Abstract
Background
Zinc is an essential trace element that has an enormous role in regulation of physiological processes whose deviant value leads to malfunction in the body. So, establishing a country specific reference value is needed to serve as a standard for the interpretation of laboratory results during clinical decision making.
Objective
The objective of this study was to determine the reference value of serum zinc level of adult population in Bangladesh.
Materials and methods
The overnight fasting blood was collected from 154 apparently healthy individuals aged 18 to 65 years, from a rural community after considering several criteria. Graphite furnace atomic absorption spectrophotometry (GF-AAS) method was used for serum zinc analysis. The 2.5th and 97.5th percentiles of zinc level were calculated for the reference value according to the recommendations of the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC).
Results
The estimated reference range of serum zinc level in sample population was 60-120 μg/dL, where the range was 59-125 μg/dL for male and 50-103 μg/dL for female. Significant differences of serum zinc level between male and female (p<0.001) was observed. However, there was no significant correlation between age of the respondents and serum zinc level (r=0.110, p>0.05).
Conclusion
The estimated reference range for serum zinc level in adult population of Bangladesh can serve as a useful indicator for clinical decision making.
Key words: reference value, zinc, healthy adults, Bangladesh
INTRODUCTION
Zinc, an inorganic micronutrient, has significant effects on the metabolism of proteins, lipids and carbohydrates through enzymatic activity. Moreover, it has effects on growth regulation, sexual maturation and function, taste acuity, psychogenetic function, maintaining epithelial integrity, wound healing, and immunity (1, 2).
Both deficiency and excessive accumulation of zinc might result in multiple dysfunctions in the body (3). In this context, a reference value helps physicians for screening the serum zinc status and making a clinical decision (4). Age, gender, ethnicity, geo-chemical factors and altitude of the population have influence in fixing the reference value (4, 5, 6). The reference values used in most of the laboratories of the developing countries are obtained either from the values of scientific literature or manufacturers’ manuals (7), which is also applicable for Bangladesh. The International Federation for Clinical Chemistry and Laboratory Medicine (IFCC), and the Clinical and Laboratory Standards Institute (CLSI) recommended that every laboratory should have fixed reference values for bio-analytes (8). Thus, the study aimed to determine the reference value of serum zinc levels in Bangladeshi adult population of a selected rural area.
MATERIALS AND METHODS
Study area
This study was conducted in Sirajdikhan, a sub-district of Munshiganj district of Bangladesh, located approximately 29 km southeast of the capital city Dhaka. This agro-based area is about 180 km2 with the geographical extension of 23°30’ and 23°41’ latitude, and 90°14’ and 90°27’ longitude. The inhabitants of this region have limited food diversity and mostly rely on rice and vegetables with supplemented fish or meat (9).
Study participants and data collection
Around 200 healthy controls from another project on tuberculosis were considered as participants of this study (Author’s manuscript, unpublished). These healthy controls were selected from a community during November to December 2012 considering several selection criteria. Participants with a known history of malabsorption syndrome, nephrotic syndrome, tuberculosis, chronic liver disease, diabetes mellitus, neoplasm and chronic renal failure were excluded. In addition, participants having a history of blood transfusion within the last three months, hospitalized in the past month, participants on zinc medication, pregnant and lactating women, and women taking oral contraceptive pill were also excluded. A semi-structured questionnaire was administered to collect data which included age, sex, socioeconomic status, educational level and food habit of the participants. Informed written consent was obtained from each participant before the interview. After overnight fasting, 6 ml blood was collected aseptically via anti-cubital venepuncture to estimate serum zinc, serum albumin, serum creatinine and blood glucose level. Serum creatinine, serum albumin and fasting blood sugar were done in order to exclude respondents having chronic renal disease, chronic liver disease and diabetes mellitus, respectively. Among all the participants, seven participants had elevated serum creatinine value (>1.4 mg/dL), five had low serum albumin (<3.5 gm/dL) and another five had elevated blood glucose levels (≥7.0 mmol/L) (10). Chest radiography and Mantoux test (MT) were done for the exclusion of tuberculosis. Moreover, 22 participants were excluded due to haemolyzed blood samples through visual inspection. Finally, data from 161 participants were considered for analysis.
