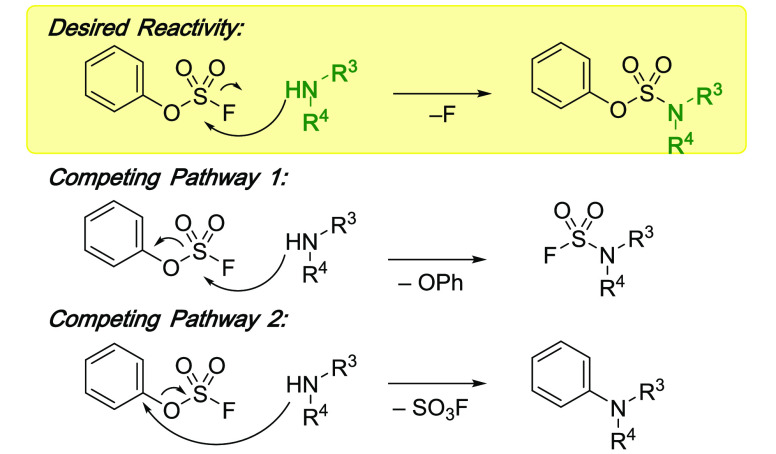
Abstract
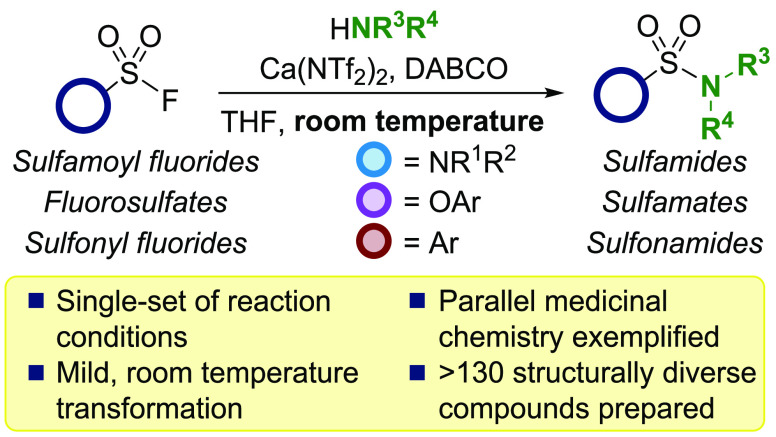
A method to activate sulfamoyl fluorides, fluorosulfates, and sulfonyl fluorides with calcium triflimide and DABCO for SuFEx with amines is described. The reaction was applied to a diverse set of sulfamides, sulfamates, and sulfonamides at room temperature under mild conditions. Additionally, we highlight this transformation to parallel medicinal chemistry to generate a broad array of nitrogen-based S(VI) compounds.
Interest in sulfur(VI) fluorides has grown immensely since Sharpless and co-workers introduced the concept of sulfur–fluoride exchange (SuFEx) chemistry in 2014.1,2 For example, SuFEx has been applied extensively in chemical biology and medicinal chemistry with application toward protein labeling, protease inhibition, and drug target discovery.3 In polymer chemistry, sulfur(VI) fluorides have been employed to generate novel polysulfates and polysulfonates, materials that have unique properties with promising applications.1,4,5 Furthermore, advancements in fluorosulfurylation chemistry and the development of new reagents to install the −SO2F group have spurred additional exploration into these functional groups.6−12 However, employment of sulfur(VI) fluorides as synthetic precursors toward nitrogen-based sulfonylated compounds is underdeveloped.1,13−18
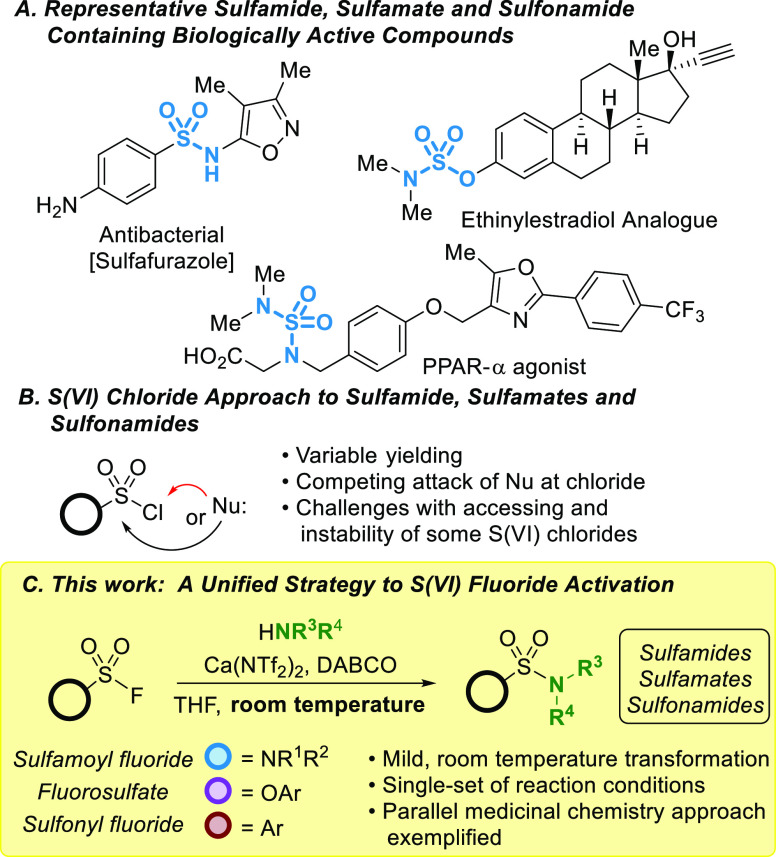
Nitrogenous sulfur(VI) compounds are well represented among small molecule drugs. For example, sulfonamides make up 25% of all sulfur-based FDA approved drugs, with therapeutic applications for multiple indications (Figure 1A).19
Figure 1.
(A) Biologically active nitrogenous S(VI) compounds; (B) S(VI) chloride-based approach to synthesize sulfonamides, sulfamates, and sulfamides; (C) our room-temperature method using Ca(NTf2)2 and DABCO to synthesize sulfamides, sulfamates, and sulfonamides from S(VI) fluorides.
