Abstract
Background
Breast implant illness (BII) after aesthetic breast augmentation remains a poorly defined syndrome encompassing a wide spectrum of symptoms. While previously published series have observed overall symptomatic improvement after breast implant removal,1–3 there is a lack of studies evaluating changes in specific symptoms over time. The purpose of this study was to gain an understanding of symptoms associated with BII, and to evaluate how these symptoms change after removal of breast implants and total capsulectomy (explantation). We hypothesized that patients presenting with BII would experience both immediate and sustained improvement in constitutional symptoms after explantation.
Methods
A retrospective study of all patients who underwent explantation by a single surgeon over 2 years was conducted. Repeated-measures analysis of variance accounting for dependency was used to compare symptoms before and after surgery. Multivariate analyses and linear regression models were used to examine the impact of patient- and implant-related factors on changes in symptoms.
Results
Seven hundred fifty patients met inclusion criteria. Mean preoperative survey score (26.19 ± 11.24) was significantly different from mean postoperative survey score at less than 30 days (9.49 ± 7.56) and greater than 30 days (9.46 ± 7.82, P < 0.001). Patients with a BMI greater than 30 or those with clinically detectable contracture on examination showed greater improvement on their survey scores (P = 0.039, 0.034, respectively).
Conclusions
Although BII encompasses a large range of symptoms, subjects in this study demonstrated significant and sustained improvement in 11 common symptom domains. This improvement was demonstrable within the first 30 days postoperatively and was maintained beyond 30 days. The study demonstrated a strong association of explantation and specific symptom improvement within the patient population studied. Future investigation will further elucidate possible biologic phenomena to better characterize the pathophysiology and mechanism of BII.
Key Words: breast implant illness, human adjuvant disease, breast implant, explantation
Breast augmentation remains a popular cosmetic procedure in the United States and represented an estimated 1.2 billion dollar industry in 2016.4 The recent major 2019 recall of specific breast implants due to published associations with anaplastic large cell lymphoma5 has highlighted safety concerns with cosmetic breast implants. Although implant-related complications, such as capsular contracture, implant rupture and malposition are well described,6,7 Breast implant illness (BII) remains a poorly defined and controversial complication. First described in the early 1960s as a human adjuvant disease and recently coined as BII, BII is a constellation of symptoms, unbound by a single organ system, which begins after the placement of breast implants. These symptoms may include, but are not limited to, fatigue, arthralgia, myalgia, cognitive impairment, dry eyes and mouth, alopecia, skin lesions, and Raynaud syndrome.1,3,8–19
A possible pathophysiology of BII is an autoimmune or inflammatory reaction that occurs in response to a stimulating agent (silicone), and presents as a wide range of symptoms with similarities to connective tissue disease.20 In the early 1980s, multiple case reports and small case series associated silicone breast implants with constitutional symptoms21 and coined the illness “human adjuvant disease.” The rise in these publications led to large class action lawsuits against implant manufacturers and an eventual FDA moratorium on silicone breast implants in the 1990s.20 However, throughout the 1990s, multiple large-scale studies disproved an association between breast implants and connective tissue disease, resulting in liberalization of FDA restrictions. More recent reports have suggested that, for a subset of women experiencing constitutional symptoms consistent with BII, removal of the breast implants and associated capsules significantly reduces their symptoms.1,3,20 These studies, however, tend to group symptoms as a whole instead of examining the validity of individual symptoms.
Given the multiple reports of constitutional symptoms associated with breast implants, further investigation into individual symptoms and BII is warranted. We hypothesized that patients presenting with BII symptoms would demonstrate significant, sustained, and reproducible improvement in all tested symptom domains after explantation of the implants and total capsule removal. The aim of this study is to quantify symptoms associated with BII and study changes in these symptoms following explantation.
