Abstract
The pressure to deliver new medicines to the patient continues to grow along with increases in compound failure rate, thus putting the current R&D model at risk. Analysis has shown that increasing the three-dimensionality of potential drug candidates decreases the risk of failure and improves binding selectivity and frequency. For this reason many workers have taken a new look at the power of photochemistry as a means to generate novel sp3 rich scaffolds for use in drug discovery programs. Here we report the design, synthesis, and computational structural analysis of a series of 2,4-methanoprolines having inherent 3D character (PMI and PBF scores) significantly higher than that of the broader AbbVie Rule of 3 (Ro3) collection.
Keywords: Drug discovery, photochemistry, sp3 rich scaffolds, substituted bridged pyrrolidine fragments, 3D character
In their seminal paper entitled “Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success”, Lovering et al. hypothesized that the shift to high-throughput synthetic practices had resulted in more achiral, aromatic compounds.1 This was supported by Walters et al., who analyzed the types of molecules that had been made by medicinal chemists over the 50 years preceding 2009. It showed quite conclusively the dramatic rise in the proportion of molecules containing sp2–sp2 couplings. The authors attributed this trend away from sp3 character to the introduction of new methods for sp2–sp2 couplings and the adaptation of these methods to high-throughput synthesis, utilized widely in optimization programs and in archive “enrichment” campaigns (Figure 1).2
Figure 1.
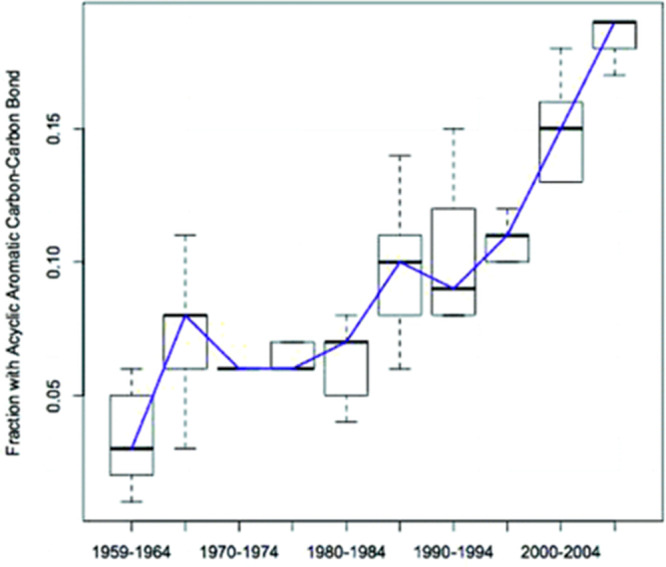
Influence of sp2–sp2 coupling chemistries on the molecules published in the Journal of Medicinal Chemistry between 1959 and 2009. Data are shown as the fraction of molecules published in each 5-year period containing at least one acyclic–aromatic carbon–carbon bond. Reprinted from ref (2). Copyright 2011 American Chemical Society.
Lovering et al. focused on carbon bond saturation as defined by fraction sp3 (Fsp3) where Fsp3 = (number of sp3 hybridized carbons/total carbon count) as a simple and interpretable measurement of the complexity of molecules prepared as potential drug candidates. They went on to demonstrate that complexity (as measured by Fsp3) correlates with success as compounds transition from discovery, through clinical testing, to drugs (see Figure 2). They further demonstrated that saturation correlates with solubility, an experimental physical property important to success in the drug discovery setting.1
Figure 2.
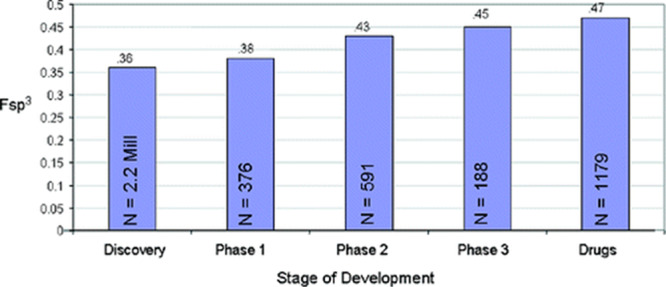
Mean Fsp3 for compounds in different stages of development. **P value <0.001. Reprinted from ref (1). Copyright 2009 American Chemical Society.
