This review article discusses current challenges to the diagnosis and management of patients with bacterial vaginosis.
Supplemental digital content is available in the text.
Abstract
Despite the availability of a number of oral and intravaginal antibiotic medications for the treatment of bacterial vaginosis (BV), management of this condition remains challenging. Recurrent BV occurs in >50% of patients receiving guideline-recommended treatments. This may be due to persistence or resurgence of the BV biofilm after treatment cessation, failure to reestablish an optimal vaginal microbiome after treatment, reinfection from an untreated sexual partner, or a combination of these factors. Nonadherence to multidose BV therapies may potentially contribute to recurrent BV, although there are no published data that directly assess the role of nonadherence to poor treatment outcomes and recurrent BV. There is a need for studies of BV treatment adherence in real-world settings as well as studies to explore the relationship between treatment adherence and recurrence. This review explores challenges associated with diagnosing and treating BV, current multidose antibiotic treatment options, newer single-dose treatment options, and ways to potentially maximize treatment success for this common vaginal infection.
Bacterial vaginosis (BV) is the most common cause of vaginal discharge and is characterized by a shift in the vaginal microbiota from lactobacilli to facultative and strict anaerobic bacteria1; however, its etiology is controversial. The overall prevalence of BV in North America in women of reproductive age is 27.4%, with a higher prevalence in black (33.2%) and Hispanic women (30.7%) than in white (22.7%) or Asian women (11.1%).2 Other characteristics associated with an increased risk of BV include new or multiple male sexual partners, partner concurrency, a female sexual partner with BV symptoms, being herpes simplex virus type 2 seropositive, and smoking, whereas consistent condom use is associated with a decreased risk.3–7
Epidemiological data strongly suggest that BV is sexually transmitted.8–10 However, there remains a critical need to determine whether BV results from acquisition of a keystone pathogen or a polymicrobial consortium of bacteria that are sexually transmitted.11,12 A key factor in BV is the occurrence of a multispecies biofilm on vaginal epithelial cells containing abundant Gardnerella vaginalis, smaller amounts of Atopobium vaginae, and other undefined bacterial species.13,14 The BV biofilm becomes metabolically inactive upon treatment, which leads to decreased susceptibility to antibiotics; this may contribute to high BV recurrence rates after therapy.15 Nonadherence to antibiotic treatment may also contribute to recurrent BV, although there are no published data that directly assess the role of nonadherence to treatment and recurrent BV.16 This review explores challenges associated with diagnosing and treating BV, current multidose antibiotic treatment regimens, newer single-dose treatment options, potential barriers to treatment adherence, and ways to potentially maximize treatment success.
DIAGNOSIS OF BV