METHODS
A retrospective study evaluating patients requesting removal of their breast implants from 2017 to 2018 (24 months) was conducted. The study was approved by the Institutional Review Board (Case SPARTA IRB 20181267) prior to its initiation. Data collected included patient demographics, comorbidities, implant size, fill, texture, and symptoms. Inclusion criteria included all patients who presented to a single practice location requesting breast implant removal due to presumed symptoms related to the implant. Exclusion criteria included patients requesting implant exchange, implant pocket exchange, implant replacement, or patients treated with partial capsulectomy. The plastic surgery practice from which this cohort was drawn consists primarily of BII patients, referred by a variety of sources including BII support groups, former patients, rheumatologists, functional medicine providers, and naturopathic providers. Patient symptoms on all patients were quantified using a patient-reported outcome measure rating 11 common symptoms both preoperatively and at each postoperative visit. The 11 symptoms included on the survey with this exact wording were: (1) numbness and tingling in the extremities; (2) joint and/or muscle pain; (3) hair loss; (4) memory loss/cognitive problems; (5) dry eyes and/or blurred vision; (6) chronic fatigue; (7) breast pain; (8) rashes and/or hives; (9) food sensitivity/intolerance; (10) flu-like symptoms and/or low-grade fever; (11) difficulty breathing. Patients were asked to rate symptoms on a scale from 0 (absent) to 5 (very severe).
All patients were treated using a standardized surgical technique of total capsulectomy and implant removal further referred to as explantation in this manuscript. Capsule on the chest wall was routinely removed to the maximum extent possible; this included both anterior and posterior capsules of the implant. For patients with multiple sets of implants with capsules located in both subglandular and submuscular planes, both current and previous capsules were removed. For patients with extracapsular rupture, all silicone granuloma were removed following mapping by ultrasound examination by a radiologist experienced in detecting silicone granuloma. Partial capsulectomy was not performed on any of the study patients.
Patient demographics are reported in a descriptive, deidentified fashion to evaluate characteristics of patients who underwent removal of breast implants. Statistical analyses were performed using SAS Enterprise Guide, Version 7.1 (SAS Institute, Cary, NC). Repeated-measures analysis of variance accounting for dependency were used to compare preoperative and postoperative survey scores at less than 30 days and greater than 30 days postoperatively. Univariate and multivariate associations of various clinical, demographic and implant-related factors were assessed using linear regression models for their impact on change in survey scores. A P value of less than 0.05 was considered significant.
RESULTS
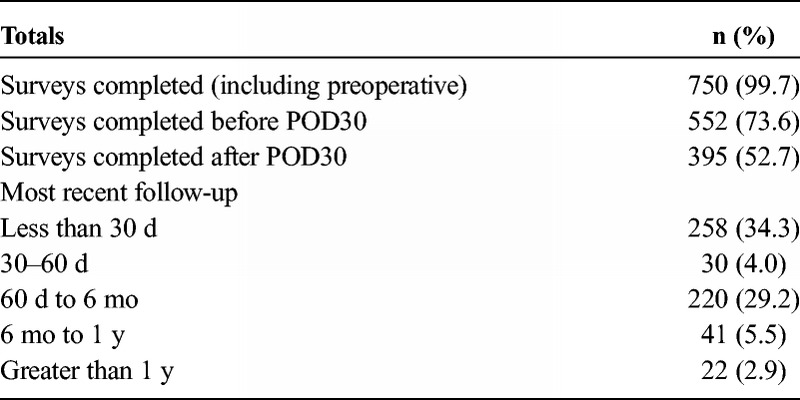
A total of 752 patients who underwent explantation including total capsulectomy from 2017 to 2018 were included in this analysis. Two (0.3%) subjects were excluded from the study as they did not complete any preoperative or postoperative surveys, leading to a total of 750 patients. Seven hundred fifty (99.7%) patients completed a preoperative survey, 552 (73.6%) completed a postoperative survey before postoperative day 30, and 395 (52.7%) completed a postoperative survey after postoperative day 30 (range, 30–1075 days; median, 138 days). In terms of follow-up, 258 subjects had their most recent follow-up before postoperative day 30, with 313 subjects following up after postoperative day 30. Table 1 illustrates the survey and follow-up data in greater detail.
TABLE 1.
Patient Survey and Follow-up Data
Patients' demographic and clinical characteristics are described in Table 2. Average patient age at surgery was 45.9 years (SD = 10.8). Average age of implant at time of explantation was 12.6 years (SD = 8.6). Implant fill and texture was diverse among our cohort: 313 (41.7%) of 750 had saline implants, 413 (55.1%) had silicone implants; 555 (74.0%) had smooth implants; 150 (20.0%) had textured implants; 6 (0.8%) had polyurethane-coated implants, either smooth or textured. Four hundred sixteen (55.4%) patients had clinically detectable contracture on examination on either side. Two hundred fifty-four (33.9%) patients had at least one self-reported preexisting illness, which is categorized mainly by organ system in Table 2.
TABLE 2.