Sulfamides and sulfamates are also valuable motifs; however, they are comparatively underexplored, despite their presence in a multitude of biologically active compounds.20 The most common approach to nitrogen-based sulfur(VI) compounds relies on the isolation or in situ generation of sulfur(VI) chlorides, such as sulfonyl chlorides (−SO2Cl), chlorosulfates (−OSO2Cl), and sulfamoyl chlorides (−NSO2Cl).1,21 Although widely used, there are several challenges with their application. While some sulfonyl chlorides are commercially available, the synthesis of sulfonyl chlorides with more complex architectures can be challenging due to the harsh synthetic conditions required to access these compounds and their inherent instability.21b Moreover, in the presence of nucleophiles, S(VI) chlorides can undergo competing addition to the chlorine or sulfur atom, dehydrochlorination, and hydrolysis (Figure 1B).1,21−23
In contrast, the corresponding S(VI) fluorides have remarkable hydrolytic, redox, and thermal stability, rendering them attractive alternatives to S(VI) chlorides.21 Despite innovation in their synthesis, there are still barriers to the broader application of S(VI) fluorides in organic chemistry. A key challenge is the reduced reactivity at the sulfur center compared to other S(VI) halides.1 Furthermore, the canonical S(VI) fluorides—sulfonyl fluorides (−SO2F), fluorosulfates (−OSO2F), and sulfamoyl fluorides (−NSO2F)—have considerably different reactivity at the sulfur center due to the reduced electrophilicity of the sulfur atom as the C–S bond is replaced with more resonance-donating atoms. Notably, disubstituted sulfamoyl fluorides require forcing conditions to undergo sulfur-fluoride exchange,24 limiting a common method toward their application in SuFEx chemistry.25
We recently reported a Ca(NTf2)2-mediated activation of sulfonyl fluorides to generate sulfonamides13 and envisioned a similar Lewis acid approach could be employed to activate less reactive sulfamoyl fluorides and fluorosulfates to access sulfamides and sulfamates, respectively. To date, a unified approach to enable SuFEx chemistry across a broader array of S(VI) fluorides does not exist, limiting their adoption as synthetic precursors. Herein, we report a high-yielding, unified method to access sulfamides, sulfamates, and sulfonamides through the room-temperature activation of sulfamoyl fluorides, fluorosulfates, and sulfonyl fluorides with calcium triflimide and DABCO (Figure 1C).
