Abstract
Purpose
Mulberry leaf extract has exerted better antidiabetic activities, while the effects of major active components in mulberry leaf extract are still unclear. Cryptochlorogenic acid (CCA) as the major active component in mulberry leaf extracts was investigated herein.
Materials and Methods
Rats were treated with 50mg/kg streptozotocin for the establishment of diabetic model in vivo, and cells were treated with 33.3 mM glucose for the establishment of cell model in vitro. HE staining assay was performed for observation of pancreatic pathology and aldehyde fuchsin staining assay for examining islet cell numbers. The iron content was detected via Perls staining assay with iron assay kit (ab83366). The malondialdehyde (MDA), glutathione (GSH) and oxidized glutathione (GSSG) were detected by corresponding kits. Real-time quantitative polymerase chain reaction (RT-qPCR) was performed for assessment of gene level and Western blot for measurement of protein expression level. The cell survival was detected via CCK-8 assay.
Results
The blood glucose level, iron content, accumulation of lipid peroxides and islet injury in diabetic model were all improved by CCA via a concentration-dependent manner. CCA functions via inhibition of ferroptosis by activation of cystine/glutamate transporter system (XC−)/glutathione peroxidase 4(GPX4)/Nrf2 and inhibition of nuclear receptor coactivator 4 (NCOA4) in diabetes.
Conclusion
CCA exerted excellent antidiabetic effects via inhibition of ferroptosis, so it may be a promising agent for diabetes therapy, providing a new avenue for diabetes treatment.
Keywords: cryptochlorogenic acid, diabetes, XC-/GPX4, ferroptosis, Nrf2, NCOA4
Introduction
Diabetes with high prevalence has become the heavy burden, threatening people’s health worldwide. By 2019, the prevalence for diabetes has been over four hundred million as presented by the federation statistics of international diabetes, and it is still in an uptrend.1 It is known to all that chronic diabetes could cause many complications such as retinopathy, kidney disease, diabetes foot, heart disease and so forth.2–6 Therefore, the timely treatment is extremely important.
There are two types of diabetes: type I diabetes and Type II diabetes. Impairment of islet β cells along with insufficient insulin secretion leads to type I diabetes. Simultaneously, insulin resistance is the main cause of type II diabetes.7 Therefore, it is valuable for us to screen the effective drug that has therapeutic effects on islet β cells dysfunction and insulin resistance.
Oxidative stress has been confirmed as one of the major causes of destroyed insulin secretion, insulin resistance and glucose utilization, contributing to the propagation of diabetes.8 Cystine/glutamate transporter system (XC−)/glutathione peroxidase-4 (GPX4) is a vital anti-oxidant system. XC-system, which is composed of SLC7A11 and SLC3A2L subunits, functions as a provider of GSH biosynthesis, and GPX4 as GSH-dependent enzyme could decrease the level of reactive oxygen species in cells. The disorder of this axis could lead to ferroptosis finally.9 Ferroptosis is peroxidation-induced non-apoptotic cell death in ROS or iron-dependent manner, which is a new found cell death avenue.10 Inhibition of ferroptosis is reported to exert protective effect on myocardial ischemia/reperfusion injury in diabetes.11 In addition, the antioxidant defense of islets is weaker than other tissues, making it susceptible to the regulatory abnormality of ROS generation.12,13 The islet function and islet viability are also reported to be compromised by ferroptosis.14 Therefore, ferroptosis is a vital target in diabetes.
In diabetes, high glucose is the final manifestation either caused by insufficient insulin secretion or insulin resistance. High glucose is able to influence the functions of SLC7A11 and SLC3A2L, which results in XC-/GPX4 disorder.15 Glucose metabolism is closely related to iron metabolism.16–18 High glucose could cause overload of iron, which leads to ferroptosis finally.19 Furthermore, overload of iron is capable of giving rise to insulin resistance.20 Ferroptosis functions via an iron-dependent manner. Thus, ferroptosis is a vital therapeutic target in diabetes.
Many Chinese herbs extracts have exerted varieties of excellent bioactivities and the exacts of mulberry leaf have exerted efficient hypoglycemic effect.21–23 The extracts of Mulberry leaf are confirmed to have regulatory effects on glycolipid metabolic abnormalities.24 In addition, the extracts of mulberry leaf also exerted attenuative effects on insulin resistance and inflammation.25,26 While the effect and mechanism of major active constituents of mulberry leaf in diabetes is still not clear. Cryptochlorogenic acid (CCA) is an active compound in mulberry leaf, and its effects and mechanism in diabetes remain unknown. Therefore, in this study, we first explored the effects of cryptochlorogenic acid and further investigated its mechanism in diabetes.
Materials and Methods
The Establishment of Animal Models with Diabetes and Treatment
Sixty Sprague-Dawley (SD) rats with weights ranging from 250–270 g were obtained from experimental animal center of Xiamen university. The animal experiment was approved by the Xiamen hospital of traditional Chinese medicine and this study is in line with the guidelines for laboratory animal welfare of Xiamen hospital of traditional Chinese medicine and “3R” principle. All the rats were housed in the environment with relative humidity of 50–60%, at the constant temperature (22 ± 2) °C and had access to the food and water freely before experiment. For diabetes model group, fasting was performed for 12h before experiment. The rats (ten rats per group) were assigned into Control group, Model (DM) treated with 50mg/kg streptozotocin (STZ) via abdominal injection, positive control group and experimental groups. The blood glucose level, which is served as the indicator for the diabetes, was monitored herein. The glucose level after modeling is above 16.7mmol/l, supporting that the modeling is successful. Then, the rats were treated with different concentrations of CCA (Sifeng biotechnology co. LTD, Hefei, China, 98%) via intragastric administration. For positive control group, the diabetes models were treated with 3mg/kg rosiglitazone (RH) via intragastric administration. For experimental groups, the diabetes models in CCA-low group were treated with 15mg/kg CCA, and those in CCA-mid group were treated with 30mg/kg CCA, while those in CCA-high group were treated with 60mg/kg CCA. The CCA and RH were treated for one and two weeks and then variables indexes were measured at one week or two weeks.
