro[azetidine]-2-oxoindoline derivatives as fusion inhibitors for treatment of RSV infection. ACS Med. Chem. Lett. 2018, 9, 94–97. 10.1021/acsmedchemlett.7b00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S.; Hong D.; Wang B.; Zheng X.; Miao K.; Wang L.; Yun H.; Gao L.; Zhao S.; Shen H. C. Discovery of imidazopyridine derivatives as highly potent respiratory syncytial virus fusion inhibitors. ACS Med. Chem. Lett. 2015, 6, 359–362. 10.1021/acsmedchemlett.5b00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S.; Li C.; Chen D.; Zheng X.; Yun H.; Gao L.; Shen H. C. Discovery of methylsulfonyl indazoles as potent and orally active respiratory syncytial virus (RSV) fusion inhibitors. Eur. J. Med. Chem. 2017, 138, 1147–1157. 10.1016/j.ejmech.2017.07.032. [DOI] [PubMed] [Google Scholar]
- Zheng X.; Wang L.; Wang B.; Miao K.; Xiang K.; Feng S.; Gao L.; Shen H. C.; Yun H. Discovery of piperazinylquinoline derivatives as novel respiratory syncytial virus fusion inhibitors. ACS Med. Chem. Lett. 2016, 7, 558–562. 10.1021/acsmedchemlett.5b00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan R.; Stray K.; Anderson F.; Perron M.; Mackman R.; Svarovskaia E.; et al. Analysis of Presatovir (GS-5806) Resistance Emergence in Human Healthy Adult Subjects Experimentally Infected with Respiratory Syncytial Virus. IDWeek, October 7–11, San Diego, CA, 2015. [Google Scholar]
- Perron M.; Stray K.; Kinkade A.; Theodore D.; Lee G.; Eisenberg E.; Sangi M.; Gilbert B. E.; Jordan R.; Piedra P. A.; et al. GS-5806 inhibits a broad range of respiratory syncytial virus clinical isolates by blocking the virus-cell fusion process. Antimicrob. Agents Chemother. 2016, 60, 1264–1273. 10.1128/AAC.01497-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D.; Lee S.; Thakkar D. V.; Luo M.; Moore L. M.; Plemper K. R. Cross-resistance mechanism of respiratory syncytial virus against structurally diverse entry inhibitors. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, e3441–3449. 10.1073/pnas.1405198111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M.; Galvin H. D.; Haw T. Y.; Nutsford A. N.; Husain M. Drug resistance in influenza A virus: the epidemiology and management. Infect. Drug Resist. 2017, 10, 121–134. 10.2147/IDR.S105473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscona A. Global Transmission of Oseltamivir-Resistant Influenza. N. Engl. J. Med. 2009, 360, 953–956. 10.1056/NEJMp0900648. [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Sasaki T.; Tamura Y.; Ogata Y.; Kawaguchi T.; Kurosaka J.; Sugaya Y.; Iwakiri K.; Takahashi R.; Sugiyama H.; Kanuma K. Design and Synthesis of 2-(1-Alkylaminoalkyl)pyrazolo[1,5-a]pyrimidines as New Respiratory Syncytial Virus Fusion Protein Inhibitors. Chem. Pharm. Bull. 2020, 68, 345–362. 10.1248/cpb.c19-00895. [DOI] [PubMed] [Google Scholar]
- Kanuma K.; Kawaguchi T.; Kurosaka J.; Yamaguchi T.; Ogata Y.; Iwakiri K.. Pyrazolo[1,5-a]pyrimidine Compound. World Intellectual Property Organization, WO/2016/148145, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.