In a later paper, Lovering described how increasing complexity reduces promiscuity and Cyp450 inhibition. Increased promiscuity has been linked to toxicity and candidate failure.3
Further to the positive impact of increasing fraction sp3 (Fsp3) on attrition rates, Clemons et al. examined compounds from different sources (commercial, academic, natural) for their protein-binding behaviors and found that these behaviors correlate with general trends in stereochemical and shape descriptors for these compound collections. Increasing the content of sp3-hybridized and stereogenic atoms relative to compounds from commercial sources, which comprise the majority of current screening collections, improved binding selectivity, and frequency.4
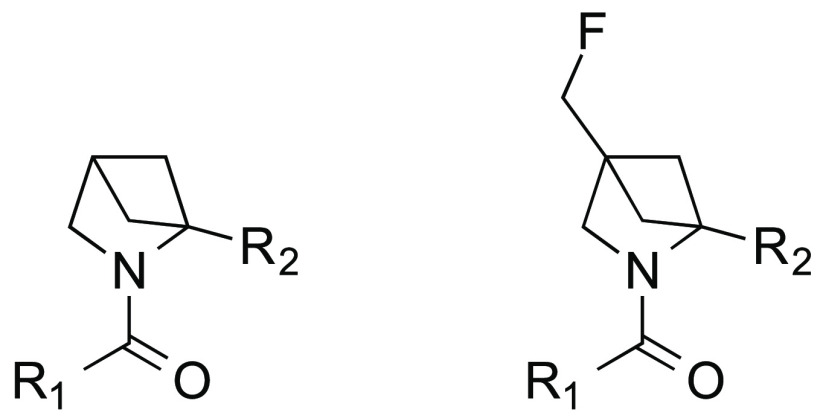
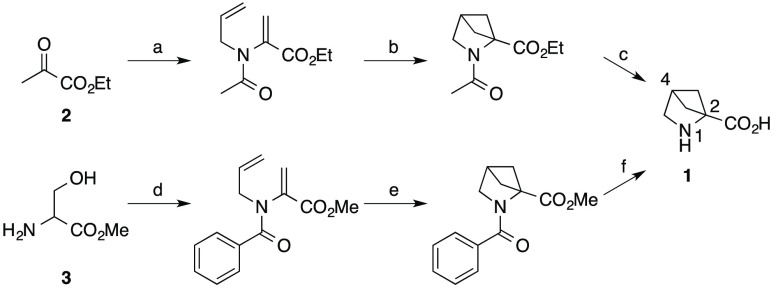
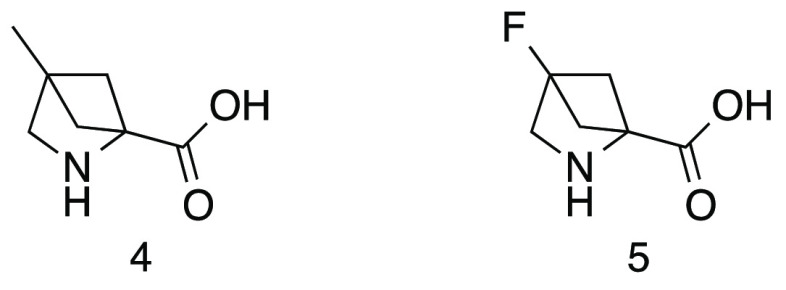
A well-established approach to the generation of high quality small molecular weight leads for drug discovery programs is by the application of fragment based methods. We were prompted to look at synthetic methodologies that could be utilized to efficiently generate novel, complex, sp3 rich fragments. For a number of years, we have been investigating the use of photochemistry, in particular [2 + 2] cycloadditions, as a source of desirable starting points for medicinal chemistry.5 We have, like other workers, focused some of our efforts on analogues of 2,4-methanoproline 1. 2,4-Methanoproline 1 was first isolated from the seeds of Ateleia herbert smithii Pittier, a tree found in Costa Rica,6 and is prepared in a simple sequence of reactions from ethyl pyruvate 2 or serine 3 (Scheme 1) via intramolecular [2 + 2] olefin photocycloaddition reactions.7,8 The utility and conformational properties of 2,4-methanoproline 1 as a replacement for d- or l-proline have been studied; its N-acetyl, methyl ester was found to show a large prevalence for a trans-amide conformation more akin to primary amino acids than to d- or l-proline.9 This makes it a potentially interesting amino acid for inclusion in therapeutic peptides and the basis for the design of fragment libraries. A number of 2-position variants of 2,4-methanoproline have been reported recently10 and a small number of 4-substituted analogues, such as the 4-methyl 4 and 4-fluoro 5 derivatives (Figure 3).9 We embarked on the synthesis of novel 2-substituted and 2,4-substituted analogues of interest in their own right but which were further utilized to prepare diverse N-substituted amide and urea parallel libraries with a view to exploring their utility as fragments in a screening library. The compounds were designed to examine the three-dimensional molecular shape and vectors produced by extended substituents at the N-1, 2 and 4-postions.