Cell Model of Diabetes
The INS-1 cells (1×105 cells/well) (Xinzhou Biotechnology Company, Shanghai, China) were seeded in 96-well plates in the DEME medium with 10%FBS. The cells were assigned into control (Con) group treated with 5 mM glucose, high glucose (HG) group treated with 50mM glucose, and experimental groups. For experimental groups, the cells induced by high glucose were treated with different concentrations of CCA (10 μM, 25 μM and 50 μM), including CCA-low group, CCA-mid group and CCA-high group, for 24h.
HE Staining Assay
The rats from different groups were sacrificed. Then the pancreatic tissues were taken out and washed. After desiccation, the tissues were cut into pieces and immersed in the 4% paraformaldehyde for fixation. After rinsing and dehydration with gradient ethanol, the tissues were made into sections. After dewaxing and hydration, the sections were stained with haematoxylin for 10min and after rinse, eosin was added for 1min. Neutral glue was used to seal the sections. Optical microscope was used for observation of histopathological condition.
Aldehyde Fuchsin Staining Assay
Aldehyde fuchsin staining assay was performed according to the previous literature.27 Briefly, after dewaxing and rehydration, the sections were oxidized by Lugol’s iodine. Then sodium thiosulphate was used for decoloration. Gormori’s aldehyde fuchsin solution and orange G, which are prepared as described in the literature, were used to stain the sections.27
Measurement of Iron Content
The Iron Assay kit (ab83366) was used for detection of iron content according to the instructions of manufacturer. To investigate the degree of iron deposit in the pancreas, Perls staining assay was performed. Xylene I and xylene II were used for dewaxing of the sections. After 15min, alcohol solutions gradient concentrations were used for hydration. Then an equal volume of potassium ferrocyanide (10%) and HCl (20%) were mixed five minutes for preparing the Perls’ staining solution. The prepared Perls’ staining solution was used for staining the sections for twenty-five minutes. The sections after rinse were observed by the optical microscope.
Assessment of MDA, GSH, GSSG and GPX4
The levels of MDA, GSH and GSSG were detected by corresponding kits according to the corresponding protocols of manufacturer. Malondialdehyde (MDA) assay kit E2019 (Applygen Technologies Inc) was used for MDA detection in the cell and tissue. GSH/GSSG Ratio Detection Assay II (Fluorometric - Green) (ab205811) was applied for measurement of GSH, GSSG and GSH/GSSG. Glutathione peroxidase 4(GPX4) kit was used for the detection of GPX4 (Runyu biotechnology co. LTD, Shanghai, China).
Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
The GPX4, SLC7A11, SLC3A2L, TFR1, Nrf2 and NCOA4 gene levels were detected via PCR assay. A RNeasy Mini Kit (Qiagen) was used for the extraction of total RNAs according to the manufacture’s protocols. M-MLV Reverse Transcriptase (Promega) was used for DNA synthesis. 7900HT Fast Real-Time PCR System (Applied Biosystems) was applied for PCR assay. The conditions of PCR were shown as follows: 95°C for 5 mins, then 40 cycles of 95°C, 15 s for denaturation, 60°C, 60s for annealing at and 70°C, 25s for extension. GAPDH served as the internal reference. The 2−ΔΔCT method was used for calculation of the relative gene level.
Western Blot
For cells, the total proteins were placed into RIPA lysis buffer (Cat No. CW2333S, CWBIO) for extraction. For the pancreatic tissues, the tissues were washed and homogenized in RIPA buffer. The BCA Protein Assay Kit (Cat No. CW0014S, CWBIO) was used for the measurement of the protein concentrations. The samples were loaded on 10% SDS-PAGE for separation. Then the proteins were blot on PVDF membrane (Millipore). Then the membranes after being blocked with skim milk (5%) were reacted with the primary antibodies against GPX4 (#ab219592, Abcam), SLC7A11 (#ab175186, Abcam), SLC3A2 (# PA5-96401, thermo Fisher scientific), TFR1 (#ab1086, Abcam), Nrf2 (#ab31163, Abcam) and NCOA4 (#PA5-96398, thermo Fisher scientific). Then the membranes were incubated with the secondary antibody. The ECL Western Blotting Analysis System (Amersham) was applied for detection of the membranes. ImageJ software was used for calculation of the band density.
CCK-8 Assay
The cell survival was detected by CCK-8 assay. Cell Counting Kit-8 (Dojindo) was used according to the instructions of manufacturer. The cells were seeded in the 96-well plates at a density of 1×105 cells per well. After treatment in the different groups, each well of the cells were incubated with ten microliters of CCK-8. The absorbance at 450nm which represents the cell viability, was detected by using the microplate reader.
Statistical Analysis
All the data herein were shown as mean±SD and analyzed by SPSS 20.0 software (SPSS Inc., Chicago, IL, USA). The significant difference between groups was determined via one-way or two-way of variance (ANOVA) test. P < 0.05 was deemed as significant difference.
