Abstract
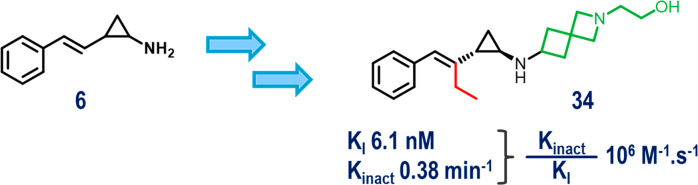
Leveraging the catalytic machinery of LSD1 (KDM1A), a series of covalent styrenylcyclopropane LSD1 inhibitors were identified. These inhibitors represent a new class of mechanism-based inhibitors that target and covalently label the FAD cofactor of LSD1. The series was rapidly progressed to potent biochemical and cellular LSD1 inhibitors with good physical properties. This effort resulted in the identification of 34, a highly potent (<4 nM biochemical, 2 nM cell, and 1 nM GI50), and selective LSD1 inhibitor. In-depth kinetic profiling of 34 confirmed its covalent mechanism of action, validated the styrenylcyclopropane as an FAD-directed warhead, and demonstrated that the potency of this inhibitor is driven by improved non-covalent binding (KI). 34 demonstrated robust cell-killing activity in a panel of AML cell lines and robust antitumor activity in a Kasumi-1 xenograft model of AML when dosed orally at 1.5 mg/kg once daily.
Keywords: LSD1, KDM1A, histone demethylase, irreversible inhibition, styrenylcyclopropane, AML
The dynamic organization of the genome in eukaryotic cells is regulated in large part by diverse post-translational modifications of histones, which control access to DNA and ultimately alter gene expression.1,2 The identification of recurrent genetic alterations in genes encoding histone-modifying enzymes in cancer cells of all types3 suggests that manipulation of chromatin structure is a strategy commonly employed by cancer cells to invoke transcriptional programs that prevent differentiation and promote proliferation. Lysine-specific demethylase LSD1 (also known as KDM1A) acts on histone H3 both as a transcriptional corepressor through demethylation of lysine-4 (H3K4) and as a coactivator by targeting the methylated lysine-9 residue (H3K9).4,5 These activities have been shown to play an essential role in a variety of normal physiological processes, including cell proliferation,6 hematopoietic differentiation,7 chromosome segregation,8 spermatogenesis,9 stem cell pluripotency,10 and embryonic development.11 LSD1 is highly expressed in many cancer types,12 where it plays a key role in oncogenic processes, including compromised differentiation, proliferation, migration, and invasion as well as metabolic reprogramming.13,14
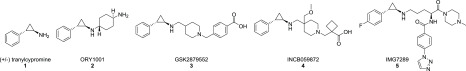
LSD1 has drawn considerable and increasing attention as a therapeutic target in human malignancies because of its involvement in a variety of disease states, including acute myelogenous leukemia (AML),15 small-cell lung cancer,16 and myelofibrosis.17 This interest has resulted in the advancement of multiple LSD1 inhibitors into clinical trials (see Figure 1). Many of these inhibitors are derived from tranylcypromine (1), a mechanism-based covalent inhibitor of monoamine oxidase A and B (MAO A and B) that demonstrates weak activity against LSD1. These clinical-stage inhibitors support LSD1 inactivation via selective reduction of the LSD1-bound flavin adenine dinucleotide (FAD) cofactor.
Figure 1.
Tranylcypromine and related LSD1 inhibitors.
Given our interest in targeting disease-relevant chromatin regulatory mechanisms for therapeutic applications, we began a hit-finding campaign with the goal of identifying novel covalent inhibitors of LSD1. LSD1 has a catalytic domain that is structurally homologous with those of MAOs, which utilize a non-covalently bound FAD as a cofactor.18 The similarity in the catalytic and structural properties of LSD1 and MAO A and B prompted the investigation of anti-MAO chemotypes as potential LSD1 inhibitors.19 A survey of the MAO literature identified propargylamines and cyclopropylamines as functional groups capable of labeling the FAD cofactor of the MAOs. Using these cyclopropylamines and propargylamines to inform our design, we generated an initial hit-finding collection that included MAO inhibitors, commercially available cyclopropylamine and propargylamine compounds, and de novo designs. Screening of this collection using an LSD1 enzymatic assay (see the Supporting Information) identified several interesting cyclopropylamine derivatives as starting points, including spirocyclopropylamines20,21 and styrenylcyclopropylamine 6. Herein we report our efforts to optimize 6 because of its impressive potency compared with 1.