Misdiagnosis is a major challenge to effective management and treatment for women with BV. Women with BV report being misdiagnosed with vulvovaginal candidiasis (VVC) or not being tested for BV when they present with vaginal symptoms.17 Telephone diagnosis is not accurate for diagnosis of BV, and health care providers may require additional education on the diagnostic criteria for BV.18 Incorrect self-diagnosis of BV as candidiasis is also a concern18,19 and may lead to inappropriate self-treatment with over-the-counter antifungals or home remedies, which may select for more resistant Candida strains, exacerbate recurrences and complications, lead to increased costs and office visits, and/or make it difficult to implement appropriate treatment.17–21
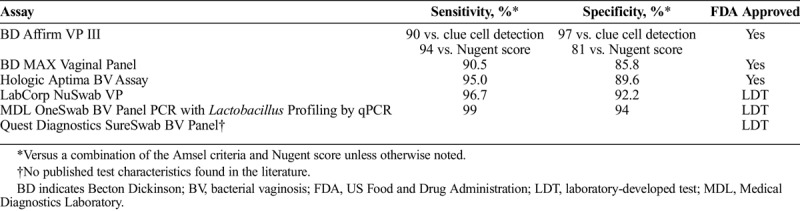
In clinical settings, at least 3 of 4 Amsel criteria are necessary for a diagnosis of BV: homogenous, thin, grayish white vaginal discharge; vaginal pH >4.5, positive whiff amine test result; and clue cells present on a wet mount of vaginal fluid.10 Bacterial vaginosis is more rigorously defined by determining the Nugent score on a Gram stain of vaginal secretions. Scores of 0 to 3 are graded as Lactobacillus predominant vaginal microbiota, 4 to 6 as intermediate microbiota, and 7 to 10 as BV.22 Compared with the Nugent score, the sensitivity and specificity of the Amsel criteria range from 37% to 70% and from 94% to 99%, respectively (Table 1).23 In the United States, several commercially available molecular diagnostic assays are available for the diagnosis of BV in women.23 One of these is the Becton Dickinson BD Affirm VP III assay, which is a DNA hybridization probe assay for the detection of G. vaginalis. Additional commercially available molecular diagnostic assays for BV diagnosis in symptomatic women include 5 nucleic acid amplification tests. These are the Becton Dickinson BD MAX Vaginal Panel, Hologic Aptima BV, LabCorp NuSwab VG, Medical Diagnostics Laboratory OneSwab BV Panel PCR with Lactobacillus Profiling by qPCR, and the Quest Diagnostics SureSwab BV Panel, all of which detect multiple bacterial species through multiplex PCR (Table 1).23–25 Although these assays are associated with higher sensitivity and specificity than the Amsel criteria (Table 1), they are not point-of-care tests and are more costly.23
TABLE 1.
Commercially Available Molecular Assays for the Diagnosis of Bacterial Vaginosis in Women in the United States23–25
CURRENTLY AVAILABLE BV TREATMENT REGIMENS
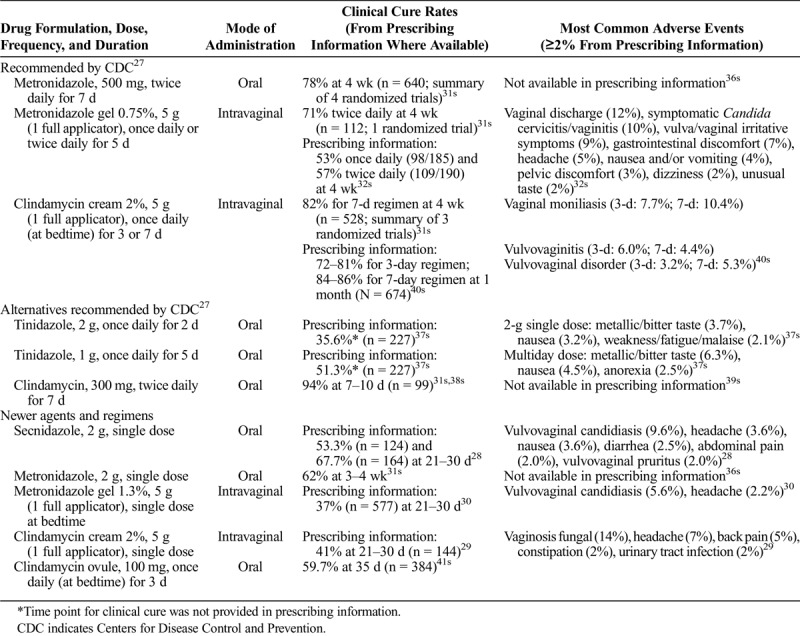
Currently recommended and alternative regimens for BV according to the 2015 US Centers for Disease Control and Prevention sexually transmitted disease treatment guidelines as well as the 2018 European guidelines on the management of vaginal discharge include multidose oral metronidazole, tinidazole, or clindamycin in addition to multidose intravaginal of metronidazole or clindamycin.26,27 Newer single-dose treatment options include oral secnidazole, a 5-nitroimidazole Food and Drug Administration (FDA) approved in 2017 in the United States as a single-dose granule formulation,28 and 2 intravaginal treatments: 2% extended-release clindamycin cream and 1.3% vaginal metronidazole gel (Table 2).16,23,24,28–30,31s–41s Supplementary Table 1 (http://links.lww.com/OLQ/A485) lists the mechanism of action of metronidazole, tinidazole, secnidazole, and clindamycin.28–30,32s,36s,39s–41s
TABLE 2.