Scheme 1. Synthesis of 2,4-Methanoproline 1.
Reagents and conditions: (a) Allylamine then AcCl, Et3N, benzene (22%); (b) hv, acetone (55%); (c) aq KOH (72%); (d) PhCOCl, Et3N, DCM, then NaH, allyl Br, DMF (80%); (e) hv, acetophenone, MeCN (88%); (f) 6 N HCl (99%).
Figure 3.
4-Substituted 2,4-methanoproline analogues.
N-1,2-Substituent Variations
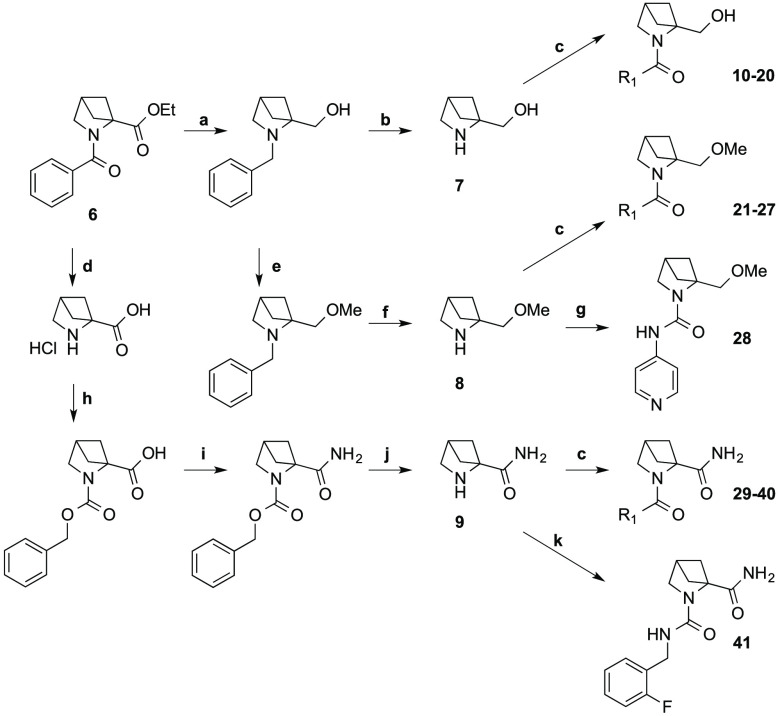
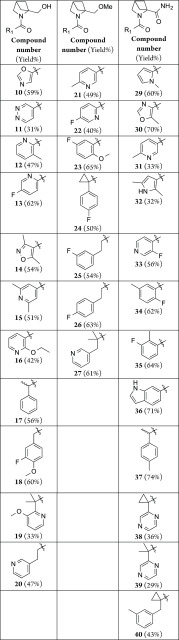
The N-benzoyl, ethyl ester of 2,4-methanoproline 6 was prepared by the method of Malpass et al.11 This compound was used to prepare a diverse range of proline derivatives; the 2-hydroxylmethyl 7, the 2-methoxylmethyl 8, and the 2-carboxamido 9 analogues (Scheme 2), from which a novel array of N-substituted amides and ureas were prepared using parallel synthesis methods (Table 1).
Scheme 2. Synthesis of 2-Substituent Variations of 2,4-Methanoproline.
Reagents and conditions: (a) LiAlH4, THF (78%); (b) ammonium formate, Pd/C, EtOH (94%); (c) DIPEA, HATU, DCM, R1 amine (See Table 1 for yields) ; (d) 6 N HCl (76%); (e) NaH, MeI, THF (81%); (f) ammonium formate, Pd/C, EtOH (92%); (g) 4-isocyanopyridine, DCM (39%); (h) CbzCl, aq Na2CO3, (80%); (i) aq ammonia, HATU DIPEA, DCM (80%); (j) ammonium formate, Pd/C, EtOH (90%); (k) 2-fluorobenzyl isocyanate, DCM (44%).