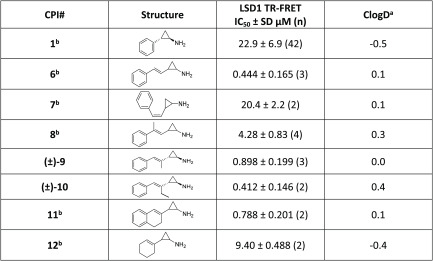
The initial medicinal chemistry efforts explored substitution and modification of the styrenyl group (Table 1). The (Z)-styrene analogue 7 lost significant activity (>40-fold) against LSD1, demonstrating that the geometry of the styrene is critical for productive binding. Subsequently, substitution of the styrene by incorporating a methyl group at the 1- or 2-position afforded 8 and 9, respectively. There was clear preference for substitution at the 2-position over the 1 position. The 2-substituted compound 9 demonstrated similar inhibition of LSD1 relative to the unsubstituted starting point 6 while being ClogD neutral. Extension from the methyl group to an ethyl group resulted in 10, a compound with activity comparable to that of the methyl-substituted analogue 9, although the ethyl group had a modest impact on the physical properties relative to 6 (ΔClogD = 0.3). In addition to substitution, more dramatic modifications to the styrenyl group were investigated, including cyclization to form dihydronaphthyl derivative 11 and removal of the phenyl ring to afford cyclohexene 12. The dihydronaphthyl derivative retained activity similar to that of the ethyl-substituted compound, but removal of the aryl ring as in 12 resulted in a >10-fold loss in activity. Further optimization of this series focused upon exploration of the amine substitution on styrenylcyclopropylamines 6 and 9.
Table 1. Exploration of the Styrene.
Calculated using Stardrop 6.6.
Mixtures of diastereomers.
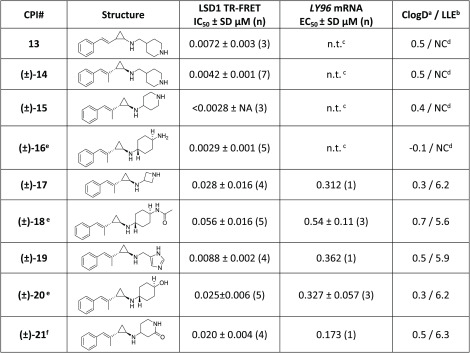
Our initial N-substituted analogues appended a 4-piperidinylmethyl group to the unsubstituted styrenyl scaffold, as in compound 13 (Table 2). These initial compounds demonstrated good potency (<10 nM) in our biochemical assay. Further profiling of 13 identified poor plasma stability as a potential liability. This observation was made when attempting to collect plasma protein binding (PPB) information, as it was observed that ≤5% of the parent compound remained after incubation in plasma. Efforts to address this issue focused upon substitution of the styrene, as we hypothesized that this group contributed to the poor stability. The methyl-substituted analogue 14 was synthesized to test this hypothesis, and encouragingly, 14 had biochemical potency comparable to that of 13 and demonstrated increased plasma stability across species.
Table 2. N-Substitution of Early Styrenylcyclopropylamine Analogues.
Calculated using Stardrop 6.6.
LLE = −log(LY96 EC50) – ClogD.
n.t. = not tested.
NC = not calculated.
Single unknown diastereomer.
Mixture of diastereomers.
With promising activity demonstrated by 14, we began a systematic investigation of amine substituents on the methyl-substituted scaffold (Table 2). Our goal in the optimization process was to improve the biochemical and cellular potency while ensuring that these compounds remained in drug-like chemical space. A variety of saturated heterocycles were investigated, including piperidine (15), trans-cyclohexylamine (16), and azetidine (17). These initial analogues were highly potent biochemical LSD1 inhibitors. Interestingly, analogues containing a linker consisting of four or five bond lengths between the two amine atoms (e.g., 14–16) demonstrated superior potency in the biochemical assay compared with analogues with shorter linkers between the two amine atoms (e.g., 17).