Results
The Blood Glucose Level Was Reduced by CCA in a Concentration-Dependent Manner
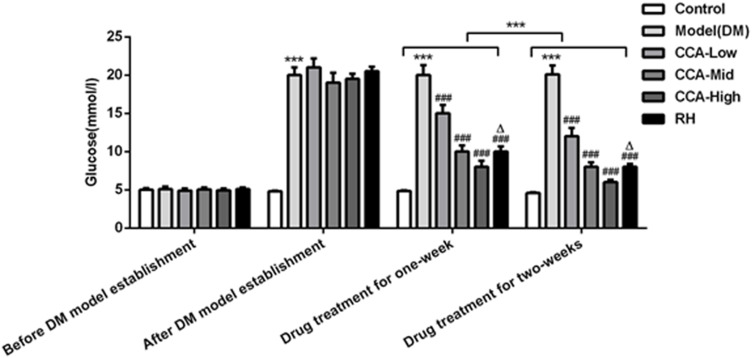
Before establishment of the diabetes models, the blood glucose levels were almost the same between groups (Figure 1). After the establishment of the diabetes models, the blood glucose levels in the experimental groups including model group, CCA-low group, CCA-mid group, CCA-high group and RH group were higher than the blood glucose in control group, indicating that the diabetes model was established successfully. Then, we further investigated the effects of CCA on the rats with diabetes. Our data revealed that the blood glucose level was reduced by CCA treatment for one week in a concentration-dependent manner in contrast to model group, and the level was reduced more significantly after CCA treatment for two weeks. The hypoglycemic effect of CCA at high concentrations is superior to RH during one week or two weeks.
Figure 1.
The effects of CCA on blood glucose level in vivo. The blood glucose level in the study groups before/after DM model establishment and drug treatment for one week/two weeks. ***P < 0.001 vs. control group; ###P < 0.001 vs. model group; ΔP < 0.05 vs. CCA-high group.
CCA Has Protective Effects on Impairment of Pancreas in Rats with Diabetes in a Concentration-Dependent Manner
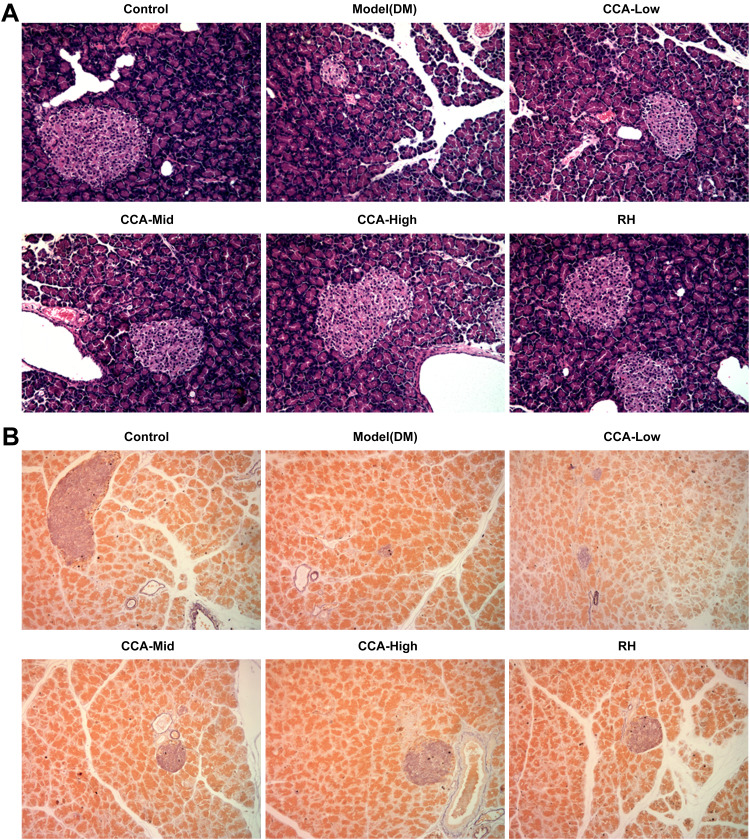
The islet in the pancreas of rats in the control group had normal architecture and is sharply marginated as well as regularly round or oval (Figure 2A and B). While in the model group, the boundary of the islet is not clear and the pathological change is comprehensive atrophy of islet. While in the CCA treatment group, the islet is well-formed regenerating by CCA in a concentration-dependent manner. Simultaneously, CCA at high concentrations had better regeneration effects on islet injury than RH.
Figure 2.
The effects of CCA on pancreatic pathology and islet cell numbers in vivo. The pancreatic pathology and islet cell numbers evaluated by hematein–eosin HE staining (A) and aldehyde fuchsin staining assay (B) respectively in the different groups.