Determination of serum zinc level
Prior to sample collection, all plastic wares were made free from metallic contamination. The screw capped plastic tubes were opened and immersed in detergent water for at least half an hour, washed thoroughly in running tap water and then air-dried. These tubes and caps were later immersed sequentially in diluted nitric acid (20% v/v) for 24 hours, rinsed thoroughly and washed thrice in de-ionized water. Afterwards, the tubes and caps were again air-dried and capped. Then the screw capped plastic tubes were stored in capped plastic container and used only once. About 2 ml blood was collected and transferred immediately in clean deionized screw capped plastic tubes. The samples were transported from the study area to the laboratory on the same day. A cool ice box with liquid nitrogen containing ice pack was used for transporting the tubes.
Serum was obtained from blood after centrifugation at 1300-1350 g force for 5 minutes. The serum was kept in clean metal free polypropylene tubes and stored at -20°C until analysis. It was subjected to a single freeze-thaw cycle at the time of analysis. The tests were done at the Department of Biochemistry, Bangabandhu Sheikh Mujib Medical University (BSMMU) by graphite furnace atomic absorption spectrometer (GFAAS) (Shimadzu model 6650; 1996, Japan) (11) equipped with deuterium (D2) lamp background correction system (12). A zinc hollow cathode lamp was operated at an 8-mA (milliampere) intensity with a spectral width of 0.5 nm and the selected analytical wavelength for zinc was 213.9 nm. All samples were diluted (1:50) using diluent (matrix modifier- MgN03 10ml: Deionized water 90 ml) and analysed in triplicate.
The linearity of the calibration curve was in strong positive correlation (r=0.9993). The lowest limit of detection of serum zinc in the current method was 250 ppb (parts per billion) (25.0 μg/dL). The inter-assay CVs of serum zinc were within 5% for normal range and within 10% for pathological range. The level of serum zinc was expressed in μg/dL from ppb value.
Ethical consideration
The study protocol was approved by the Institutional Review Board of BSMMU, Dhaka, Bangladesh (2012/12497) in accordance with the Declaration of Helsinki.
Statistical methods
The normality assumption of serum zinc level was done by Shapiro-Wilk test (W value=0.881, p<0.000) and found not in Gaussian distribution. Tukey’s method was then employed to remove outliers which reduced the sample size to 154 from 161(13). A graphical presentation of the data distribution on serum zinc level before and after outlier removal is depicted in Figure 1. Recommended statistical approach of IFCC–CLSI was used to estimate the reference value of serum zinc (8). Reference values of serum zinc level for male and female were estimated by Robust method due to inadequate sample size (8,14).
Figure 1.
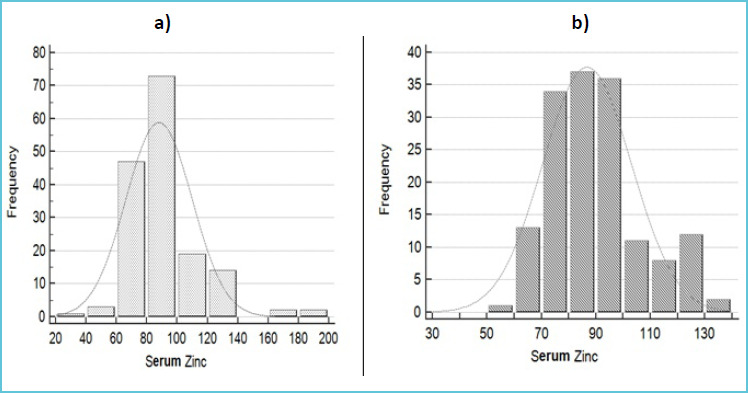
Normal distribution curve on a) original data of serum zinc values (μg/dL) (n=161) and b) that of after outlier treatment (n=154)
Descriptive analysis was done on respondents’ socio-demographic variables. Frequency and percentages were estimated as summary measures for the categorical variables and arithmetic mean, standard deviation, median and percentile were used to describe the continuous variables. The measure of association for the categorical variables was calculated by chi-square test, while Mann-Whitney U test and ANOVA for continuous variables. Spearmen correlation was used for correlation of the continuous variables.