Azetidine 17 was subjected to a cell target engagement assay. Compound-induced changes in expression of the LSD1 target gene lymphocyte antigen 96 (LY96) were measured in human MV4–11 AML cells (see the Supporting Information).22 In the LY96 target engagement assay, compound 17 demonstrated good activity (<1 μM), simultaneously validating our triage scheme and confirming the activity of the styrenylcycloprane scaffold in cellular settings. While the LSD1 TR-FRET assay was initially useful in SAR analysis, many of the N-substituted analogues could not be differentiated with this assay. Thus, we transitioned to driving the program on the basis of the LY96 assay data, including the use of this data to calculate the lipophilic ligand efficiency (LLE)23 and track the optimization of this series.
Next, nonbasic substituents were investigated, including amide (18), imidazole (19), alcohol (20), and lactam (21). These nonbasic substituents were designed to probe whether an explicit hydrogen-bond donor could replace the basic amine heterocycles present in earlier analogues. While the monobasic compounds afforded promising activity in the biochemical assay, these analogues did not significantly improve upon the initial cellular activity seen with azetidine 17 and were net neutral with respect to LLE.
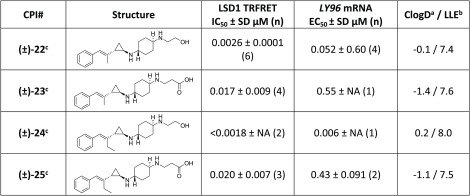
In search of improved activity in the LY96 assay, we focused additional effort around substitution of the distal amine. Initially, we introduced polar groups, such as the ethanol group in 22 or the acid as in 23. The ethanol group in 22 increased the potency in both our biochemical assay and LY96 gene induction assay relative to earlier compounds. This change also resulted in an impressive LLE of 7.4 for compound 22. Introduction of an acid, as in 23, resulted in a large (10-fold) potency loss in the LY96 assay, but the dramatic decrease in the ClogD resulted in a net improvement in the LLE (Table 3, 22 and 23).
Table 3. Comparison of Methyl and Ethyl Styrenylcyclopropylamines.
Calculated using Stardrop 6.6.
LLE = −log(LY96 EC50) – ClogD.
Single unknown diastereomer.
Further exploration of the scaffold focused on ethyl-substituted styrene analogues of 22 and 23 in an effort to modestly increase their ClogD. Synthesis of the ethyl analogues of these compounds afforded ethanol 24 and acid 25. Introduction of the ethyl group afforded improved activity in the cellular assay for ethanol analogue 24 (∼9-fold for 24 vs 22). The improvement in cellular activity offset the increased lipophilicity, as demonstrated by an increase in LLE in going from 22 to 24. Interestingly, introduction of the ethyl group did not have the same effect on the carboxylic acid analogue, as compound 25 and compound 23 demonstrated similar activities in the LY96 assay.
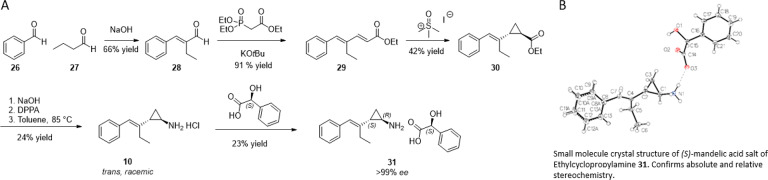
With the impressive LY96 activity and LLE observed with compound 24, we focused our efforts on the ethyl-substituted styrenylcyclopropylamine scaffold. To enable this effort, we developed a scalable synthesis of the enantioenriched cyclopropylamine building block (Scheme 1). The synthesis began with the condensation of benzaldehyde (26) and butanaldehyde (27) to afford enal 28. Reaction of the Horner–Wadsworth–Emmons reagent with enal 28 afforded diene 29 in excellent yield. This diene was then treated with the Corey–Chaykovsky reagent to afford cyclopropane 30 in modest yield. Hydrolysis of the ester followed by a Curtius rearrangement and workup afforded racemic trans-cyclopropane 10. Resolution of 10 with (S)-mandelic acid afforded the enantioenriched cyclopropylamine (1R,2S)-31. The absolute stereochemistry of 31 was confirmed by small-molecule X-ray crystallography.