CCA Improved the Iron Overload in Rats with Diabetes in a Concentration-Dependent Manner
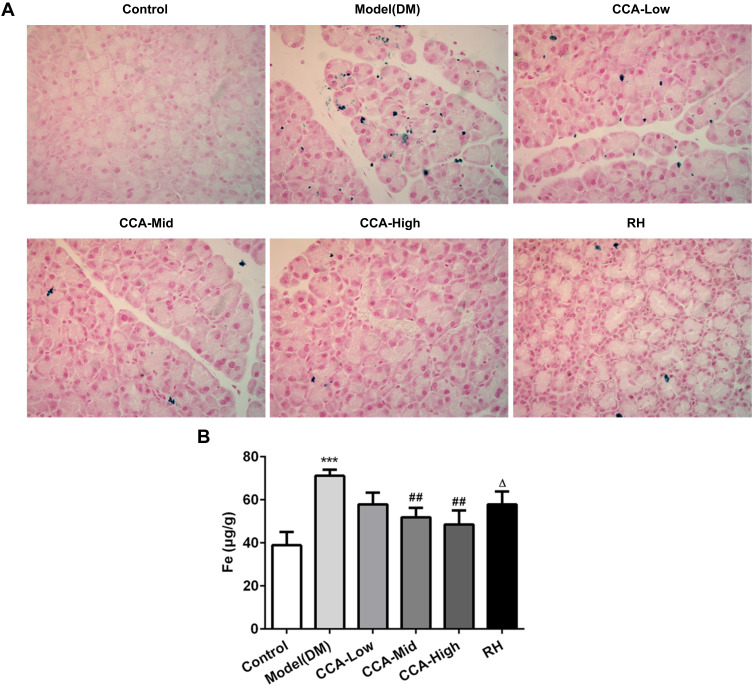
Iron overload was reported to contribute to insulin resistance.20 The role of CCA on iron content in rats with diabetes was also investigated. In the control group, there is no sign of iron deposit. The iron deposits occurred widely in the pancreatic tissue of the model group (Figure 3A). The iron deposits were improved by CCA in a concentration-dependent manner. The iron deposits were improved more significantly by CCA than RH. To further confirm the results, the iron content was also explored. Compared with control, the iron content was reduced by CCA in a concentration-dependent manner, more significantly at high concentrations (Figure 3B). Furthermore, CCA at high concentrations had better attenuative effects on iron deposits than RH. All the results were consistent, supporting that CCA had alleviative effects on iron deposits.
Figure 3.
The effects of CCA on iron content in vivo. The iron deposit evaluated by Perls stain (A) and the iron content detected by iron assay kit (ab83366) (B) in the different groups. ***P < 0.001 vs. control group; ##P < 0.01 vs. model group; ΔP < 0.05 vs. CCA-high group.
The Effects of CCA on Antioxidant Activities in Rats with Diabetes
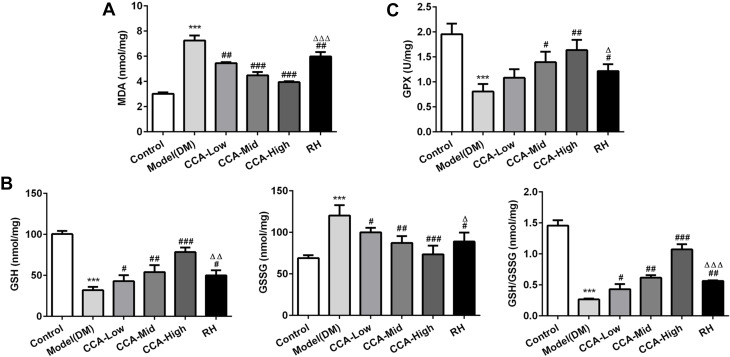
The MDA, GSH, GSSG and GSH/GSSG as indicators of oxidative effects were evaluated herein. The MDA as lipid peroxide and GSSG as the oxidized form of GSH were all elevated in the model group and further reduced by CCA in a concentration-dependent manner (Figure 3). The GSH, GSH/GSSG and GPX4 were all reduced in the model group when compared with control and the levels of these anti-oxidant indicators in rats with diabetes were further increased by CCA in a concentration-dependent manner (Figure 4A–C). As indicated by the data, the CCA at high concentrations has better enhanced anti-oxidant effects than RH.
Figure 4.
The effects of CCA on MDA, GSH, GSSG and GSH/GSSG level in vivo. The MDA level in the study groups (A), the levels of GSH, GSSG and GSH/GSSG in the different groups (B); the GPX4 level in the study groups (C). ***P < 0.001 vs. control group; #P < 0.05, ##P < 0.01 and ###P < 0.001 vs. model group ; ΔP < 0.05 ΔΔP < 0.01 and ΔΔΔP < 0.001vs. CCA-high group.
The Effects of CCA on Related Protein Expressions in Ferroptosis
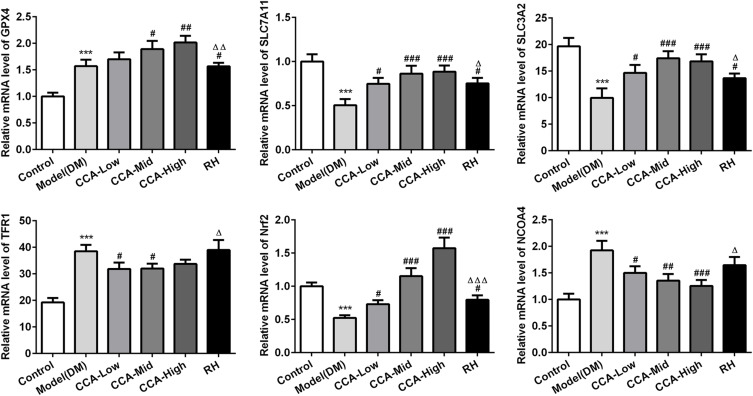
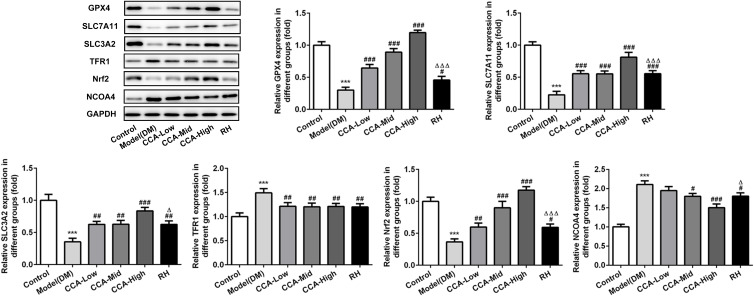
The major proteins or pathway related to ferroptosis, including XC-/GPX4, TFR1, Nrf2 and NCOA4 were all investigated herein (Figures 5 and 6). The Transferrin receptor 1 (TFR1) is a vital player in internalizing the transferrin-bound iron.28 In this research, TFR1 was elevated in model group when compared with control. The TFR1 level in the model group was further reduced by CCA, but this effect was very weak, indicating that TFR1 is not the target protein of CCA in rats with diabetes. Nrf2 activation was confirmed to have protective effects against ferroptosis.29 Herein, Nrf2 was activated by CCA in a concentration-dependent manner, while the effects of RH on Nrf2 were not clear, suggesting that the effects of CCA in diabetes may be achieved via activation of Nrf2. Inhibition of XC-/GPX4 induces ferroptosis.30 In this study, due to oxidative stress was induced by diabetes, the GPX4 gene level was increased by diabetes, while the GPX4 protein was decreased in the diabetes model group in contrast to the control group. The GPX4 level in the model group was increased significantly by CCA via a concentration-dependent manner, confirming that the protective effects of CCA in diabetes were achieved via elevating GPX4, resulting in inhibition of ferroptosis. NCOA4 is of paramount importance in ferritin turnover and iron homeostasis and NCOA4 resulted in overload of iron.31 In the current study, compared with control, the NOCA4 was increased significantly in diabetes model. The CCA have inhibitory effects on NOCA4 activation via a concentration-dependent manner, indicating that inhibition of NOCA4 may be one of the mechanisms for the effects of CCA on diabetes.