Table 1. N-1 Amide Derivatives of 2-Substituent Variations of 2,4-Methanoproline.
N-1,2,4-Substituent Variations
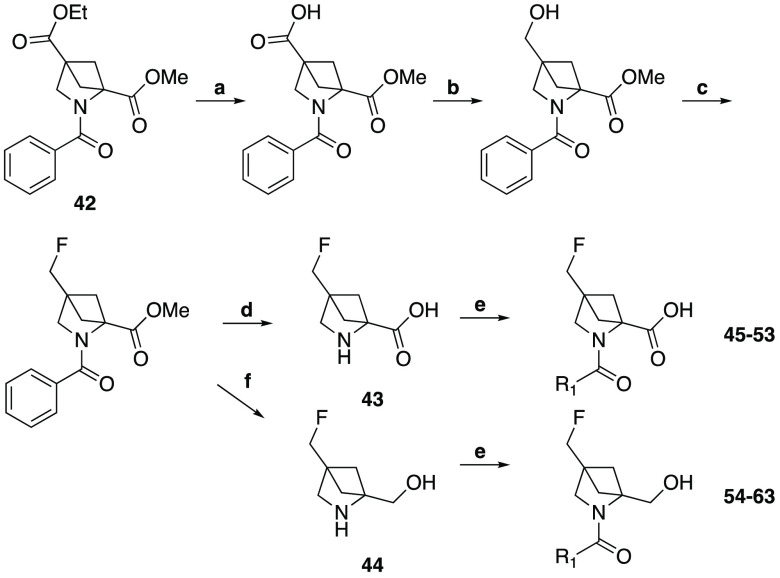
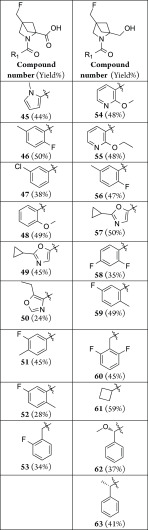
The 4-carboxylic acid derivative of 2,4-methanoproline was prepared as its N-benzoyl, 4-ethyl, 2-methyl diester 42 by the method of Esslinger et al.12 This compound was used to prepare the 4-fluoromethyl derivative 43 and the 4-fluoromethyl-2-hydroxylmethyl derivative 44 (Scheme 3), from which a further novel array of N-substituted amides were prepared using parallel synthesis methods (Table 2). This sequence involved the selective saponification of the 4-ethyl ester in the presence of the 2-methyl ester using lithium hydroxide in a THF water mixture, followed by selective reduction of the carboxylic acid function and fluorination of the alcohol product.
Scheme 3. Synthesis of 2,4-Substituent Variations of 2,4-Methanoproline.
Reagents and conditions: (a) LiOH, THF/H2O (84%); (b) BH3THF, THF (84%); (c) DAST, DCM (68%); (d) TFA, H2O (43%); (e) DIPEA, HATU, DCM, R1 amine (See Table 2 for yields); (f) LiAlH4, THF then ammonium formate, Pd/C, EtOH (40%).
Table 2. N-1 Amide Derivatives of 2,4-Substituent Variations of 2,4-Methanoproline.