In the past, studies evaluating treatments for BV have been inconsistent in their definition of cure, most only using 1 week for follow-up.22,42s The Amsel or Nugent criteria provide an objective assessment of cure, and 3- or 4-week follow-up periods may provide a better measure of efficacy than earlier follow-up.22 The 2016 guidance by the US FDA on developing drugs for the treatment of BV recommends evaluating clinical cure at 7 to 14 days after the first day of treatment, with clinical cure defined as resolution of abnormal vaginal discharge, negative whiff test result, and clue cells <20% per high-power field on wet mount.43s In addition, the 2016 US FDA guidance recommends that only patients with a Nugent score of ≥7 should be enrolled in clinical trials evaluating BV treatments to increase the reliability of diagnosis.43s
EFFICACY AND SAFETY PROFILES OF CURRENT BV TREATMENT REGIMENS
Recommended and Alternative Regimens
In studies comparing 7-day oral metronidazole (500 mg twice daily) to 7-day intravaginal clindamycin cream (2%, 5 g once daily) and 5-day intravaginal metronidazole gel (0.75%, 5 g twice daily), 4-week cure rates were comparable at approximately 70% to 80% (Table 2).31s However, these studies varied in whether the 3 components of their cure definitions included resolution of symptoms, homogeneous vaginal discharge, pH, amine odor, and/or clue cells.31s Moreover, none of these studies used a Nugent score ≥7 in their inclusion criteria, as all were conducted before the 2016 US FDA guidance.31s,43s It should also be noted that the prescribing information for the 5-day intravaginal metronidazole gel reported a lower 4-week cure rate: 53% for daily dosing and 57% for twice-daily dosing.32s In nonpregnant women, clindamycin (oral, cream, or ovule) and metronidazole (oral) had similar rates of treatment failure, but metronidazole had a higher rate of adverse events, including metallic taste, nausea, and vomiting.44s A randomized, double-blind study comparing oral metronidazole 500 mg and tinidazole 500 mg and 1 g, each twice daily for 7 days, showed similar cure rates among the 3 treatment groups (64.1%, 67.5%, and 61.9% at 1 month, respectively).45s There was a higher incidence of bad taste with tinidazole 1 g (41.8%) than with metronidazole 500 mg (11.0%) or tinidazole 500 mg (15.2%).45s The incidence of vaginal yeast infection was similar among the 3 treatment arms (24.5%–29.3%).45s
Single-Dose Oral and Intravaginal Regimens
Secnidazole is a recently approved single-dose oral treatment option for BV. The convenience of a single oral dose is a potential benefit of secnidazole compared with other oral regimens.46s A randomized, double-blind, phase 3, noninferiority study conducted in Europe showed that a single 2-g oral dose of secnidazole (in a sachet formulation) was as effective as 7 days of metronidazole 500 mg twice daily for treatment for women with BV, with cure rates at day 28 of 60.1% and 59.5%, respectively.46s In that study, BV cure was defined as both clinical and microbiological cure.46s In a randomized, double-blind, placebo-controlled phase 3 study in the United States, clinical outcome responder rates at 21 to 30 days were 53.3% with 2 g oral secnidazole (in a granule formulation) versus 19.3% with placebo (P < 0.001); clinical cure rates were 64.0% and 26.4%, respectively, based on the 2016 US FDA guidance, with resolution of abnormal vaginal discharge, normal whiff test result, and clue cells <20% at days 7 to 14. Assessment was limited to patients with Nugent scores of ≥7 at baseline.28,47s Another randomized, double-blind, placebo-controlled study reported clinical responder rates at 21 to 30 days of 67.7% with secnidazole versus 17.7% with placebo (P < 0.001).28 Vaginal yeast infections were the most common treatment-related adverse events in randomized clinical trials of secnidazole (2.8%–4.8% with 2 g secnidazole, 1.4%–3.1% with placebo)47s,48s and in an open-label, single-arm phase 3 study evaluating the safety of 2 g secnidazole in women or adolescents with BV (5.3%).49s The safety profile of secnidazole is well established based on clinical practice experience during more than 30 years of use globally and is consistent with the safety profile for the 5-nitroimidazole class.50s