Figure 5.
The effects of CCA on related proteins in ferroptosis in vivo. The levels of SLC7A11, SLC3A2, GPX4, TFR1, Nrf2 and NCOA4 evaluated by PCR in the study groups. ***P < 0.001 vs. control group; #P < 0.05, ##P < 0.01 and ###P < 0.001 vs. model group; ΔP < 0.05, ΔΔP<0.01 and ΔΔΔP < 0.001vs. CCA-high group.
Figure 6.
The effects of CCA on related proteins in ferroptosis in vivo. The levels of SLC7A11, SLC3A2, GPX4, TFR1, Nrf2 and NCOA4 evaluated by Western blot in the study groups. ***P < 0.001 vs. control group; #P < 0.05, ##P < 0.01 and ###P < 0.001 vs. model group; ΔP < 0.05 and ΔΔΔP < 0.001vs. CCA-high group.
The Effects and Mechanism of CCA in Diabetes in vitro
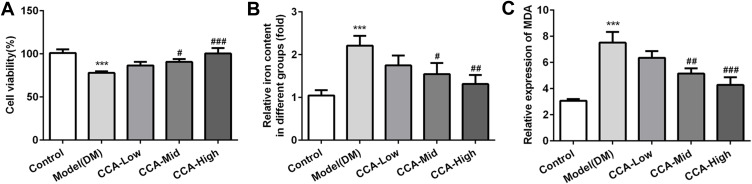
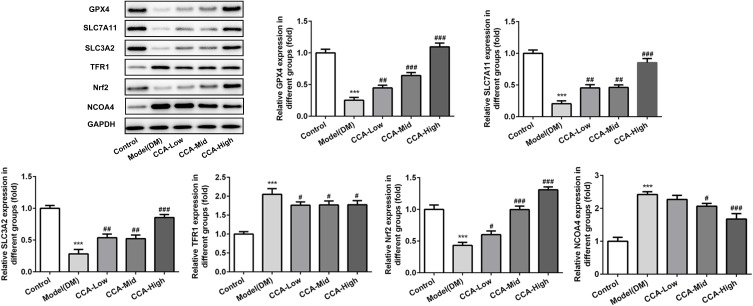
To further investigate the effects of CCA in the cells, we established the cell model of diabetes using high glucose. As seen by the data, consistent with the results in vivo in the diabetes model, the cell survival was decreased and MDA level as well as iron content were increased in cell model of diabetes, indicating that the cell model was established successfully (Figure 7A–C). After CCA treatment, the cell viability was increased and the MDA level as well as iron content was decreased by CCA in a concentration-dependent manner. The results in cell model were in line with those in rats model of diabetes. The effects of CCA in vivo and vitro are consistent, all supporting that CCA had protective effects against diabetes injury. The data in the cells revealed that the related proteins including SLC7111, SLC3A2 and GPX4 in anti-oxidant system and Nrf2 were decreased by high glucose and further elevated by CCA in a concentration-dependent manner (Figure 8), suggesting that the effects of CCA in cell model of diabetes was achieved via activation of XC-/GPX4 system. However, the effects of CCA on the level of TFR were not obvious, suggesting that the TFR was not the main mechanism for the effects of CCA. NCOA4 as mediator of ferroptosis was elevated by high glucose when compared with control and this effect was reversed by CCA in a concentration-dependent manner, indicating that inhibition of NCOA4 may be one of the mechanisms for the effects of CCA. In summary, the effects of CCA in diabetes were mainly achieved via activation of XC-/GPX4 system as well as Nrf2 and inhibition of NCOA4.
Figure 7.
The effects of CCA on cell viability, iron content and MDA level. The cell survival (A), iron content (B) and MDA level (C) in the study groups. ***P < 0.001 vs. control group; #P < 0.05, ##P < 0.01 and ###P < 0.001 vs. model group.
Figure 8.