Applying our previously reported method utilizing excess amine in tert-amylOH generated sulfamide 3 in 70% yield (Table 1, entry 1); however, sulfamoyl fluoride 1 was not fully consumed in the reaction, and additional heating at 60 °C for 48 h did not further improve the conversion. Increasing the temperature and equivalents of Ca(NTf2)2 improved the yield, although the reactions still did not fully consume sulfamoyl fluoride 1 (entries 2 and 3). We identified that increasing the concentration to 1 M had a marked effect, affording a 96% yield of sulfamide 3, with full consumption of starting material (1) in only 2 h (entry 4).
Table 1. Optimization Studiesa.
| entry | amine 2 (equiv) | Ca(NTf2)2 (equiv) | base (equiv) | solvent (conc, M) | temp (°C) | time (h) | yield (%) |
|---|---|---|---|---|---|---|---|
| 1 | 2 | 1 | t-amylOH (0.2) | 60 | 24 | 70 | |
| 2 | 2 | 1 | t-amylOH (0.2) | 90 | 24 | 89 | |
| 3 | 2 | 1.1 | t-amylOH (0.2) | 100 | 24 | 92 | |
| 4 | 2 | 1.1 | t-amylOH (1.0) | 90 | 2 | 96 | |
| 5 | 1.05 | 1.1 | t-amylOH (1.0) | 90 | 2 | 52 | |
| 6 | 1.05 | 1.1 | DIPEA (1.5) | t-amylOH (1.0) | 90 | 2 | 95 (54)b |
| 7 | 1.05 | 1.1 | DABCO (1.5) | t-amylOH (1.0) | 90 | 2 | 96 (88)b |
| 8 | 1.05 | 1.1 | DABCO (1.5) | THF (0.5) | rt | 24 | 93 |
| 9 | 1.05 | 0 | DABCO (1.5) | THF (0.5) | rt | 24 | NRc |
Reaction scale: sulfamoyl fluoride 1 (0.35 mmol, 1.0 equiv).
Reaction was performed in the presence of 5% water by volume.
No reaction.
We next explored the amine nucleophile and found that decreasing the equivalents (2 equiv → 1.05 equiv of amine 2, entry 5) required the addition of a base to drive the reaction to completion (e.g., DIPEA or DABCO, entries 6 and 7). With DIPEA, the yield varied depending on the bottle of tert-amylOH employed. We surmised that this could be a result of varying amounts of water present in the solvent. Indeed, the addition of 5% water (by volume) significantly decreased the yield from 95% to 54% (entry 6). Notably, when DABCO was employed, the inclusion of 5% water in the reaction had a minimal effect, yielding 88% yield of the desired sulfamide 3 (entry 7). This result led us to hypothesize that DABCO may have an expanded role, beyond acting solely as a base in the reaction, and led us to investigate additional reaction parameters.1,26,27
Toward this end, we explored the effect of various bases, solvents, and Lewis acids on the transformation (see the Supporting Information (SI) for details). DABCO proved exceptional as compared to other bases explored. With DABCO, we no longer observed a preference for an alcoholic solvent, the requirement for high concentration was less pronounced, and the reaction proceeded at room temperature when using THF as a solvent (entry 8). Evaluating a selected set of Lewis acids further established Ca(NTf2)2 as particularly effective across a variety of substrates (vide infra). In our screen we found that 0.55 equiv of Ca(NTf2)2, and calcium triflate Ca(OTf)2 could generate sulfamide 3 in good yields, although in both cases the reactions required higher temperatures and were not as general across different amine substrates. Lower equivalents of Ca(NTf2)2 resulted in significantly reduced yield of 3 precluding catalysis (see the SI for details). Further decreasing the equivalents of Ca(NTf2)2 led to incomplete conversion to 3. It is noteworthy that in the absence of calcium the reaction does not proceed (entry 9).