Scheme 1. Synthesis of (1R,2S)-31.
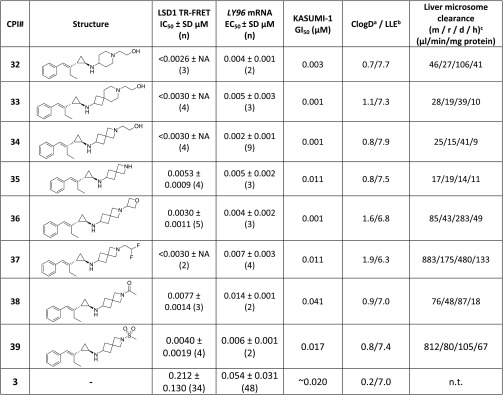
Continuing our SAR efforts on the ethyl-substituted styrenylcyclopropylamine scaffold, we re-examined the impact of the linker between the two amines on the activity and ADME properties. We kept the ethanolamine fragment in place and surveyed a variety of saturated heterocyclic linkers, including piperidine (32), 4,6-spiro (33), and 4,4-spiro (34). These heterocyclic linkers in compounds 32–34 afforded highly active LSD1 inhibitors with TR-FRET IC50 < 3 nM, LY96 EC50 < 10 nM, and LLE ≥ 7.
With the impressive potency of the ethyl-substituted styrenylcyclopropane compounds in the LY96 target engagement assay, we began triaging compounds in our phenotypic assay (see the Supporting Information). In the phenotypic assay we assessed the compounds for growth inhibition in the Kasumi-1 cell line upon 12 days of compound treatment. For these inhibitors there was a very good correlation among all three assays (TR-FRET IC50, LY96 EC50, and phenotypic GI50). While multiple inhibitors afforded potent target engagement and phenotypic activity, we chose to focus on the 4,4-spiro system for further exploration because of the promising liver microsome (LM) data and high LLE of compound 34.
A subset of the 4,4-spiro analogues synthesized are presented in Table 4. Unsubstituted spiro analogue 35 afforded a potent LSD1 inhibitor in the TR-FRET, LY96, and phenotypic assays. Additional substitutions aimed at modulating the basicity of the amine, as with oxetane 36 and difluoroethyl 37, afforded two highly potent LSD1 inhibitors with good activity in the phenotypic assay. These two compounds showed attenuated basicity of the distal amine by >3 pKa units,24 demonstrating that modulated bases can serve as potent LSD1 inhibitors. Next, we tested the requirement for a second basic site in this spiro- scaffold by synthesizing acetamide 38 and sulfonamide 39. Both monobasic compounds afforded potent activity in the LY96 assay with good activity in the phenotypic assay. Additional profiling of the attenuated bases 36 and 37 and the monobasic compounds 38 and 39 demonstrated a significant decrease in microsomal stability compared with compound 34. Compound 34 was selected for further characterization because of its impressive combination of potency, LLE, and low molecular weight.
Table 4. Structure–Activity Relationship on (1R,2S)-31.
Calculated using Stardrop 6.6.
LLE = −log(LY96 EC50) – ClogD.
m/r/d/h = mouse/rat/dog/human.
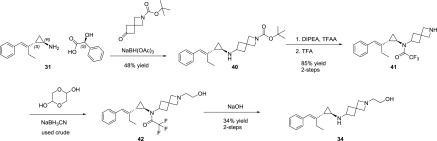
The synthesis of 34 began from styrenylcyclopropylamine 31(25) via reductive amination with tert-butyl 6-oxo-2-azaspiro[3.3]heptane-2-carboxylate (Scheme 2). This afforded spirocyclic cyclopropylamine 40 in good yield. Protection of the cyclopropylamine as the trifluoroacetamide followed by Boc deprotection afforded amine 41. This material was then subjected to reductive amination with glycolaldehyde dimer to afford the penultimate intermediate 42. Removal of the trifluoroacetamide group under basic conditions followed by purification afforded 34 in good yield.