Single-dose intravaginal treatments may provide an alternative for patients who desire greater convenience than multidose regimens. The 2 US FDA-approved single-dose intravaginal treatments are 2% extended-release clindamycin cream and single-dose 1.3% vaginal metronidazole gel. Extended-release clindamycin cream (2%) and 7-day clindamycin cream were compared in a randomized, single-blind, active-controlled study.51s At the test-of-cure visit (21–30 days after treatment), clinical and microbiological BV cure rates were 64.3% and 56.5%, respectively, with the single-dose cream versus 63.2% and 57.7%, respectively, with the 7-day regimen.51s However, these results were based on a per-protocol and not intent-to-treat analysis, using data collected from only 46% of enrolled participants with evaluable data.51s In another randomized, double-blind, placebo-controlled study, lower clinical and microbiological cure rates at 21 to 30 days after treatment with the single-dose clindamycin cream (41.0% and 44.9%, respectively) were obtained.29 The most common adverse events were VVC and vulvovaginal pruritus (14.4% and 4.2% with 2% extended-release clindamycin cream vs. 10.2% and 3.0% with the 7-day regimen, respectively).51s The other approved single-dose intravaginal treatment, 1.3% metronidazole gel, was compared with vehicle control in a randomized, double-blind study.52s,53s Clinical and microbiological cure rates were 37.0% and 19.5%, respectively, with single-dose 1.3% metronidazole gel at the test-of-cure visit (days 21–30) versus 26.7% and 7.7%, respectively, with the vehicle control.30,53s The most common adverse event was VVC (5.6% vs. 3.2% with the control).53s With the exception of secnidazole, it is important to note that the efficacy of the 2 single-dose intravaginal creams has not been directly compared with multidose BV treatments.
RECURRENT BV
Although short-term cure rates are generally comparable with currently recommended treatments for BV, studies with longer follow-up indicate high rates of recurrence.54s The consequences of unresolved BV include adverse obstetric outcomes including premature rupture of membranes and preterm labor/delivery, increased risk of acquisition of HIV and other sexually transmitted infections, and development of precancerous cervical lesions, which may be related to its role in the persistence of human papillomavirus.55s–58s Recurrent BV can also have a significant psychosocial impact on women, affecting sexual relationships and quality of life.59s,60s More than half of patients treated with oral metronidazole for BV experience recurrence: 58% reported recurrence by 1 year in a prospective study of oral metronidazole 400 mg twice daily for 7 days, and 52% reported recurrence at a mean follow-up of 6.9 years in a long-term observational study following oral metronidazole 500 mg 3 times daily for 10 days.33s,61s Clindamycin cream seems to have relapse rates comparable to oral metronidazole, but long-term studies are lacking.33s,44s Short-term recurrence rates (at 1 and 2 months) were 30% to 40% with tinidazole 500 mg twice daily for 7 days and approximately 20% with 1 mg tinidazole twice daily for 7 days.45s In a dose-ranging study of intravaginal metronidazole gel, the rate of symptom recurrence ranged from 21.4% (with 1.3% gel for 5 days) to ≥50% (with 0.75% gel for 5 days or 1.3% gel for 1 or 3 days).52s The similar recurrence rates of intravaginal formulations of clindamycin and metronidazole administered as a single dose compared with a multidose course of metronidazole indicate that patient preference for mode and frequency of treatment administration could play a role in treatment selection.