Computational Analysis
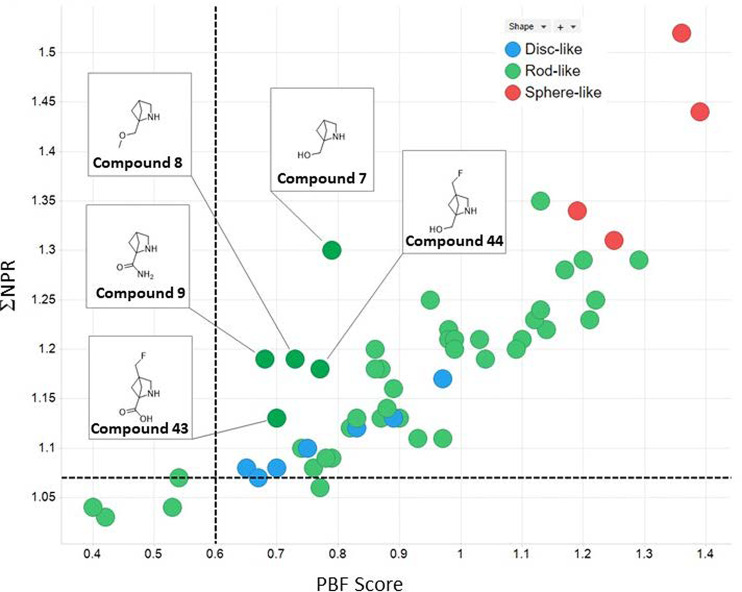
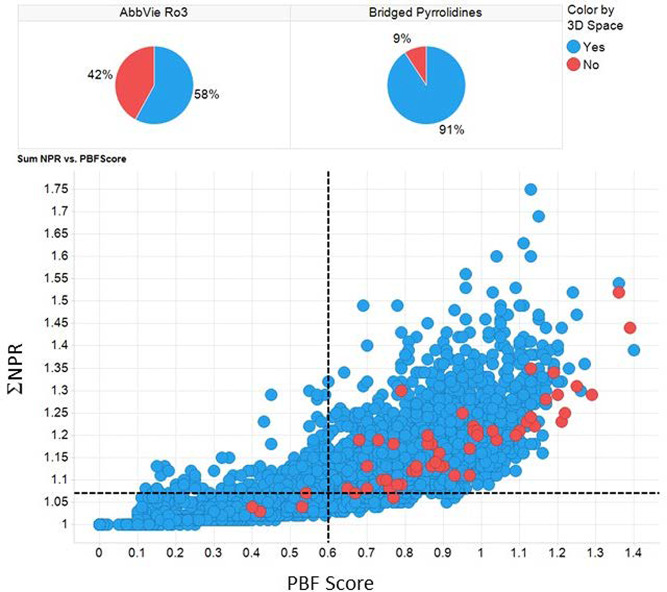
In order to investigate the in-silico properties of these molecules, the structures were uploaded into AbbVie’s design platform. In addition to a range of physicochemical properties, the Principal Moments of Inertia (PMI) and the Plane of Best Fit (PBF) scores were calculated using methods described in the literature.13,14 These descriptors were then used in combination to map the 3-dimensional space of these compounds. Plotting the sum of the normalized PMIs versus the PBF score, the compounds within the region of the graph defined by ΣNPR (Sum of the Normalized Principal Moments of Inertia (NPR1 and NPR2)) ≥ 1.07 and PBF Score ≥0.6 are deemed, by Firth et al., to reside in 3D space by virtue of two independent 3D descriptors. Figure 4 shows this plot for 54 of the bridged pyrrolidines, with the corresponding unfunctionalized methanoproline intermediates labeled. Note that the shape was assigned using an approximation based on the relative values of NPR1 and NPR2.
Figure 4.
Plot of the normalized PMI versus PBF score for all 54 fragments.
All but 5 of these fragments sit in 3D space with all of the intermediates resting comfortably in this region of space. A comparison of the relative level of 3-dimensionality with other fragments within the AbbVie Ro3 fragment collection is shown in Figure 5.
Figure 5.
% of compounds in 3D space for AbbVie’s Ro3 fragment library vs bridged pyrrolidines.
Overall the % of 3D character of the bridged pyrrolidines is significantly higher (91%) than that of the broader AbbVie Rule of 3 (Ro3) collection (58%) which is testament to the proclivity of this scaffold to produce compounds with a higher degree of 3D character within Ro3 chemical space. This is similar to pyrrolidines which are common scaffolds in drugs and known to enhance the 3D nature of fragments, with enabling chemistries employed to maximize both 2 and especially 3D diversity of fragment space.15 It is interesting to note that the unsubstituted methanoproline intermediates are highly saturated with proportionate levels of Fsp3; however, for the majority of the corresponding substituted amide products, the level of 3 dimensionality increases as the level of saturation decreases (Figure 6).