The effects of CCA on related proteins in ferroptosis in vitro. The levels of SLC7A11, SLC3A2, GPX4, TFR1, Nrf2 and NCOA4 evaluated by Western blot in the study groups. ***P < 0.001 vs. control group; #P < 0.05, ##P < 0.01 and ###P < 0.001 vs. model group.
Discussion
Mulberry leaf extract has exerted excellent therapeutic effects on diabetes. Mulberry leaf extract has antidiabetic activities in mice via enhancing glucose disposal.32 It is also beneficial to diabetes patients in glycemic control.33 In addition, the mulberry leaf extract also decreases the insulin concentrations in adults with normal blood glucose.34 There are still multiple modes of action in the effects of mulberry leaf extract in diabetes needing to be illuminated. Furthermore, the components in the extracts are complex and the effects and mechanism of each component remain unknown. Cryptochlorogenic acid as one of the main active components in mulberry leaf extract was investigated in this manuscript. Our data revealed that Cryptochlorogenic acid had protective effects against injuries induced by diabetes, mainly via improving ferroptosis caused by Xc-/GPX4 dysfunction.
The glycemic control and pancreas islet functions are extremely vital for patients with diabetes. Better glycemic control could reduce complications and delay the progression of diabetes.35,36 Therefore, we first established the diabetes model and investigated the effects of CCA on the blood glucose level in the diabetes. Before the establishment of diabetes model, the blood glucose in the different groups are nearly the same between groups, while after the establishment of the diabetic model, the blood glucose in all the experimental groups except control group were all elevated, indicating the successful establishment of the diabetes model. Our data showed that the glucose level was reduced by CCA in a concentration-dependent manner and the CCA at a high concentration has better Hypoglycemic effect than RH.
The degree of pancreatic injury is closely related to the insulin secretion and blood glucose levels. Our data showed that CCA have protective effects on pancreatic injury in diabetes, particularly superior to RH at high concentrations. Recently, research has reported that islets are vulnerable to ferroptosis and ferroptosis inhibitors can restore the islet function in vitro.14 In this study, the islets were severely injured in diabetes, which were well-formed regenerating by CCA with increasing concentration, which may be related to the ferroptosis inhibition effects of CCA. As Ferroptosis is iron-dependent, we further investigated the iron content and iron deposition degree. As revealed by our data, the iron deposits and iron content were increased in the diabetic rats and this effect was further alleviated by CCA, confirming that CCA had inhibitory effects on iron deposition.
There are mainly two avenues causing ferroptosis: one is that accumulation of lipid peroxides is caused via GPX4 insufficient catalyzed by iron, the other is the dysfunctions of iron metabolism.37–39 MDA and GSSG as lipid peroxides were increased in diabetes and decreased by CCA, suggesting the anti-oxidant effects of CCA. GSH as indicators of antioxidant agents and a cofactor of GPX4 were decreased in diabetes, which were further increased by CCA, confirming the antioxidant effects of CCA. The GPX4 activity was decreased in diabetes, which could also be elevated by CCA. The high lipid peroxides level and decreased GPX level in the diabetes indicated the existence of ferroptosis.
The related protein in ferroptosis was further explored. System xc− which is composed of SLC7A11 and SLC3A2, served as the provider of GSH synthesis and GPX as a GSH-dependent enzyme is the primary neutralizer of lipid peroxides.40 In the current study, we found that SLC7A11, SLC3A2 and GPX4 was reduced in diabetes model in vivo and vitro, which were all increased by CCA via a concentration-dependent manner. Our data suggested that dysfunction of the xc−/GPX4 axis resulted in ferroptosis in diabetes, which was alleviated by CCA. CCA was proved to have protective effects in diabetes via inhibition of ferroptosis.
As the iron metabolism disorder is another inducer for ferroptosis, the effects of CCA on related proteins in iron metabolism were also explored. The TFR1 as the important iron regulator worked by uptake of transferrin-bound iron into the cells.28,41 Herein, CCA elevated the TFR1 level, but this effect was not obvious, indicating that the TFR1 was not the main avenue for the effects of CCA in diabetes. Nrf2 is a crucial protein for iron homeostasis and activation of Nrf2 elevates iron storage protein ferritin.42 In addition, the anti-oxidant system which complements antioxidant functions of GPX, can also be activated by Nrf2.42,43 Our results showed that Nrf2 induced by diabetes is elevated by CCA, confirming that Nrf2 was one of the main action avenues in diabetes. NCOA4 as a selective cargo receptor worked by degradation of ferritin and overexpression of NCOA4 enhanced the sensitivity to ferroptosis.44 In this research, the NCOA4 induced by diabetes was higher and this effect was reduced by CCA significantly in a concentration-dependent manner, confirming that NCOA4 was also one of the main pathways that acted by CCA for inhibition of ferroptosis in the diabetes.
Conclusion
In this research, we first investigated the effects of CCA, which is a main active component of Mulberry leaf extracts. Our results revealed that CCA have hypoglycemic and protective effects, particular at high concentrations, which is superior to RH. The effects of CCA in diabetes were realized by inhibition of ferroptosis via activation of xc−/GPX4/Nrf2 and inhibition of NCOA4. The findings in this study provide a new avenue and lay the foundations for further research.
Disclosure
The author declares no conflicts of interest in this work.