The most significant risk factor for recurrent BV is a regular sexual partner.33s Recurrence may also be due to persistence or resurgence of BV-associated bacteria in the BV biofilm, or failure to reestablish an optimal vaginal microbiome dominated by Lactobacillus species after treatment.54s,62s,63s Vaginal colonization and extravaginal reservoirs of BV-associated bacteria are also risk factors for persistent and recurrent BV.34s,64s Patient nonadherence to multidose therapy could also potentially contribute to recurrent BV, although there are no published data that directly assess the role of nonadherence to treatment and recurrent BV. This role for adherence has been identified in treatment of other bacterial infections.65s,66s
CHALLENGES IN BV MANAGEMENT
Restoring Normal Vaginal Microbiota
The need to restore normal vaginal microbiota is a major challenge associated with BV treatment.62s Although up to 300 bacterial species may be present in BV, the most common microorganisms associated with BV include G. vaginalis, A. vaginae, Mobiluncus species, Prevotella species, Leptotrichia/Sneathia species, Megasphaera species, and bacteria in the Clostridiales order.54s,67s–69s Although G. vaginalis was previously believed to be the sole cause of BV, this has not been definitively established.67s An additional hypothesis for the pathogenesis of BV is that it is caused by a polymicrobial consortium of microorganisms that are sexually transmitted.12 In the original study on the BV biofilm, G. vaginalis was found to constitute 60% to 95% of biofilm mass, in addition to smaller amounts of Atopobium (1%–40% of biofilm mass) and Lactobacillus species (0.01%–5% of biofilm mass).14 The increased rate of BV in African American women may be related to differences in their vaginal microbiome compared with that of women of European ancestry.70s Healthy, reproductive-aged African American women are more likely to have a vaginal microbiome dominated by G. vaginalis and other BV-associated bacteria.71s
An optimal vaginal microbiome includes Lactobacillus species, which produce lactic acid and hydrogen peroxide and prevent the growth of BV-associated bacteria.42s,63s Beneficial Lactobacillus species in the vagina also produce bacteriocins, which inhibit the growth of other bacteria.22,42s L. crispatus is the vaginal lactobacilli most strongly associated with optimal vaginal microbiota.63s,72s Probiotics have been studied as adjuncts to traditional antibiotic therapy for BV in an attempt to reestablish optimal vaginal microbiota67s,73s and to prevent recurrent BV, with marginal results.74s–77s Several of the probiotics studied include a yogurt drink containing L. crispatus, L. gasseri, L. jensenii, and L. rhamnosus73s; a vaginal capsule containing L. rhamnosus, L. acidophilus, and Streptococcus thermophilus74s; a vaginal pessary containing L. acidophilus75s; and a vaginal capsule or applicator containing L. crispatus.76s,77s Overall, results from probiotic studies have been mixed and additional studies are needed to determine the efficacy of probiotics in preventing or treating BV.
Sustained cure and effectiveness against BV-associated sequelae and the high rate of recurrence without antibiotic-associated adverse events may be obtained through approaches combining antimicrobials with biofilm-disrupting agents. One such investigational agent, TOL-463, is a boric acid–based vaginal anti-infective enhanced with ethylenediaminetetraacetic acid that specifically targets vaginal bacterial and fungal biofilms.78s It has been designed as a dual-indication treatment of both BV and VVC. In a phase 2, investigator-blinded study of TOL-463 inserts and gel, clinical cure rates of BV at test of cure were 59% (95% confidence interval [CI], 41%–75%) for TOL-463 insert and 50% (95% CI, 31%–69%) for TOL-463 gel. For VVC, clinical cure rates were 92% (95% CI, 67%–99%) for TOL-463 insert and 81% (95% CI, 57%–93%) for TOL-463 gel. Both products were safe and well tolerated with no secondary cases of VVC; vulvovaginal burning was the most common adverse event (9.6%).78s
Another potential newcomer to the anti-BV armamentarium is astodrimer 1% gel, a microbicidal vaginal gel that may hold promise for the prevention of recurrent BV.79s Although not an antibiotic, astodrimer belongs to a group of topical microbicides known as polyanion-based entry inhibitors. In 2 phase 3, double-blind, multicenter trials, astodrimer sodium gel was associated with a lower rate of BV recurrence than placebo. Clinical cure rates at days 9 to 12 were 50.4% (59/117) versus 16.5% (19/115; P < 0.001) in study 1 and 56.7% (68/120) versus. 21.4% (25/117; P < 0.001) for study 2 of astodrimer versus placebo. Adverse events were generally mild and self-limiting.79s
Treatment Adherence and BV