Principle component analysis was adopted to measure the wealth index based on the household characteristics: type of wall, roof, floor and toilet; utilities: cooking fuel, source of light and water; ownership of the house; and crowding index (15).
The socio-economic status (SES) was categorized as lower, middle and upper based on the wealth index (16). Descriptive analysis was done by SPSS version 24 (Statistical Package, Chicago, USA), and “reference Intervals” of R Programming language (R Software version: 3.51) (17) was used to determine outlier and reference interval. A p value of less than 5 percent was considered as significant.
RESULTS
Socio-demographic profile and zinc value
Among the 154 healthy participants, ages 18 to 65 [mean (SD) age 33.2 (11.9) years], 53 percent were male, and 47 percent were female. Approximately 50 percent of the respondents were normal in weight [BMI mean (SD): 22.2(1.9) kg/m2], while 40 percent respondents were overweight [BMI mean (SD): 26.9 (1.4) kg/m2]. However, no significant weight difference was found between male and female respondents. Around three fourth of the respondents consumed meat 1-3 times, 60 percent consumed fish 4-6 times and half of the respondents consumed egg 1-3 times on a regular week, while the food consumption behaviour of male and female was almost identical (pvalue>0.05). Among all respondents, 16.9 percent belonged to lower SES, 42.2 percent to middle SES and 40.9 percent to upper SES.
The mean (SD) value of serum zinc level was 85.5 (16.0) μg/dL. This value showed significant difference between male [mean (SD): 92.0 (15.3) μg/dL] and female [mean (SD): 78.0 (13.8) μg/dL, p value<0.05]. However, serum zinc level had no significant correlations with age (spearman correlation, r=0.110; p>0.05) and BMI (r=-0.052; p>0.05).
The reference value of serum zinc level among the adult participants of this study was 60-120 μg/dL. The estimated reference intervals of serum zinc level for both genders are summarized in Table 1.
Table 1.
Measured reference intervals of serum zinc value (μg/dL)
| Population categories | Median | 2.5th – 97.5th percentile | 90% CI of lower limit (2.5th percentile) |
90% CI of upper limit (97.5th percentile) |
|---|---|---|---|---|
|
Total (n=154) |
85.0 | 60-120 | 55-65 | 120-135 |
|
Male (n=83) |
90.0 | 59-125 | 54-63 | 118-131 |
|
Female (n=71) |
75.0 | 50-103 | 43-54 | 97-110 |
P-value for male vs. female: <0.0001 (Mann-Whitney U test);
Reference interval calculated using Robust algorithm and confidence intervals (CI) calculated by bootstrapping, R = 5000.
Comparison with published studies
A comparative picture of serum zinc level of different studies conducted in various countries is shown in Table 2.
Table 2.