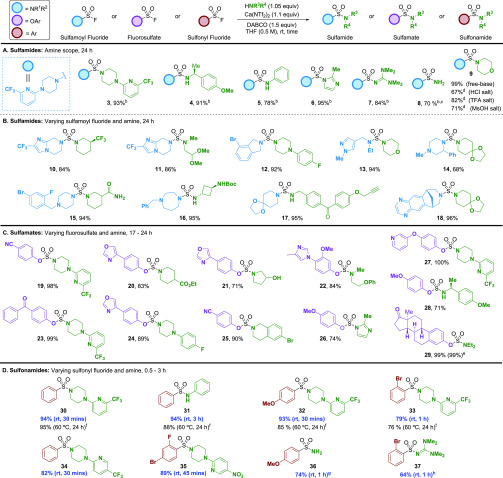
We next investigated the generality of the sulfamide preparation by exploring a diverse set of amine nucleophiles with sulfamoyl fluoride 1 (Scheme 1A). Primary and secondary amines, as well as weaker amine nucleophiles such as aniline, methylimidazole and tetramethyl guanidine, readily underwent SuFEx to produce sulfamides (3–7). Ammonia was also a competent nucleophile, generating sulfamide 8 with a free amino group. In addition, amine salts (i.e., HCl, TFA, and MsOH) of morpholine were tolerated, giving sulfamide 9, albeit with a reduction in yield compared to the free base. To further evaluate the versatility of the reaction, we paired a diverse array of sulfamoyl fluorides with various amines (Scheme 1B, 10–18). The reaction was compatible with a broad range of functional groups (e.g., primary amide, halides, alkyne, etc.) and acid sensitive functionality (e.g., Boc and acetal protecting groups), as well as multiple heterocyclic motifs.
Scheme 1. Scope of Sulfamide, Sulfamate, and Sulfonamide Formation.
S(VI) fluoride (1.0 equiv), amine (1.05 equiv), Ca(NTf2)2 (1.1 equiv), DABCO (1.5 equiv) in THF (0.5 M) at rt for time indicated.
No reaction in the absence of Ca(NTf2)2
2.0 equiv of 7 N NH3 in MeOH was used as the amine source.
The corresponding amine salt of morpholine was used, along with 3.0 equiv of DABCO.
Reaction was conducted on a 1.2 mmol scale in fluorosulfate for 24 h at room temperature.
Amine (2.0 equiv), Ca(NTf2)2 (1.0 equiv), t-amylOH (0.2 M), 60 °C; see ref (13).
2 M solution of NH3 in iPrOH was used.
1.1 equiv of TMG was used.
Fluorosulfates pose additional challenges, as multiple reaction pathways can be envisioned upon reaction with amine nucleophiles (Figure 2).3b Indeed, 4-cyanophenyl fluorosulfate in the presence of DABCO and amine 2 (excluding Ca(NTf2)2) resulted in significant side-product formation, including products outlined in Figure 2 (see the SI for details). Remarkably, inclusion of Ca(NTf2)2 into the reaction mixture completely rescued the transformation, affording sulfamate 19 in 98% yield. A structurally diverse set of sulfamates were synthesized with secondary amines and phenolic fluorosulfates in excellent yield (Scheme 1C, 19–29). Notably, the reaction was run on a 1.2 mmol scale with the synthesis of estrone-derived sulfamate 29 in near-quantitative yield. Utilizing a primary amine with the electron-poor 4-benzoylphenyl fluorosulfate resulted in elimination of the phenol (see the SI for details);24 however, on a more electron-rich substrate (i.e., 4-methoxyfluorosulfate) the desired product 28 could be isolated with only a minor amount of side product formation.