Adherence rates from studies with BV treatments are not available for all drugs and formulations.16,33s–35s For metronidazole (500 mg twice daily for 7 days), adherence rates were 50% using a personal digital assistant and 68.3% using a paper diary.16 At least 80% were self-reported.33s,35s For metronidazole gel 75%, 5 g once or twice daily for 5 days, adherence rates were 77.6% using a personal digital assistant and 88.5% using a paper diary16; 93% were self-reported.34s As with other conditions treated by antibiotics, nonadherence to antibiotics for BV could potentially lead to drug resistance among BV-associated bacteria, which can adversely affect patient outcomes and result in a reduction in the number of effective antibiotics.65s Many of the currently recommended oral and intravaginal treatments for BV are associated with a number of drawbacks. Women with recurrent BV have reported frustration with rapid recurrence after initially effective 7-day regimens with side effects that may be difficult to tolerate; as a result, they may self-treat unless the episode is symptomatic or severe.17 Potential barriers to adherence to oral treatments for BV may include gastrointestinal complaints, including nausea, stomach cramps, and diarrhea; bad (metallic) taste; and difficulty swallowing tablets.17,80s,81s For intravaginal treatments, barriers to adherence include product properties such as messiness, leakage, the need to reuse applicators, and lifestyle restrictions, such as refraining from intercourse while on therapy.80s,82s For intravaginal treatments that contain mineral oil (i.e., clindamycin ovule or cream), the use of condoms or diaphragms is not recommended for 3 or 5 days.27,80s Duration of therapy is another potential barrier to adherence for both oral and intravaginal treatments for BV,80s with most of the guideline-recommended and alternative treatments requiring up to 5 or 7 days of treatment.26,27 In a survey of women with a history of BV that compared the use of a 3-day clindamycin ovule with that of a 5-day metronidazole intravaginal gel, the 3-day duration was cited as the most important feature of the ovule, due in part to better adherence.80s Although a shorter course of therapy may correspond with improved adherence, it is important to note that there are no published studies that directly assess the role of nonadherence in BV treatment failure and recurrence.
The fact that most BV clinical studies rely on self-report likely underestimates the rate of nonadherence to multidose regimens.16 Patient self-report is a subjective method of measuring adherence and is considered less accurate than other methods, such as directly measuring medication taking or using electronic devices to indirectly measure medication taking; it is common for patients to overestimate their adherence level.83s Electronic devices, such as Medication Events Monitoring System (MEMS), are an accurate method for measuring adherence in clinical trials.83s–85s However, the high cost of MEMS limits its use in larger clinical trials or routine clinical practice.83s For practical reasons, it is also more difficult to use it for nonplanned, short-term therapies, which is a typical case of antibiotic treatment of BV. In a review of 117 studies comparing methods of measuring adherence, nonelectronic methods overestimated mean adherence compared with MEMS: 17% for self-report, 8% for pill count, and 6% for rating by a health care provider, caregiver, or parent.84s
Studies that use electronic devices to monitor adherence should be performed to assess adherence to treatment in women with BV. Moreover, it should be recognized that adherence is likely to be higher for patients enrolled in clinical trials compared with patients who take antibiotic courses for BV on their own. There is also a need for more studies of adherence in the general population of women with BV, namely, women who are not enrolled in clinical trials, for a real-world perspective on adherence. In addition, there is a need for well-designed studies to assess the role of treatment nonadherence in BV treatment failure and recurrence.
MAXIMIZING CLINICAL SUCCESS IN THE TREATMENT OF BV
Patient preference and tolerability, along with efficacy, should be considered when prescribing treatments for BV.19 Given its high recurrence rates, further steps should be taken to improve the diagnosis, treatment, and management of patients with BV. The main barrier to improvements in diagnosis and treatment is that the exact etiology of BV remains controversial. Until this is determined, these issues will continue to be a challenge. Treatment rates and the prevention of recurrent BV may be enhanced by results of studies using both antibiotics, biofilm-disrupting agents, and/or probiotics to treat BV as well as to restore normal vaginal microbiota and prevent recurrent infections. Recently available single-dose oral and intravaginal therapies may provide more convenient alternatives for patients not wanting to take multidose regimens. However, there are no studies to date that demonstrate superior efficacy of single-dose therapies to multidose regimens for BV. Nevertheless, health care providers should consider the patient's perspective when prescribing a treatment regimen for BV to optimize adherence as well as clinical outcomes.