Review of reference values of zinc concentration in various countries
| Reference | Country | Sample | Age (year) |
Analytic Method | Statistical parameter | Zinc level (μg/dL)# |
|---|---|---|---|---|---|---|
| Present study | Bangladesh | 154 | 18-65 | GF-AAS | P2.5-P97.5 | 60-120 |
| Alimonti et al(19) | Italy | 110 | 20-61 | ICP-AES | P5-P95 | 59.7-102.8 |
| Forrer et al(20) | Switzerland | 110 | - | ICP-MS | P5-P95 | 63.7-100.4 |
| Rahil-Khazen et al (21) | Norway | 141 | 21-87 | ICP-AES | P2.5-P97.5 | 71.26-108.53 |
| McMaster et al (22) | Northern Ireland | 499111 | 25-64 | AAS | P5-P95 | Survey 1: 60.82-96.13 Survey 2: 66.05-111.18 |
| Hussain et al (23) | Pakistan | 450 | 20-29 | Flame AAS | P2.5-P97.5 | 75.01-240.14 |
| Abiaka et al (24) | Kuwait | 560 | 15-80 | Flame AAS | Range (mean±2SD)* |
59.50-151.03 |
| Rükgauer et al (25) | Germany | 68 | 22-75 | AAS | mean±2SD | 108.53±40.54 |
| Grandjean et al (26) | Denmark | 200 | - | Flame-AAS | P2.5-P97.5 | 53.61-102.65 |
P = percentile;
# All zinc levels were calculated in μg/dL by conversion factor. (1 μg/dL = 0.153μmol/L or 1 μg/L= 10 μg/dL);
* Range from frequency distribution plot;
ICP-AES: Inductively Coupled Plasma Spectrometry with Atomic Emission;
ICP-MS: Inductively Coupled Plasma-Mass Spectrometer;
GF-AAS: graphite furnace atomic absorption spectrometer.
DISCUSSION
A number of factors needs to be considered for determining the reference interval of bio-analytes. In this regard, the study participants were enrolled through accounting several selection criteria, and also controlling intra-individual variation by specimen collection timing, fasting duration and exercise restriction. Quality control was also ensured in sample collection, storage and analytic procedures. Finally, an appropriate statistical method, with outlier treatment, was followed for the measurement of reference interval of the bio-analytes (18).
The notable finding of the present study, considering the reference range of serum zinc in the normal adult population, was found as 60-120 μg/dL, after taking the necessary precautions in order to avoid the potential pre-analytical contamination while collecting samples, storing them and preparing for instrumental analysis. The scarcity of data on the standard reference range of serum zinc in Bangladesh makes the finding incomparable in the local context. In comparison to the finding of the current study, several other studies revealed almost similar reference ranges of serum zinc level (19–22) while few studies also reported inconsistent findings (23–26). Grandjean et al (26) reported lower reference range of serum zinc level than the present study, but higher reference range was also reported by some other studies (23, 25). Different results of serum zinc reference level are subjected to variation of laboratory techniques (23, 25, 26), statistical procedures (20, 25) and eco-environmental factors.
The mean serum zinc concentration was statistically higher in male (about 18%) than female (p<0.001). In congruent with this study, male gender was reported to have more zinc concentration than female (19, 24). As an explanation, high concentration of zinc was reported in the prostate gland and semen to maintain normal physiology of sperm (27). This condition makes adult men 3 times more recommended to zinc consumption than women (28). On the contrary, few studies found no significant difference in respect of gender (24) or opposing higher zinc value in female (29).
A non-significant correlation between zinc values and participants’ age was observed in this study (r=0.110; p>0.05). Analogously, the similar non-linear relationship was also detected by McMaster et al (22). However, Li et al found an increasing tendency of serum zinc in different age groups (30). Similarly, Zhang et al also reported increased value of serum zinc in subjects older than 50 years, but decreasing tendency before the age of 50, with any unnoticeable change of zinc value in different age groups (31). Another study even showed descending zinc values with ascending age (21).
This study warrants following limitations: the result of this study is difficult to generalize as the samples were taken from a selective rural area. After gender partitioning, the sample size became smaller. So, the gender specific reference ranges may not be representative for the whole population. However, the study findings recommend the importance of measuring reference values of every biochemical analytes with larger sample size ensuring proper quality. The study also recommends reduction of dependency on literature or test kit reference value for clinical decision making.
CONCLUSION
The result of this study provides important information about the reference value of serum zinc in adult population of Bangladesh.