Patient Demographics and Clinical Characteristics

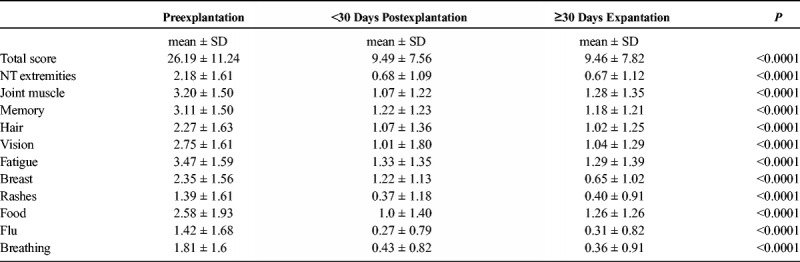
Comparisons of symptom ratings before and after explantation are described in Table 3. There was a significant change in all 11 symptom domains rated by subjects from the preoperative period to both postoperative time periods (P < 0.001). There was no significant change in symptoms from less than 30 days postoperatively to greater than 30 days postoperatively, demonstrating that this improvement was sustained beyond the immediate postoperative period.
TABLE 3.
Symptom Ratings Before and After Breast Implant Removal Surgery at <30 and >30 Days Postoperatively
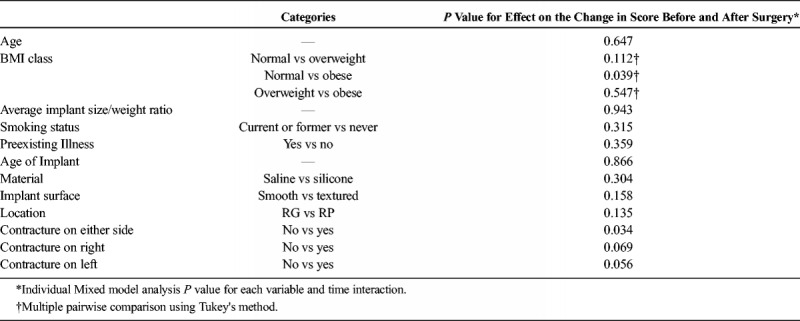
Univariate and multivariate associations of patient and implant-related factors are outlined in Table 4 and describe the impact of these factors on change in symptom scores. There was a significant correlation of body mass index (BMI) and the presence of contracture on either side with improvement in total survey score. Patients with a BMI greater than 30 (obese) demonstrated greater improvement in survey scores when compared with those with a BMI greater than 25 (normal) (17.4 vs 20.3, P = 0.039). Furthermore, patients with clinically detectable contracture demonstrated statistically significantly greater improvement in symptoms compared with those without contracture on examination (18.6 vs 17.3, P = 0.028).
TABLE 4.
Univariate and Multivariate Association With Change in Survey Score After Surgery (Significant P Values Highlighted)
DISCUSSION
The results of our study demonstrate both an immediate and sustained improvement across 11 common symptom domains following removal of breast implants and total capsulectomy in patients presenting for implant removal for presumed BII. In addition, our findings suggest that certain patient (BMI) and implant (presence of contracture) characteristics may be associated with greater symptom improvement after explantation.
Although the mechanism of BII remains unknown, multiple theories suggest an inflammatory process triggered by the introduction of silicone.3,21 This can be seen in a routine histologic analysis of the capsules of one of our subjects, which reveals infiltration of inflammatory cells into the tissues surrounding the implant (Figs. 1A–B).
FIGURE 1.
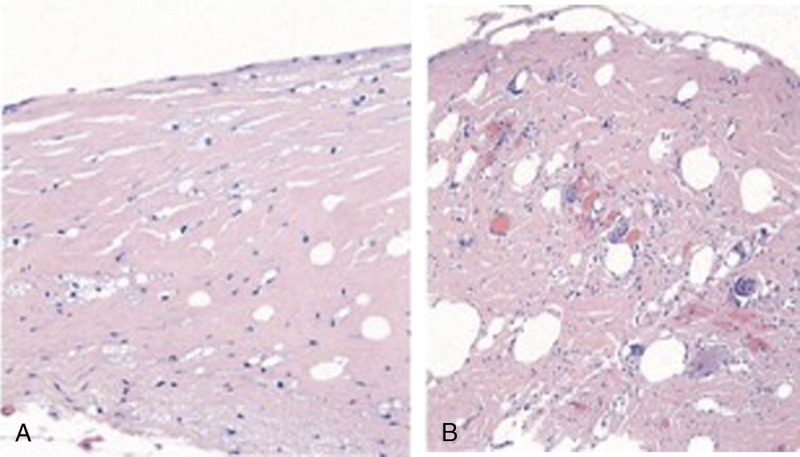
A and B, Histologic analysis of capsules surrounding an intact (A) and ruptured (B) implant. (A) Patchy mild foam cell reaction and minimal chronic inflammatory infiltrate; (B) Mild to moderate cell reaction and foreign body giant cell reaction in association with silicone.