References
- 1.L’Heveder R, Nolan T. International diabetes federation. Diabetes Res Clin Pract. 2013;101(3):349–351. doi: 10.1016/j.diabres.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 2.Stana D, Potop V, Istrate SL, et al. Variability of diabetic macular edema in correlation with hypertension retinopathy in patients with diabetes mellitus and essential hypertension. Rom J Ophthalmol. 2019;63(4):327–338. doi: 10.22336/rjo.2019.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li T, Xu X, Xu Y, et al. PPARG polymorphisms are associated with unexplained mild vision loss in patients with type 2 diabetes mellitus. J Ophthalmol. 2019;2019:5284867. doi: 10.1155/2019/5284867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.John P, Yadla M. Noninvasive method of differentiating diabetic nephropathy and nondiabetic renal disease using serum bone morphogenetic protein-7 and transforming growth factor-beta 1 levels in patients with type-2 diabetes mellitus. Saudi J Kidney Dis Transpl. 2019;30(6):1300–1309. doi: 10.4103/1319-2442.275474 [DOI] [PubMed] [Google Scholar]
- 5.Jnana A, Muthuraman V, Varghese VK, et al. Microbial community distribution and core microbiome in successive wound grades of individuals with diabetic foot ulcers. Appl Environ Microbiol. 2020;86(6). doi: 10.1128/AEM.02608-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butler J, Handelsman Y, Bakris G, Verma S. Use of sodium-glucose co-transporter-2 inhibitors in patients with and without type 2 diabetes: implications for incident and prevalent heart failure. Eur J Heart Fail. 2020. doi: 10.1002/ejhf.1708 [DOI] [PubMed] [Google Scholar]
- 7.Zaccardi F, Webb DR, Yates T, Davies MJ. Pathophysiology of type 1 and type 2 diabetes mellitus: a 90-year perspective. Postgrad Med J. 2016;92(1084):63–69. doi: 10.1136/postgradmedj-2015-133281 [DOI] [PubMed] [Google Scholar]
- 8.Rehman K, Akash MSH. Mechanism of generation of oxidative stress and pathophysiology of type 2 diabetes mellitus: how are they interlinked? J Cell Biochem. 2017;118(11):3577–3585. doi: 10.1002/jcb.26097 [DOI] [PubMed] [Google Scholar]
- 9.Conrad M, Friedmann Angeli JP. Glutathione peroxidase 4 (Gpx4) and ferroptosis: what’s so special about it? Mol Cell Oncol. 2015;2(3):e995047. doi: 10.4161/23723556.2014.995047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Latunde-Dada GO. Ferroptosis: role of lipid peroxidation, iron and ferritinophagy. Biochim Biophys Acta Gen Subj. 2017;1861(8):1893–1900. doi: 10.1016/j.bbagen.2017.05.019 [DOI] [PubMed] [Google Scholar]
- 11.Li W, Li W, Leng Y, Xiong Y, Xia Z. Ferroptosis is involved in diabetes myocardial ischemia/reperfusion injury through endoplasmic reticulum stress. DNA Cell Biol. 2020;39(2):210–225. [DOI] [PubMed] [Google Scholar]
- 12.Bottino R, Balamurugan AN, Bertera S, Pietropaolo M, Trucco M, Piganelli JD. Preservation of human islet cell functional mass by anti-oxidative action of a novel SOD mimic compound. Diabetes. 2002;51(8):2561–2567. doi: 10.2337/diabetes.51.8.2561 [DOI] [PubMed] [Google Scholar]
- 13.Bottino R, Balamurugan AN, Tse H, et al. Response of human islets to isolation stress and the effect of antioxidant treatment. Diabetes. 2004;53(10):2559–2568. doi: 10.2337/diabetes.53.10.2559 [DOI] [PubMed] [Google Scholar]
- 14.Bruni A, Pepper AR, Pawlick RL, et al. Ferroptosis-inducing agents compromise in vitro human islet viability and function. Cell Death Dis. 2018;9(6):595. doi: 10.1038/s41419-018-0506-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koppula P, Zhang Y, Shi J, Li W, Gan B. The glutamate/cystine antiporter SLC7A11/xCT enhances cancer cell dependency on glucose by exporting glutamate. J Biol Chem. 2017;292(34):14240–14249. doi: 10.1074/jbc.M117.798405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang LY, Lao WQ, Meng Z, et al. Analysis of the influence of iron overload in glucose metabolism in thalassemia major patients. Zhonghua Er Ke Za Zhi. 2017;55(6):419–422. doi: 10.3760/cma.j.issn.0578-1310.2017.06.005 [DOI] [PubMed] [Google Scholar]
- 17.Ma W, Feng Y, Jia L, et al. Dietary iron modulates glucose and lipid homeostasis in diabetic mice. Biol Trace Elem Res. 2019;189(1):194–200. doi: 10.1007/s12011-018-1446-3 [DOI] [PubMed] [Google Scholar]
- 18.Stechemesser L, Eder SK, Wagner A, et al. Metabolomic profiling identifies potential pathways involved in the interaction of iron homeostasis with glucose metabolism. Mol Metab. 2017;6(1):38–47. doi: 10.1016/j.molmet.2016.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shu T, Lv Z, Xie Y, Tang J, Mao X. Hepcidin as a key iron regulator mediates glucotoxicity-induced pancreatic beta-cell dysfunction. Endocr Connect. 2019;8(3):150–161. doi: 10.1530/EC-18-0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jahng JWS, Alsaadi RM, Palanivel R, et al. Iron overload inhibits late stage autophagic flux leading to insulin resistance. EMBO Rep. 2019;20(10):e47911. doi: 10.15252/embr.201947911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung HA, Islam MD, Kwon YS, et al. Extraction and identification of three major aldose reductase inhibitors from Artemisia montana. Food Chem Toxicol. 2011;49(2):376–384. doi: 10.1016/j.fct.2010.11.012 [DOI] [PubMed] [Google Scholar]