Scheme 2. Synthesis of 34.
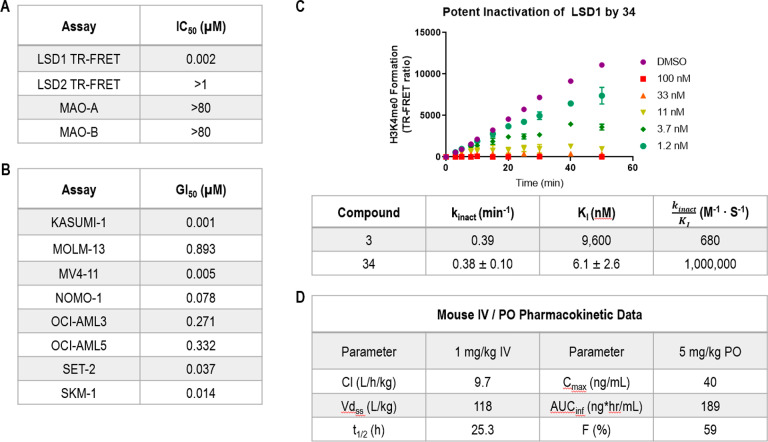
Further characterization of 34 in a panel of FAD-utilizing enzymes confirmed that this compound was highly selective for inhibition of LSD1, with no activity detected against the homologous enzyme LSD2 and the related monoamine oxidases MAO-A and MAO-B (Figure 2A). Additional biochemical characterization of 34 demonstrated that this molecule displays time-dependent inhibition of LSD1 biochemical activity, corroborating its hypothesized covalent mechanism of inhibition (Figure 2C). This covalent inhibition occurs via a two-step mechanism consisting of an initial reversible non-covalent binding event followed by covalent inactivation of the FAD cofactor, in accordance with previous characterization of tranylcypromine-based LSD1 inhibitors.14,26 Interestingly, the ∼1500-fold improved inactivation efficiency (kinact/KI) of 34 over 3 is driven through the reversible non-covalent binding event, while the observed rate of inactivation, kinact, is comparable to that of tranylcypromine-based 3.14,27
Figure 2.
Further characterization of 34.
Profiling of compound 34 in a panel of human AML cell lines resulted in dose-dependent effects on cell growth, with five out of eight cell lines found to be highly sensitive to inhibitor treatment (GI50 < 100 nM). In vivo characterization of 34 began with a single-dose PK experiment in mice (Figure 2D). This PK experiment revealed that 34 is a high-clearance, high-volume compound with acceptable oral bioavailability to enable in vivo efficacy studies.
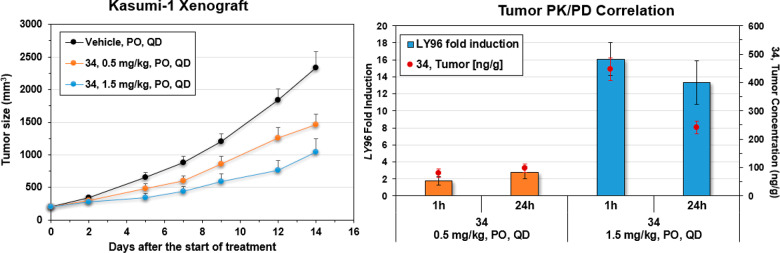
In a Kasumi-1 xenograft model, 34 demonstrated significant and dose-dependent tumor growth inhibition (TGI) when administrated at 0.5 mg/kg and 1.5 mg/kg PO QD (Figure 3) for 14 days. Both doses were well-tolerated, with ≤5% body weight loss observed at 1.5 mg/kg PO and no significant body weight changes at 0.5 mg/kg PO (see the Supporting Information). Compound exposure and target engagement were measured in the plasma and tumor samples at 1 and 24 h after the last dose. Target engagement was monitored via induction of LY96 in the tumor, and a good correlation among induction of LY96, compound concentration in the tumor, and TGI was observed.