With this in mind, one may expect silicone implants to cause a more profound inflammatory effect compared with saline implants. However, silicone is actually present in the shells of many saline implants and can be seen as nonrefractile silicone elastomers in pathology slides.22 This may explain why removal of both saline and silicone implants leads to similar improvement in symptoms (Table 4, P = 0.304).
Capsular contracture is one of the most commonly identified complications of breast augmentation with some studies publishing rates of approximately 20%.23 In our study, we noted that 55% of the patients (Table 2) had some degree of contracture. These patients experienced more significant improvement as compared with patients without contracture. Possible mechanisms include the mechanical nature of contracture that may result in significant physical symptoms including breast pain, muscle aches and pains, and difficulty breathing related to chest wall restrictive movement. Alternatively, capsular contracture can occur secondary to an inflammatory reaction7; thus, a particular subset of patients with capsular contracture on examination may represent a cohort with a disproportionate inflammatory response to a foreign body. The inflammatory response theory remains a plausible theory to support removal of the foreign body (implant) in BII patients. Similar mechanisms may be responsible for why obese patients experience greater improvement with explantation than patients with a normal BMI. As obesity is considered to be a proinflammatory state,24 these patients may mount a greater inflammatory response to a trigger such as silicone.
The results of this study are subject to several limitations. The retrospective nature of the study can introduce data collection bias. For example, symptoms included on the survey reflect the most common symptoms as determined by the senior author's experience and may not represent all symptoms reported in association with breast implants. Future prospective study will include a wide range of symptoms to fully understand the nature of BII. This study is also limited by potential sampling bias, as patients requesting explantation due to the severity of their symptoms may not be representative of the overall augmented/reconstructed population. Furthermore, both preexisting conditions and symptom severity in this study are dependent on patient reports and are, thus, subject to recall bias and subjectivity; there is also no method by which to determine causality. This study also does not eliminate confounding factors such as comorbidities in its analysis. In addition, this study is limited by its quantity of long-term follow-up data, as follow-up beyond 6 months decreases significantly. This indicates the need for continued prospective study. Despite these limitations, this study quantitatively measured individual symptoms both preexplantation and at different time points postexplantation in women presenting with symptoms of BII and identified consistent improvements in symptoms after explantation in a large cohort. Efforts to balance subjectivity included a consistent ranking system for rating symptoms before and after surgery. As mentioned, an additional strength of this study is its sample size, with greater than 99% of patients completing any survey, 73.6% of patients completing a survey before 30 days postoperatively and 52.7% of patients completing a survey after 30 days postoperatively.
CONCLUSIONS
Patients presenting with symptomatic implant BII had significant immediate and sustained improvement in 11 common symptoms after removal of the implant and capsule. Future research efforts will include long-term prospective studies, as well as identification and examination of objective measures in symptomatic patients.
Footnotes
Conflicts of interest and sources of funding: none declared.
A.K. is a recipient of research funds from KLS Martin.
REFERENCES
- 1.Maijers MC, de Blok CJ, Niessen FB, et al. Women with silicone breast implants and unexplained systemic symptoms: a descriptive cohort study. Neth J Med. 2013;71:534–540. [PubMed] [Google Scholar]
- 2.Magnusson MR, Cooter RD, Rakhorst H, et al. Breast Implant Illness: A Way Forward. Plastic and reconstructive surgery. 2019;143:74S–81S. doi: 10.1097/PRS.0000000000005573. [DOI] [PubMed] [Google Scholar]
- 3.Colaris MJL, de Boer M, van der Hulst RR, et al. Two hundreds cases of ASIA syndrome following silicone implants: a comparative study of 30 years and a review of current literature. Immunol Res. 2017;65:120–128. doi: 10.1007/s12026-016-8821-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Research GV Breast Implants Market Analysis By Product (Silicone Breast Implants, Saline Breast Implants), By Shape (Round, Anatomical), By Application (Reconstructive Surgery, Cosmetic Surgery), By End-use, And Segment Forecasts, 2018–2025. <https://www.grandviewresearch.com/industry-analysis/breast-implants-market> (2017).