Supplementary Material
For further references, please see “Supplemental References,” http://links.lww.com/OLQ/A485.
Footnotes
Acknowledgments: The authors were responsible for all content and editorial decisions, and received no honoraria related to the development of this manuscript. Both authors contributed to the research, writing, and reviewing of all drafts of this manuscript and approved the final version. Editorial support in the preparation of this manuscript was provided by Phase Five Communications, funded by Symbiomix Therapeutics, LLC, a Lupin Pharmaceuticals company.
Conflict of Interest and Sources of Funding: C.A.M. has received research grant support from the National Institute of Allergy and Infectious Diseases, is a consultant for Lupin Pharmaceuticals and BioFire Diagnostics, and has received honoraria from Lupin Pharmaceuticals, Cepheid, Becton Dickinson, and Roche Diagnostics. P.K. reports no conflict of interest.
Editorial support in the preparation of this manuscript (i.e., providing PDF copies of manuscripts reviewed, formatting of the manuscript text and tables, and management of the references) was provided by Phase Five Communications, funded by Symbiomix Therapeutics, LLC, a Lupin Pharmaceuticals company. The findings and conclusions in this manuscript are those of the authors and do not represent the views of Symbiomix Therapeutics, LLC, or Lupin Pharmaceuticals.
Author contributions: Both authors contributed to the literature review, writing, and reviewing of all drafts of this manuscript and approved the final draft. They also contributed to revising the manuscript per the reviewers' suggestions.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (http://www.stdjournal.com).
REFERENCES
- 1.Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001–2004 National Health and Nutrition Examination Survey data. Obstet Gynecol 2007; 109:114–120. [DOI] [PubMed] [Google Scholar]
- 2.Peebles K, Velloza J, Balkus JE, et al. High global burden and costs of bacterial vaginosis: A systematic review and meta-analysis. Sex Transm Dis 2019; 46:304–311. [DOI] [PubMed] [Google Scholar]
- 3.Fethers KA, Fairley CK, Hocking JS, et al. Sexual risk factors and bacterial vaginosis: A systematic review and meta-analysis. Clin Infect Dis 2008; 47:1426–1435. [DOI] [PubMed] [Google Scholar]
- 4.Kenyon CR, Buyze J, Klebanoff M, et al. Association between bacterial vaginosis and partner concurrency: A longitudinal study. Sex Transm Infect 2018; 94:75–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradshaw CS, Walker SM, Vodstrcil LA, et al. The influence of behaviors and relationships on the vaginal microbiota of women and their female partners: The WOW Health Study. J Infect Dis 2014; 209:1562–1572. [DOI] [PubMed] [Google Scholar]
- 6.Vodstrcil LA, Walker SM, Hocking JS, et al. Incident bacterial vaginosis (BV) in women who have sex with women is associated with behaviors that suggest sexual transmission of BV. Clin Infect Dis 2015; 60:1042–1053. [DOI] [PubMed] [Google Scholar]
- 7.Abbai NS, Nyirenda M, Naidoo S, et al. Prevalent herpes simplex virus-2 increases the risk of incident bacterial vaginosis in women from South Africa. AIDS Behav 2018; 22:2172–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forcey DS, Vodstrcil LA, Hocking JS, et al. Factors associated with bacterial vaginosis among women who have sex with women: A systematic review. PLoS One 2015; 10:e0141905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muzny CA, Schwebke JR. Gardnerella vaginalis: Still a prime suspect in the pathogenesis of bacterial vaginosis. Curr Infect Dis Rep 2013; 15:130–135. [DOI] [PubMed] [Google Scholar]
- 10.Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis: Diagnostic criteria and microbial and epidemiologic associations. Am J Med 1983; 74:14–22. [DOI] [PubMed] [Google Scholar]