Figure 2.
Possible modes of fluorosulfate reactivity with amines.
Our previous work demonstrated the conversion of sulfonyl fluorides to sulfonamides using Ca(NTf2)2 with various amines; however, the reaction required elevated temperature (60 °C) and long reaction time (24 h). Applying the new Ca(NTf2)2/DABCO combination had an exceptional effect: phenylsulfonyl fluoride undergoes the SuFEx reaction to form sulfonamide 30 in 94% yield in less than 30 min at room temperature (Scheme 1D). Encouraged by these results, we revisited a series of amine nucleophiles and sulfonyl fluorides for comparison. In all cases, comparable or improved yields were obtained, along with a dramatic increase in reaction rate, at a lower reaction temperature (i.e., sulfonamides 30–33). Further scope was exemplified by varying the electronics on the sulfonyl fluoride, amine, and highlighting the use of both ammonia and tetramethyl guanidine nucleophiles (34–37). In addition, to probe the mechanism of this reaction, we conducted NMR (1H and 19F) and LCMS studies and propose a SuFEx mechanism that first involves Ca/DABCO activation of the S(VI) fluoride to form an activated N-sulfonyl-DABCO salt, that in the presence of an amine undergoes product formation (see the SI for details).27
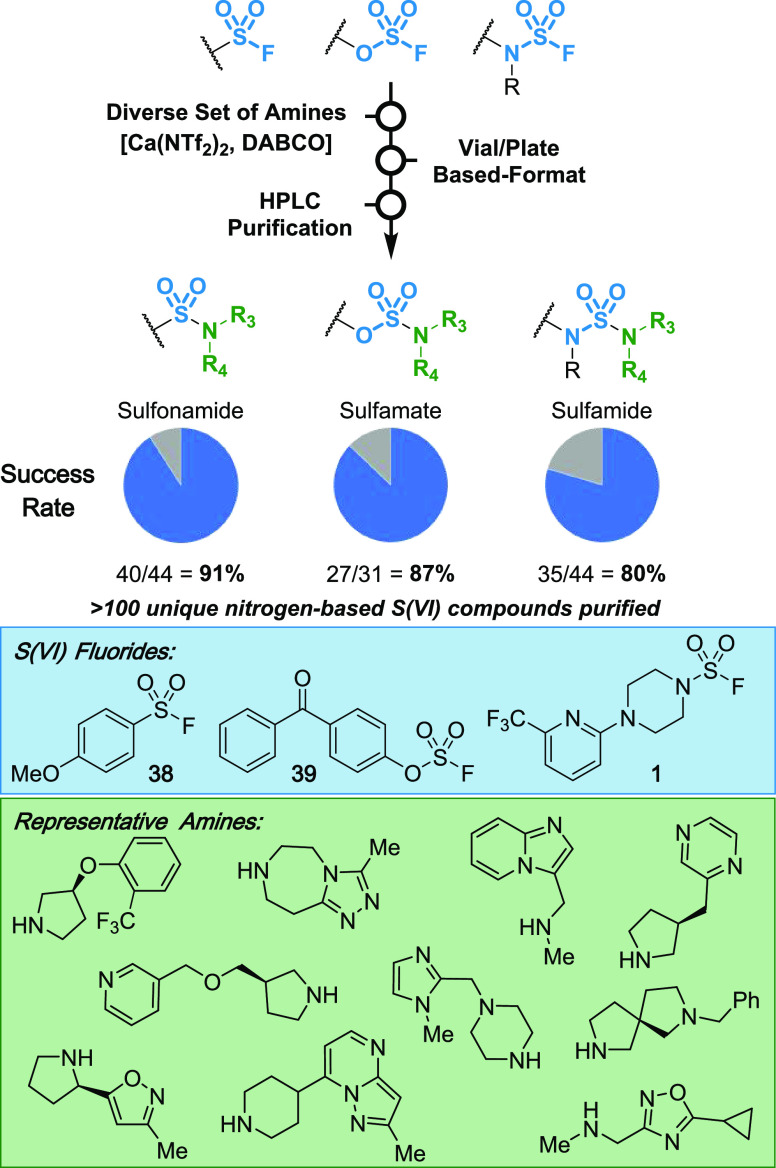
Parallel medicinal chemistry (PMC) is frequently used in drug discovery to rapidly expand SAR and optimize lead compound properties. PMC-enabled chemistry should be tolerant of a wide range of functional groups and have relatively simple reaction setup and purification. This is to facilitate the use of a plate-based (e.g., 96-well or greater) format, automated liquid handling equipment, and HPLC purification of final products, with the goal of generating numerous compounds in a more efficient manner than in singleton or batch format. To explore the utility of our Ca(NTf2)2/DABCO reaction conditions in PMC, we selected three S(VI) fluoride templates (sulfonyl fluoride 38, fluorosulfate 39, and sulfamoyl fluoride 1) and a diverse set of amine monomers (Scheme 2; see the SI for the full set of amines). Our protocol successfully translated to PMC format, generating >100 unique nitrogen-based S(VI) compounds with high overall success rate and purity across three classes of nitrogen-based S(VI) compounds (i.e., sulfonamide 40/44, sulfamate 27/31, and sulfamides 35/44 compounds). These results highlight the application of this robust transformation to a diverse set of functional groups and heterocycles.
Scheme 2. PMC of S(VI) Fluorides with Amines.