Figure 3.
Kasumi-1 xenograft data for compound 34.
Herein we have described our efforts directed toward the discovery of novel covalent inhibitors of LSD1. This research program identified the styrenylcyclopropane structure as a previously unreported mechanism-based inhibitor of LSD1 that covalently labels the FAD cofactor. This novel chemotype diversifies upon the clinically relevant tranylcypromine-based scaffolds and potentially offers access to different physical properties, FAD adducts, and biological effects. Optimization of this series culminated in the identification of compound 34, a highly potent biochemical, cellular, and efficient (LLE) inhibitor. Furthermore, we characterized the kinetics of its covalent inhibition and demonstrated that 34 has an improved inactivation efficiency relative to tranylcypromine-derived 3. This covalent inhibitor is differentiated from the current tranylcypromine-based inhibitors by its highly potent non-covalent binding (KI), which results in an impressive inactivation efficiency roughly 3 orders of magnitude greater than that of tranylcypromine-based 3. Additional characterization of 34 across a panel of AML cell lines demonstrated the broad potential utility of this mechanism in AML. Finally, 34 was shown to provide dose-dependent tumor growth inhibition in an AML xenograft model at tolerated doses.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.0c00060.
Methods and materials, experimental procedures, LSD1 biochemical assay, LSD1 Kinact/Ki studies, LSD1 LY96 assay, cell proliferation assays, and in vivo Kasumi-1 xenograft experiment (PDF)
Author Present Address
† M.D. and A.C.G.: C4 Therapeutics, 490 Arsenal Way, Suite 200, Watertown, MA 02472.
Author Present Address
‡ F.B. and S.F.B.: Foghorn Therapeutics, 100 Binney Street, Suite 610, Cambridge, MA 02142.
Author Present Address
§ A.C.: Schrödinger, LLC, 120 West 45th Street, 17th Floor, New York, NY 10036.
Author Present Address
∥ V.W.: KSQ Therapeutics, 610 Main Street North, 4th Floor, Cambridge, MA 02142.
Author Present Address
⊥ S.B.: AstraZeneca, 35 Gatehouse Drive, Waltham, MA 02451.
Author Present Address
# P.I.: Agenus, 3 Forbes Road, Lexington, MA 02421.
Author Present Address
∇ P.S.: Syros Pharmaceuticals, 620 Memorial Drive, Suite 300, Cambridge, MA 02139.
Author Present Address
○ B.K.A.: Rheos Medicines, 38 Sidney Street, Cambridge, MA 02139.
Author Present Address
◆ J.-C.H.: Goldfinch Bio, 215 First Street, 4th Floor, Cambridge, MA 02142.
Author Present Address
¶ J.E.A.: Northwestern University, 633 Clark Street, Evanston, IL 60208.
The authors declare no competing financial interest.