- 11.Schwebke JR, Muzny CA, Josey WE. Role of Gardnerella vaginalis in the pathogenesis of bacterial vaginosis: A conceptual model. J Infect Dis 2014; 210:338–343. [DOI] [PubMed] [Google Scholar]
- 12.Srinivasan S, Fredricks DN. The human vaginal bacterial biota and bacterial vaginosis. Interdiscip Perspect Infect Dis 2008; 2008:750479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Machado A, Cerca N. Influence of biofilm formation by Gardnerella vaginalis and other anaerobes on bacterial vaginosis. J Infect Dis 2015; 212:1856–1861. [DOI] [PubMed] [Google Scholar]
- 14.Swidsinski A, Mendling W, Loening-Baucke V, et al. Adherent biofilms in bacterial vaginosis. Obstet Gynecol 2005; 106(5 Pt 1):1013–1023. [DOI] [PubMed] [Google Scholar]
- 15.Swidsinski A, Mendling W, Loening-Baucke V, et al. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am J Obstet Gynecol 2008; 198:97.e91–97.e96. [DOI] [PubMed] [Google Scholar]
- 16.Bartley JB, Ferris DG, Allmond LM, et al. Personal digital assistants used to document compliance of bacterial vaginosis treatment. Sex Transm Dis 2004; 31:488–491. [DOI] [PubMed] [Google Scholar]
- 17.Bilardi J, Walker S, McNair R, et al. Women's management of recurrent bacterial vaginosis and experiences of clinical care: A qualitative study. PLoS One 2016; 11:e0151794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen-Davis JT, Beck A, Parker R, et al. Assessment of vulvovaginal complaints: Accuracy of telephone triage and in-office diagnosis. Obstet Gynecol 2002; 99:18–22. [DOI] [PubMed] [Google Scholar]
- 19.Chavoustie SE, Eder SE, Koltun WD, et al. Experts explore the state of bacterial vaginosis and the unmet needs facing women and providers. Int J Gynaecol Obstet 2017; 137:107–109. [DOI] [PubMed] [Google Scholar]
- 20.Mania-Pramanik J, Kerkar SC, Salvi VS. Bacterial vaginosis: A cause of infertility? Int J STD AIDS 2009; 20:778–781. [DOI] [PubMed] [Google Scholar]
- 21.Salah RM, Allam AM, Magdy AM, et al. Bacterial vaginosis and infertility: Cause or association? Eur J Obstet Gynecol Reprod Biol 2013; 167:59–63. [DOI] [PubMed] [Google Scholar]
- 22.Verstraelen H, Verhelst R. Bacterial vaginosis: An update on diagnosis and treatment. Expert Rev Anti Infect Ther 2009; 7:1109–1124. [DOI] [PubMed] [Google Scholar]
- 23.Coleman JS, Gaydos CA. Molecular diagnosis of bacterial vaginosis: An update. J Clin Microbiol 2018; 56:e00342–e00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aptima BV. Assay Package Insert. San Diego, CA: Hologic, Inc.; 2019. [Google Scholar]
- 25.FDA clearance of Aptima BV and Aptima CV/TV molecular assays ushers in new era of comprehensive and objective diagnostic testing for vaginitis [press release]. 2019. Available at: https://investors.hologic.com/press-releases/press-release-details/2019/FDA-Clearance-of-Aptima-BV-and-Aptima-CVTV-Molecular-Assays-Ushers-in-New-Era-of-Comprehensive-and-Objective-Diagnostic-Testing-for-Vaginitis/default.aspx. Published 2019. Accessed October 24, 2019.
- 26.Sherrard J, Wilson J, Donders G, et al. 2018 European (IUSTI/WHO) International Union against sexually transmitted infections (IUSTI) World Health Organisation (WHO) guideline on the management of vaginal discharge. Int J STD AIDS 2018; 29:1258–1272. [DOI] [PubMed] [Google Scholar]
- 27.Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 28.Solosec (Secnidazole) Oral Granules Prescribing Information. Baltimore, MD: Symbiomix Therapeutics LLC (a Lupin company), 2017. [Google Scholar]
- 29.Clindesse (Clindamycin Phosphate) Vaginal Cream, 2% Prescribing Information. Allegan, MI: Perrigo, 2014. [Google Scholar]
- 30.Nuvessa (Metronidazole Vaginal Gel 1.3%) Prescribing Information. Florham Park, NJ: Exeltis USA, Inc., 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