In conclusion, we have developed a unified Ca(NTf2)2/DABCO-mediated method that activates S(VI) fluorides, with considerably different reactivity, toward SuFEx with amines. The reaction proceeds at room temperature, a diverse array of sulfamides, sulfamates, and sulfonamides can be readily prepared, and application to parallel medicinal chemistry is exemplified. We anticipate the introduction of this Ca(NTf2)2/DABCO SuFEx method will further solidify S(VI) fluorides as privileged S(VI) synthons in synthetic chemistry.28
Acknowledgments
N.D.B. thanks Pomona College for start-up funds and Prof. Daniel O’Leary for sharing laboratory resources. C.P.W., J.G., S.N.C., S.C.K., and S.R.K. thank the Pomona SURF program and the Pomona College Department of Chemistry for their funding. S.N.C. thanks the Linares Fellowship program. C.P.W. and N.D.B. thank the ACS Scholars Program. C.P.W., N.D.B., and C.W.A. thank the Beckman Scholars Program. Additionally, we thank current and former Pfizer employees Christopher Helal, John Humphrey, and Patrick Mullins for helpful discussions.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.0c01397.
Author Contributions
This work was performed in collaboration between the Ball laboratory at Pomona College and Pfizer, Inc.
Author Contributions
§ S.M. and C.W.P are co-first authors.
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award No. R15GM134457.
The authors declare no competing financial interest.
Supplementary Material
References
- Dong J.; Krasnova L.; Finn M. G.; Sharpless K. B. Sulfur(VI) Fluoride Exchange (SuFEx): Another Good Reaction for Click Chemistry. Angew. Chem., Int. Ed. 2014, 53, 9430–9448. 10.1002/anie.201309399. [DOI] [PubMed] [Google Scholar]
- For representative reviews on sulfur–fluoride exchange, see:; a Abdul Fattah T.; Saeed A.; Albericio F. Recent advances towards sulfur (VI) fluoride exchange (SuFEx) click chemistry. J. Fluorine Chem. 2018, 213, 87–112. 10.1016/j.jfluchem.2018.07.008. [DOI] [Google Scholar]; b Revathi L.; Ravindar L.; Leng J.; Rakesh K. P.; Qin H.-L. Synthesis and Chemical Transformations of Fluorosulfates. Asian J. Org. Chem. 2018, 7, 662–682. 10.1002/ajoc.201700591. [DOI] [Google Scholar]; c Chinthakindi P. K.; Arvidsson P. I. More Than Click Reagents?. Eur. J. Org. Chem. 2018, 2018, 3648–3666. 10.1002/ejoc.201800464. [DOI] [Google Scholar]; d Barrow A. S.; Smedley C. J.; Zheng Q.; Li S.; Dong J.; Moses J. E. The growing applications of SuFEx click chemistry. Chem. Soc. Rev. 2019, 48, 4731–4758. 10.1039/C8CS00960K. [DOI] [PubMed] [Google Scholar]
- a Narayanan A.; Jones L. H. Sulfonyl fluorides as privileged warheads in chemical biology. Chem. Sci. 2015, 6, 2650–2659. 10.1039/C5SC00408J. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Jones L. H. Emerging Utility of Fluorosulfate Chemical Probes. ACS Med. Chem. Lett. 2018, 9, 584–586. 10.1021/acsmedchemlett.8b00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Zhou F.; Ren G.; Zheng Q.; Chen H.; Gao B.; Klivansky L.; Liu Y.; Wu B.; Xu Q.; Lu J.; Sharpless K. B.; Wu P. SuFEx-Based Polysulfonate Formation from Ethenesulfonyl Fluoride-Amine Adducts. Angew. Chem., Int. Ed. 2017, 56, 11203–11208. 10.1002/anie.201701160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B.; Zhang L.; Zheng Q.; Zhou F.; Klivansky L. M.; Lu J.; Liu Y.; Dong J.; Wu P.; Sharpless K. B. Bifluoride-catalysed sulfur(VI) fluoride exchange reaction for the synthesis of polysulfates and polysulfonates. Nat. Chem. 2017, 9, 1083–1088. 10.1038/nchem.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veryser C.; Demaerel J.; Bieliu̅nas V.; Gilles P.; De Borggraeve W. M. Ex-Situ Generation of Sulfuryl Fluoride for the Synthesis of Aryl Fluorosulfates. Org. Lett. 2017, 19, 5244–5247. 10.1021/acs.orglett.7b02522. [DOI] [PubMed] [Google Scholar]