- 22.Varghese SM, Thomas J. Polyphenolic constituents in mulberry leaf extract (M. latifolia L. cv. BC259) and its antidiabetic effect in streptozotocin induced diabetic rats. Pak J Pharm Sci. 2019;32(1):69–74. [PubMed] [Google Scholar]
- 23.Yu X, An X, Lu H, Wang X, Jiang Y. Hypoglycemic effects of mulberry leaf extracts on diabetic mice. Wei Sheng Yan Jiu. 2018;47(3):432–436. [PubMed] [Google Scholar]
- 24.Meng Q, Qi X, Chao Y, et al. IRS1/PI3K/AKT pathway signal involved in the regulation of glycolipid metabolic abnormalities by Mulberry (Morus alba L.) leaf extracts in 3T3-L1 adipocytes. Chin Med. 2020;15:1. doi: 10.1186/s13020-019-0281-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian S, Wang M, Liu C, Zhao H, Zhao B. Mulberry leaf reduces inflammation and insulin resistance in type 2 diabetic mice by TLRs and insulin signalling pathway. BMC Complement Altern Med. 2019;19(1):326. doi: 10.1186/s12906-019-2742-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang LW, Ji T, Su SL, et al. Pharmacokinetics of mori folium flavones and alkaloids in normal and diabetic rats. Zhongguo Zhong Yao Za Zhi. 2017;42(21):4218–4225. doi: 10.19540/j.cnki.cjcmm.20170901.008 [DOI] [PubMed] [Google Scholar]
- 27.Jennings BM. Aldehyde-fuchsin staining applied to frozen sections for demonstrating pituitary and pancreatic beta cells. J Histochem Cytochem. 1965;13:328–333. doi: 10.1177/13.5.328 [DOI] [PubMed] [Google Scholar]
- 28.Park E, Chung SW. ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis. 2019;10(11):822. doi: 10.1038/s41419-019-2064-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abdalkader M, Lampinen R, Kanninen KM, Malm TM, Liddell JR. Targeting Nrf2 to suppress ferroptosis and mitochondrial dysfunction in neurodegeneration. Front Neurosci. 2018;12:466. doi: 10.3389/fnins.2018.00466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaschler MM, Andia AA, Liu H, et al. FINO2 initiates ferroptosis through GPX4 inactivation and iron oxidation. Nat Chem Biol. 2018;14(5):507–515. doi: 10.1038/s41589-018-0031-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quiles Del Rey M, Mancias JD. NCOA4-mediated ferritinophagy: a potential link to neurodegeneration. Front Neurosci. 2019;13:238. doi: 10.3389/fnins.2019.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bae UJ, Jung ES, Jung SJ, Chae SW, Park BH. Mulberry leaf extract displays antidiabetic activity in db/db mice via Akt and AMP-activated protein kinase phosphorylation. Food Nutr Res. 2018;62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riche DM, Riche KD, East HE, Barrett EK, May WL. Impact of mulberry leaf extract on type 2 diabetes (Mul-DM): a randomized, placebo-controlled pilot study. Complement Ther Med. 2017;32:105–108. doi: 10.1016/j.ctim.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 34.Lown M, Fuller R, Lightowler H, et al. Mulberry-extract improves glucose tolerance and decreases insulin concentrations in normoglycaemic adults: results of a randomised double-blind placebo-controlled study. PLoS One. 2017;12(2):e0172239. doi: 10.1371/journal.pone.0172239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yorke E, Atiase Y. Impact of structured education on glucose control and hypoglycaemia in Type-2 diabetes: a systematic review of randomized controlled trials. Ghana Med J. 2018;52(1):41–60. doi: 10.4314/gmj.v52i1.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang W, Liu CY, Ji LN, Wang JG. Blood pressure and glucose control and the prevalence of albuminuria and left ventricular hypertrophy in patients with hypertension and diabetes. J Clin Hypertens (Greenwich). 2020. doi: 10.1111/jch.13793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kagan VE, Mao G, Qu F, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13(1):81–90. doi: 10.1038/nchembio.2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lei P, Bai T, Sun Y. Mechanisms of ferroptosis and relations with regulated cell death: a review. Front Physiol. 2019;10:139. doi: 10.3389/fphys.2019.00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishizawa H, Matsumoto M, Shindo T, et al. Ferroptosis is controlled by the coordinated transcriptional regulation of glutathione and labile iron metabolism by the transcription factor BACH1. J Biol Chem. 2020;295(1):69–82. doi: 10.1074/jbc.RA119.009548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kajarabille N, Latunde-Dada GO. Programmed cell-death by ferroptosis: antioxidants as mitigators. Int J Mol Sci. 2019;20(19). doi: 10.3390/ijms20194968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. Nat Chem Biol. 2014;10(1):9–17. doi: 10.1038/nchembio.1416 [DOI] [PubMed] [Google Scholar]
- 42.Kerins MJ, Ooi A. The roles of NRF2 in modulating cellular iron homeostasis. Antioxid Redox Signal. 2018;29(17):1756–1773. doi: 10.1089/ars.2017.7176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim YJ, Ahn JY, Liang P, Ip C, Zhang Y, Park YM. Human prx1 gene is a target of Nrf2 and is up-regulated by hypoxia/reoxygenation: implication to tumor biology. Cancer Res. 2007;67(2):546–554. doi: 10.1158/0008-5472.CAN-06-2401 [DOI] [PubMed] [Google Scholar]
- 44.Hou W, Xie Y, Song X, et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12(8):1425–1428. doi: 10.1080/15548627.2016.1187366 [DOI] [PMC free article] [PubMed] [Google Scholar]