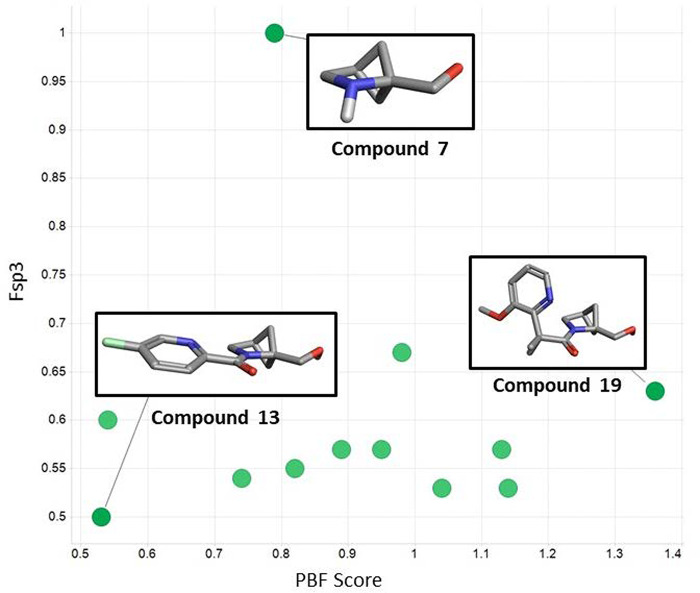
Figure 6.
Comparison of Fsp3 versus PBF score for the methanoprolinol (compound 7) derived series.
As an example, we analyzed the series of compounds from the methanoprolinol derivative (compound 7) and plotted Fsp3 versus PBF score. While the unsubstituted prolinol is fully saturated (Fsp3 = 1), the corresponding amide products have lower levels of saturation (Fsp3 < 1); however, in many cases they possess higher degrees of 3-dimensionality (see Figure 5). The 3D structures of the corresponding conformations used to calculate the 3D descriptors were visualized using Cresset’s Forge platform. It was evident from these 3D structures why compound 19 possessed a higher degree of 3D character with a sphere-like conformation, while compound 13 is a flatter, more rod-like shape. This demonstrates that Fsp3 should be used with caution when describing the relative 3-dimensionality of compounds. Overall these bridged pyrrolidines possess inherent 3D character and allow for the addition of fragments with higher degrees of favorable 3-dimesionality. In addition, this method opens the door to prospective tailoring of the 3D character of the fragment library prior to synthesis.
Conclusion
Over the coming 5–10 years the predicted positive impact of researchers synthesizing compounds with higher Fsp3 in drug discovery programs will become more apparent. Photocycloaddition reactions stand to play a significant part in generating highly desirable templates such as the 2,4-methanoproline derivatives covered here. Our computational analysis clearly demonstrates that fragments derived from 2,4-methanoproline have inherent 3D character using two independent 3D descriptors (PMI and PBF Score) and that the degree of shape and 3D character may be prospectively designed and biased toward fragments with enhanced 3D character, residing in distinct property space to that largely occupied by conventional screening libraries, thus enabling the “escape from flatland”.
Acknowledgments
The authors wish to thank the following colleagues for providing small molecule analysis data: Alaa Abdul-Sada (high resolution mass spectrometry), Iain Day (NMR), and Mark Roe (X-ray crystallography). We are also grateful to the Universities of Sussex and Bristol.
Glossary
Abbreviations
- DAST
diethylaminosulfur trifluoride
- DCM
dichloromethane
- DMF
dimethylformamide
- DIPEA
diisopropylethylamine
- HATU
1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate
- THF
tetrahydrofuran.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.0c00039.
Synthetic methods, characterization, crystallographic and computational modeling data. (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
We gratefully acknowledge support for Luke Elliott from the Engineering and Physical Sciences Research Council (EP/P013341/1).
The authors declare the following competing financial interest(s): PBC is an employee of Abbvie. BC and KIBM are employees of their respective universities, co-founders and owners of Photodiversity Ltd. The design, study content, and financial support for the study were provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the publication.