- Davies A. T.; Curto J. M.; Bagley S. W.; Willis M. C. One-pot palladium-catalyzed synthesis of sulfonyl fluorides from aryl bromides. Chem. Sci. 2017, 8, 1233–1237. 10.1039/C6SC03924C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribby A. L.; Rodríguez I.; Shariffudin S.; Ball N. D. Pd-Catalyzed Conversion of Aryl Iodides to Sulfonyl Fluorides Using SO2 Surrogate DABSO and Selectfluor. J. Org. Chem. 2017, 82, 2294–2299. 10.1021/acs.joc.7b00051. [DOI] [PubMed] [Google Scholar]
- Zhou H.; Mukherjee P.; Liu R.; Evrard E.; Wang D.; Humphrey J. M.; Butler T. W.; Hoth L. R.; Sperry J. B.; Sakata S. K.; Helal C. J.; am Ende C. W. Introduction of a Crystalline, Shelf-Stable Reagent for the Synthesis of Sulfur(VI) Fluorides. Org. Lett. 2018, 20, 812–815. 10.1021/acs.orglett.7b03950. [DOI] [PubMed] [Google Scholar]
- Laudadio G.; de A Bartolomeu A.; Verwijlen L. M. H. M.; Cao Y.; de Oliveira K. T.; Noël T. Sulfonyl Fluoride Synthesis through Electrochemical Oxidative Coupling of Thiols and Potassium Fluoride. J. Am. Chem. Soc. 2019, 141, 11832–11836. 10.1021/jacs.9b06126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.; Ball N. D.; Sammis G. M. One-pot fluorosulfurylation of Grignard reagents using sulfuryl fluoride. Chem. Commun. 2019, 55, 14753–14756. 10.1039/C9CC08487H. [DOI] [PubMed] [Google Scholar]
- Pérez-Palau M.; Cornella J. Synthesis of Sulfonyl Fluorides from Sulfonamides. Eur. J. Org. Chem. 2020, 2020, 2497–2500. 10.1002/ejoc.202000022. [DOI] [Google Scholar]
- Mukherjee P.; Woroch C. P.; Cleary L.; Rusznak M.; Franzese R. W.; Reese M. R.; Tucker J. W.; Humphrey J. M.; Etuk S. M.; Kwan S. C.; am Ende C. W.; Ball N. D. Sulfonamide Synthesis via Calcium Triflimide Activation of Sulfonyl Fluorides. Org. Lett. 2018, 20, 3943–3947. 10.1021/acs.orglett.8b01520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T.; Meng G.; Zhan X.; Yang Q.; Ma T.; Xu L.; Sharpless K. B.; Dong J. A New Portal to SuFEx Click Chemistry: A Stable Fluorosulfuryl Imidazolium Salt Emerging as an “F–SO2+” Donor of Unprecedented Reactivity, Selectivity, and Scope. Angew. Chem., Int. Ed. 2018, 57, 2605–2610. 10.1002/anie.201712429. [DOI] [PubMed] [Google Scholar]
- Thomas J.; Fokin V. V. Regioselective Synthesis of Fluorosulfonyl 1,2,3-Triazoles from Bromovinylsulfonyl Fluoride. Org. Lett. 2018, 20, 3749–3752. 10.1021/acs.orglett.8b01309. [DOI] [PubMed] [Google Scholar]
- Liu J.; Wang S.-M.; Qin H.-L. Installation of sulfonyl fluorides onto primary amides. Beilstein J. Org. Chem. 2019, 15, 1907–1912. 10.3762/bjoc.15.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F.; Wang H.; Li S.; Bare G. A. L.; Chen X.; Wang C.; Moses J. E.; Wu P.; Sharpless K. B. Biocompatible SuFEx Click Chemistry: Thionyl Tetrafluoride (SOF4)-Derived Connective Hubs for Bioconjugation to DNA and Proteins. Angew. Chem., Int. Ed. 2019, 58, 8029–8033. 10.1002/anie.201902489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.; Xiong H.; Lu F.; Ma F.; Gu Y.; Ma P.; Xu H.; Yang G. Synthesis of N-Acyl Sulfamates from Fluorosulfonates and Potassium Trimethylsilyloxyl Imidates. J. Org. Chem. 2019, 84, 15380–15388. 10.1021/acs.joc.9b02394. [DOI] [PubMed] [Google Scholar]