- 5.Loftus P. The Wall Street Journal. 2019. [Google Scholar]
- 6.Hillard C, Fowler JD, Barta R, et al. Silicone breast implant rupture: a review. Gland Surg. 2017;6:163–168. doi: 10.21037/gs.2016.09.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Headon H, Kasem A, Mokbel K. Capsular contracture after breast augmentation: an update for clinical practice. Arch Plast Surg. 2015;42:532–543. doi: 10.5999/aps.2015.42.5.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bridges AJ, Conley C, Wang G, et al. A clinical and immunologic evaluation of women with silicone breast implants and symptoms of rheumatic disease. Ann Intern Med. 1993;118:929–936. [DOI] [PubMed] [Google Scholar]
- 9.Freundlich B, Altman C, Snadorfi N, et al. A profile of symptomatic patients with silicone breast implants: a Sjögrens-like syndrome. Semin Arthritis Rheum. 1994;24:44–53. [DOI] [PubMed] [Google Scholar]
- 10.Giltay EJ, Bernelot Moens HJ, Riley AH, et al. Silicone breast prostheses and rheumatic symptoms: a retrospective follow up study. Ann Rheum Dis. 1994;53:194–196. doi: 10.1136/ard.53.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solomon G. A clinical and laboratory profile of symptomatic women with silicone breast implants. Semin Arthritis Rheum. 1994;24:29–37. [DOI] [PubMed] [Google Scholar]
- 12.Vasey FB, Havice DL, Bocanegra TS, et al. Clinical findings in symptomatic women with silicone breast implants. Semin Arthritis Rheum. 1994;24:22–28. [DOI] [PubMed] [Google Scholar]
- 13.Cuellar ML, Gluck O, Molina JF, et al. Silicone breast implant—associated musculoskeletal manifestations. Clin Rheumatol. 1995;14:667–672. [DOI] [PubMed] [Google Scholar]
- 14.Wells KE, Roberts C, Daniels SM, et al. Psychological and rheumatic symptoms of women requesting silicone breast implant removal. Ann Plast Surg. 1995;34:572–577. [DOI] [PubMed] [Google Scholar]
- 15.Shoaib BO, Patten BM. Human adjuvant disease: presentation as a multiple sclerosis-like syndrome. South Med J. 1996;89:179–188. [DOI] [PubMed] [Google Scholar]
- 16.Peters W, Smith D, Fornasier V, et al. An outcome analysis of 100 women after explantation of silicone gel breast implants. Ann Plast Surg. 1997;39:9–19. [DOI] [PubMed] [Google Scholar]
- 17.Melmed EP. A review of explanation in 240 symptomatic women: a description of explantation and capsulectomy with reconstruction using a periareolar technique. Plast. Reconstr. Surg. 1998;101:1364–1373. [DOI] [PubMed] [Google Scholar]
- 18.Cohen Tervaert JW, Kappel RM. Silicone implant incompatibility syndrome (SIIS): a frequent cause of ASIA (Shoenfeld's syndrome). Immunol Res. 2013;56:293–298. doi: 10.1007/s12026-013-8401-3. [DOI] [PubMed] [Google Scholar]
- 19.Coroneos CJ, Selber JC, Offodile AC, 2nd, et al. US FDA breast implant postapproval studies: long-term outcomes in 99,993 patients. Ann. Surg. 2019;269:30–36. doi: 10.1097/SLA.0000000000002990. [DOI] [PubMed] [Google Scholar]
- 20.Magnusson MR, et al. Breast implant illness. Plast Reconstr Surg. 2019;143:74S–81S. doi: 10.1097/prs.0000000000005573. [DOI] [PubMed] [Google Scholar]
- 21.Silver RM, Sahn EE, Allen JA, et al. Demonstration of silicon in sites of connective-tissue disease in patients with silicone-gel breast implants. Arch Dermatol. 1993;129:63–68. [PubMed] [Google Scholar]
- 22.Types of Breast Implants. <https://www.fda.gov/medical-devices/breast-implants/types-breast-implants>(.
- 23.Cunningham BL, Lokeh A, Gutowski KA. Saline-filled breast implant safety and efficacy: a multicenter retrospective review. Plast Reconstr Surg. 2000;105:2143–2149; discussion 2150–2141. doi: 10.1097/00006534-200005000-00037. [DOI] [PubMed] [Google Scholar]
- 24.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]