Supplementary Material
References
- Bannister A. J.; Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell 2007, 128, 693–705. 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Suvà M. L.; Riggi N.; Bernstein B. E. Epigenetic reprogramming in cancer. Science 2013, 339, 1567–1570. 10.1126/science.1230184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y.; Lan F.; Matson C.; Mulligan P.; Whetstine J. R.; Cole P. A.; Casero R. A.; Shi Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 2004, 119, 941–953. 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Metzger E.; Wissmann M.; Yin N.; Mueller J. M.; Schneider R.; Peters A.; Guenther T.; Buettner R.; Schuele R. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature 2005, 437, 436–439. 10.1038/nature04020. [DOI] [PubMed] [Google Scholar]
- Cho H. S.; Suzuki T.; Dohmae N.; Hayami S.; Unoki M.; Yoshimatsu M.; Toyokawa G.; Takawa M.; Chen T.; Kurash J. K.; Field H. I.; Ponder B. A.; Nakamura Y.; Hamamoto R. Demethylation of RB regulator MYPT1 by histone demethylase LSD1 promotes cell cycle progression in cancer cells. Cancer Res. 2011, 71, 655–660. 10.1158/0008-5472.CAN-10-2446. [DOI] [PubMed] [Google Scholar]
- Li Y.; Deng C.; Hu X.; Patel B.; Fu X.; Qiu Y.; Brand M.; Zhao K.; Huang S. Dynamic interaction between TAL1 oncoprotein and LSD1 regulates TAL1 function in hematopoiesis and leukemogenesis. Oncogene 2012, 31, 5007–5018. 10.1038/onc.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv S.; Bu W.; Jiao H.; Liu B.; Zhu L.; Zhao H.; Liao J.; Li J.; Xu X. LSD1 is required for chromosome segregation during mitosis. Eur. J. Cell Biol. 2010, 89, 557–563. 10.1016/j.ejcb.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Godmann M.; Auger V.; Ferraroni-Aguiar V.; Di Sauro A.; Sette C.; Behr R.; Kimmins S. Dynamic regulation of histone H3 methylation at lysine 4 in mammalian spermatogenesis. Biol. Reprod. 2007, 77, 754–764. 10.1095/biolreprod.107.062265. [DOI] [PubMed] [Google Scholar]
- Zhou H.; Li W.; Zhu S.; Joo J. Y.; Do J. T.; Xiong W.; Kim J. B.; Zhang K.; Schöler H. R.; Ding S. Conversion of mouse epiblast stem cells to an earlier pluripotency state by small molecules. J. Biol. Chem. 2010, 285, 29676–29680. 10.1074/jbc.C110.150599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster C. T.; Dovey O. M.; Lezina L.; Luo J. L.; Gant T. W.; Barlev N.; Bradley A.; Cowley S. M. Lysine-specific demethylase 1 regulates the embryonic transcriptome and CoREST stability. Mol. Cell. Biol. 2010, 30, 4851–4863. 10.1128/MCB.00521-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayami S.; Kelly J. D.; Cho H.-S.; Yoshimatsu M.; Unoki M.; Tsunoda T.; Field H. I.; Neal D. E.; Yamaue H.; Ponder B. A.; Nakamura Y.; Hamamoto R. Overexpression of LSD1 contributes to human carcinogenesis through chromatin regulation in various cancers. Int. J. Cancer 2011, 128, 574–586. 10.1002/ijc.25349. [DOI] [PubMed] [Google Scholar]
- Kosumi K.; Baba Y.; Sakamoto A.; Ishimoto T.; Harada K.; Nakamura K.; Kurashige J.; Hiyoshi Y.; Iwatsuki M.; Iwagami S.; Sakamoto Y.; Miyamoto Y.; Yoshida N.; Oki E.; Watanabe M.; Hino S.; Nakao M.; Baba H. Lysine-specific demethylase-1 contriutes to malignant behavior by regulation of invasive activity and metabolic shift in esophageal cancer. Int. J. Cancer 2016, 138, 428–439. 10.1002/ijc.29714. [DOI] [PubMed] [Google Scholar]
- Sheng W.; LaFleur M. W.; Nguyen T. H.; Chen S.; Chakravarthy A.; Conway J. R.; Li Y.; Chen H.; Yang H.; Hsu P. H.; Van Allen E. M.; Freeman G. J.; De Carvalho D. D.; He H. H.; Sharpe A. H.; Shi Y. LSD1 ablation stimulates anti-tumor immunity and enables checkpoint blockade. Cell 2018, 174, 549–563. 10.1016/j.cell.2018.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris W. J.; Huang X.; Lynch J. T.; Spencer G. J.; Hitchin J. R.; Li Y.; Ciceri F.; Blaser J. G.; Greystoke B. F.; Jordan A. M.; Miller C. J.; Ogilvie D. J.; Somervaille T. C. P. The histone demethylase KDM1A sustains the oncogenic potential of MLL-AF9 leukemia stem cells. Cancer Cell 2012, 21, 473–487. 10.1016/j.ccr.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Mohammad H. P.; Smitheman K. N.; Kamat C. D.; Soong D.; Federowicz K. E.; Van Aller G. S.; Schneck J. L.; Carson J. D.; Liu Y.; Butticello M.; Bonnette W. G.; Gorman S. A.; Degenhardt Y.; Bai Y.; McCabe M. T.; Pappalardi M. B.; Kasparec J.; Tian X.; McNulty K.; Rouse M.; McDevitt P.; Ho T.; Crouthamel M.; Hart T. K.; Concha N. O.; McHugh C. F.; Miller W. H.; Dhanak D.; Tummino P. J.; Carpenter C. L.; Johnson N. W.; Hann C. L.; Kruger R. G. A DNA Hypomethylation Signature Predicts Antitumor Activity of LSD1 Inhibitors in SCLC. Cancer Cell 2015, 28, 57–69. 10.1016/j.ccell.2015.06.002. [DOI] [PubMed] [Google Scholar]
- ClinicalTrials.gov . IMG-7289 in patients with myelofibrosis. https://www.clinicaltrials.gov/ct2/show/NCT03136185?term=LSD1 (accessed 2020-04-09).