- Scott K. A.; Njardarson J. T. Analysis of US FDA-Approved Drugs Containing Sulfur Atoms. Topics in Current Chemistry Topics in Curr. Chem. 2019, 376, 1–34. 10.1007/978-3-030-25598-5_1. [DOI] [PubMed] [Google Scholar]
- a Winum J.-Y.; Scozzafava A.; Montero J.-L.; Supuran C. T. Sulfamates and their therapeutic potential. Med. Res. Rev. 2005, 25, 186–228. 10.1002/med.20021. [DOI] [PubMed] [Google Scholar]; b Winum J.-Y.; Scozzafava A.; Montero J.-L.; Supuran C. T. Therapeutic applications of sulfamates. Expert Opin. Ther. Pat. 2004, 14, 1273–1308. 10.1517/13543776.14.9.1273. [DOI] [Google Scholar]; c Spillane W.; Malaubier J.-B. Sulfamic Acid and Its N- and O-Substituted Derivatives. Chem. Rev. 2014, 114, 2507–2586. 10.1021/cr400230c. [DOI] [PubMed] [Google Scholar]
- a Wright S. W.; Hallstrom K. N. Sulfonamides and Sulfonyl Fluorides from Heteroaryl Thiols. J. Org. Chem. 2006, 71, 1080–1084. 10.1021/jo052164+. [DOI] [PubMed] [Google Scholar]; b Bogolubsky A. V.; Moroz Y. S.; Mykhailiuk P. K.; Pipko S. E.; Konovets A. I.; Sadkova I. V.; Tolmachev A. Sulfonyl Fluorides as Alternative to Sulfonyl Chlorides in Parallel Synthesis of Aliphatic Sulfonamides. ACS Comb. Sci. 2014, 16, 192–197. 10.1021/co400164z. [DOI] [PubMed] [Google Scholar]
- Gaulton A.; Bellis L. J.; Bento A. P.; Chambers J.; Davies M.; Hersey A.; Light Y.; McGlinchey S.; Michalovich D.; Al-Lazikani B.; Overington J. P. ChEMBL: a large-scale bioactivity database for drug discovery. Nucleic Acids Res. 2012, 40, D1100–D1107. 10.1093/nar/gkr777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillane W. J.; McHugh F. A.; Burke P. O. Elimination mechanisms in the anilinolysis of sulfamoyl chlorides in chloroform and acetonitrile. J. Chem. Soc., Perkin Trans. 2 1998, 2, 13–18. 10.1039/a706410a. [DOI] [Google Scholar]
- Monosubstituted sulfamoyl fluorides are more reactive because of their ability to go through the sulfenium intermediate (see the SI for details and the following reference):Edwards D. R.; Wolfenden R. Proton-in-Flight Mechanism for the Spontaneous Hydrolysis of N-Methyl O-Phenyl Sulfamate: Implications for the Design of Steroid Sulfatase Inhibitors. J. Org. Chem. 2012, 77, 4450–4453. 10.1021/jo300386u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Employing the Sharpless MgO conditions (ref (1)) with sulfamoyl fluoride 1 and amine 2 gave sulfamide 3 in 45% yield (see the SI for details).
- Gembus V.; Marsais F.; Levacher V. An Efficient Organocatalyzed Interconversion of Silyl Ethers to Tosylates Using DBU and p-Toluenesulfonyl Fluoride. Synlett 2008, 2008, 1463–1466. 10.1055/s-2008-1078407. [DOI] [Google Scholar]
- For examples of DABCO addition to sulfonyl chlorides, see:; a Hartung J.; Hünig S.; Kneuer R.; Schwarz M.; Wenner 1,4-Diazabicyclo[2.2.2]octance (DABCO) – an Efficient Reagent in the Synthesis of Alkyl Tosylates or Sulfenates. Synthesis 1997, 1997, 1433–1438. 10.1055/s-1997-1372. [DOI] [Google Scholar]; b Fu Y.; Xu Q.-S.; Li Q.-Z.; Li M.-P.; Shi C.-Z.; Du Z. Sulfonylation of 1,4-Diazabicyclo[2.2.2]octane: Charge-Transfer Complex Triggered C–N Bond Cleavage. ChemistryOpen 2019, 8, 127–131. 10.1002/open.201800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A previous version of this manuscript was published as a preprint:Mahapatra S.; Woroch C. P.; Butler T. W.; Carneiro S. N.; Kwan S. C.; Khasnavis S. R.; Gu J.; Dutra J. K.; Vetelino B. C.; Bellenger J.; am Ende C. W.; Ball N. D.. SuFEx Activation with Ca(NTf2)2: A Unified Strategy to Access Sulfamides, Sulfamates and Sulfonamides from S(VI) Fluorides. ChemRxiv 2020, ver. 1. (accessed 2020-05-21). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.