Supplementary Material
References
- Lovering F.; Bikker J.; Humblet C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52, 6752–6756. 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- Walters W. P.; Green J.; Weiss J. R.; Murcko M. A. What do medicinal chemists actually make? A 50-year retrospective. J. Med. Chem. 2011, 54, 6405–6416. 10.1021/jm200504p. [DOI] [PubMed] [Google Scholar]
- Lovering F. Escape from Flatland 2: complexity and promiscuity. MedChemComm 2013, 4, 515–519. 10.1039/c2md20347b. [DOI] [Google Scholar]
- Clemons P. A.; Bodycombe N. A.; Carrinski H. A.; Wilson J. A.; Shamji A. F.; Wagner B. K.; Koehler A. N.; Schreiber S. L. Small molecules of different origins have distinct distributions of structural complexity that correlate with protein-binding profiles. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 18787–18792. 10.1073/pnas.1012741107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox B.; Booker-Milburn K. I.; Elliott L. D.; Robertson-Ralph M.; Zdorichenko V. Escaping from Flatland: [2 + 2] Photocycloaddition; Conformationally Constrained sp3-rich Scaffolds for Lead Generation. ACS Med. Chem. Lett. 2019, 10, 1512–1517. 10.1021/acsmedchemlett.9b00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell E. A.; Qureshi M. Y.; Pryce R. J.; Janzen D. H.; Lemke P.; Clardy J. 2,4-Methanoproline (2-Carboxy-2,4-methanopyrrolidine) and 2,4-Methanoglutamic Acid (1 - Amino-1,3-dicarboxycyclobutane) in Seeds of Ateleia Herbert smithii Pittier (Leguminosae). J. Am. Chem. Soc. 1980, 102, 1409–1412. 10.1021/ja00524a029. [DOI] [Google Scholar]
- Pirrung M. C. Total Synthesis of 2,4-Methanoproline. Tetrahedron Lett. 1980, 21, 4577–4578. 10.1016/0040-4039(80)80077-3. [DOI] [Google Scholar]
- Varnes J. G.; Lehr G. S.; Moore G. L.; Hulsizer J. M.; Albert J. S. Efficient preparation of 2,4-methanoproline. Tetrahedron Lett. 2010, 51, 3756–3758. 10.1016/j.tetlet.2010.05.054. [DOI] [Google Scholar]
- Mykhailiuk P. K.; Kubyshkin V.; Bach T.; Budisa N. Peptidyl-Prolyl Model Study: How Does the Electronic Effect Influence the Amide Bond Conformation?. J. Org. Chem. 2017, 82, 8831–8841. 10.1021/acs.joc.7b00803. [DOI] [PubMed] [Google Scholar]
- Levterov V. V.; Michurin O.; Borysko P. O.; Zozulya S.; Sadkova I. V.; Tolmachev A. A.; Mykhailiuk P. K. Photochemical In-Flow Synthesis of 2,4-Methanopyrrolidines: Pyrrolidine Analogues with Improved Water Solubility and Reduced Lipophilicity. J. Org. Chem. 2018, 83, 14350–14361. 10.1021/acs.joc.8b02071. [DOI] [PubMed] [Google Scholar]
- Malpass J. R.; Patel A. B.; Davies J. W.; Fulford S. Y. Modification of 1-substituents in the 2-azabicyclo[2.1.1]hexane ring system; approaches to potential nicotinic acetylcholine receptor ligands from 2,4-methanoproline derivatives. J. Org. Chem. 2003, 68, 9348–9355. 10.1021/jo035199n. [DOI] [PubMed] [Google Scholar]
- Esslinger C. S.; Koch H. P.; Kavanaugh M. P.; Philips D. P.; Chamberlin A. R.; Thompson C. M.; Bridges R. J. Structural Determinants of Substrates and Inhibitors: Probing Glutamate Transporters with 2,4-Methanopyrrolidine-2,4-dicarboxylate. Bioorg. Med. Chem. Lett. 1998, 8, 3101–3106. 10.1016/S0960-894X(98)00560-5. [DOI] [PubMed] [Google Scholar]
- Firth N. C.; Brown N.; Blagg J. Plane of Best Fit: A Novel Method to Characterize the Three-Dimensionality of Molecules J. J. Chem. Inf. Model. 2012, 52, 2516–2525. 10.1021/ci300293f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurer W. H. B.; Schwarz M. K. Molecular Shape Diversity of Combinatorial Libraries: A Prerequisite for Broad Bioactivity. J. Chem. Inf. Comput. Sci. 2003, 43, 987–1003. 10.1021/ci025599w. [DOI] [PubMed] [Google Scholar]
- Garner P.; Cox P. B.; Rathayake U.; Holloran N.; Erdman P. Design and Synthesis of Pyrrolidine-based Fragments That Sample Three-dimensional Molecular Space. ACS Med. Chem. Lett. 2019, 10, 811–815. 10.1021/acsmedchemlett.9b00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.