- Forneris F.; Binda C.; Battaglioli E.; Mattevi A. LSD1: oxidative chemistry for multifaceted functions in chromatin regulation. Trends Biochem. Sci. 2008, 33, 181–189. 10.1016/j.tibs.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Binda C.; Valente S.; Romanenghi M.; Pilotto S.; Cirilli R.; Karytinos A.; Ciossani G.; Botrugno O. A.; Forneris F.; Tardugno M.; Edmondson D. E.; Minucci S.; Mattevi A.; Mai A. Biochemical, Structural, and Biological Evaluation of Tranylcypromine Derivatives as Inhibitors of Histone Demethylases LSD1 and LSD2. J. Am. Chem. Soc. 2010, 132, 6827–6833. 10.1021/ja101557k. [DOI] [PubMed] [Google Scholar]
- Albrecht B. K.; Audia J. E.; Cote A.; Duplessis M.; Gehling V. S.; Good A.; Harmange J.-C.; Leblanc Y.; Nasveschuk C. G.; Taylor A. M.; Vaswani R. G. Spirocyclic compounds as LSD1 inhibitors. WO 2016123387, 2016.
- Shi Y.; Wu Y.-R.; Su M.-B.; Shen D.-H.; Gunosewoyo H.; Yang F.; Li J.; Tang J.; Zhou Y.-B.; Yu L.-F. Novel spirocyclic tranylcypromine derivatives as lysine-specific demethylase 1 (LSD1) inhibitors. RSC Adv. 2018, 8, 1666–1676. 10.1039/C7RA13097J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J.; Trojer P. Targeting histone lysine methylation in cancer. Pharmacol. Ther. 2015, 150, 1–22. 10.1016/j.pharmthera.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Leeson P. D.; Springthorpe B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nat. Rev. Drug Discovery 2007, 6, 881–890. 10.1038/nrd2445. [DOI] [PubMed] [Google Scholar]
- Morgenthaler M.; Schweizer E.; Hoffmann-Röder A.; Benini F.; Martin R. E.; Jaeschke G.; Wagner B.; Fischer H.; Bendels S.; Zimmerli D.; Schneider J.; Diederich F.; Kansy M.; Müller K. Predicting and tuning physicochemical properties in lead optimization: amine basicities. ChemMedChem 2007, 2, 1100–1115. 10.1002/cmdc.200700059. [DOI] [PubMed] [Google Scholar]
- Albrecht B. K.; Audia J. E.; Cote A.; Duplessis M.; Gehling V. S.; Harmange J.-C.; Vaswani R. G. Preparation of 2-styrylcyclopropan-1-amine derivatives as LSD1 inhibitors and uses thereof. WO 2016172496, 2016.
- Schmidt D. M.; McCafferty D. G. trans-2-Phenycyclopropylamine is a mechanism -based inactivator of the histone demethylase LSD1. Biochemistry 2007, 46, 4408–4416. 10.1021/bi0618621. [DOI] [PubMed] [Google Scholar]
- Strelow J. M. A Perspective on the Kinetics of Covalent and Irreversible Inibition. SLAS Discovery 2017, 22, 3–20. 10.1177/1087057116671509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.