REFERENCE
- 1.Shanker AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr 1998; 68:447S-63S. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- 2.Rink L. Zinc and the immune system. Proc Nutr Soc 2000;59:541-552. doi: 10.1017/S0029665100000781. [DOI] [PubMed] [Google Scholar]
- 3.Strachan S. Trace elements. Curr Anaesth Cri Care 2010;21:44-48. doi: 10.1016/j.cacc.2009.08.004. [Google Scholar]
- 4.Dosoo DK, Kayan K, Adu-Gyasi D, Kwara E, Ocran J, Osei-Kwakye K, et al. Haematological and biochemical reference values for healthy adults in the middle belt of Ghana. PloS one 2012;7:e36308 doi: 10.1371/journal.pone.0036308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horn PS, Pesce AJ. Effect of ethnicity on reference intervals. Clin Chem 2001; 48: 1802–1804. [PubMed] [Google Scholar]
- 6.Buchanan AM, Muro FJ, Gratz J, Crump JA, Musyoka AM, Sichangi MW, et al. Establishment of haematological and immunological reference values for healthy Tanzanian children in Kilimanjaro Region. Trop Med Int Health 2010; 15:1011–1021. doi: 10.1111/j.1365-3156.2010.02585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koram K, Addae M, Ocran J, Adu-Amankwah S, Rogers WO, Nkrumah FK. Population based reference intervals for common blood haematological and biochemical parameters in the Akuapem North District. Ghana Med J 2007; 41:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.IFCC CLSI, EP28-A3c document, Defining, Establishing and Verifying Reference Intervals in the Clinical Laboratory: Approved Guideline. 3rd ed, vol.28, No. 30, 2010. [Google Scholar]
- 9.Khan NI, Bruce D, Naidu R, Owens G. Implementation of food frequency questionnaire for the assessment of total dietary arsenic intake in Bangladesh: part B, preliminary findings. Environ Geochem Health 2009;31:221-238. doi:10.1007/s10653-008-9232-3. [DOI] [PubMed] [Google Scholar]
- 10.Prasad AS. Clinical and biochemical manifestation zinc deficiency in human subjects. Journal de Pharmacologie 1985: 16(4):344-352. (PMID:2419703). [PubMed] [Google Scholar]
- 11.Dey AC, Shahidullah M, Mannan MA, Noor MK, Saha L, Rahman SA. Maternal and neonatal serum zinc level and its relationship with neural tube defects. J Health Popul Nutr 2010;28(4):343-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pizent A, Telisman S. Analysis of reference materials for serum copper and zinc by flame AAS. At Spectrosc 1996;17:88-91. [Google Scholar]
- 13.Tukey JW. Box-and-Whisker Plots. in Exploratory Data Analysis. Reading, Massachusetts: Addison-Wesley, 1977: 39-43. [Google Scholar]
- 14.Geffré A, Braun JP, Trumel C, Concordet D. Estimation of reference intervals from small samples: an example using canine plasma creatinine. Vet Clin Pathol 2009;38: 477-484. doi: 10.1111/j.1939-165X.2009.00155.x. [DOI] [PubMed] [Google Scholar]
- 15.Hjelm L, Mathiassen A, Miller D, Wadhwa A. Creation of a Wealth Index - 1 - World Food Programme, 2017. Available at: https://docs.wfp.org/api/documents/WFP-0000022418/download/. Accessed 5th June 2018.
- 16.Freitas LP, Souza-Santos R, Kolte IV, Malacarne J, Basta PC. Socioeconomic status of indigenous peoples with active tuberculosis in Brazil: a principal components analysis. bioRxiv. 2018:290668 doi: 10.1101/290668. [Google Scholar]
- 17.Finnegan D. Package ‘referenceIntervals’. 2015. Available at: https://CRAN.R-project.org/package=referenceIntervals. Accessed 3rd June 2018.
- 18.Ichihara K, Kawai T. Determination of reference intervals for 13 plasma proteins based on IFCC international reference preparation (CRM470) and NCCLS proposed guideline (C28-P, 1992): Trial to select reference individuals by results of screening tests and application of maximal likelihood method. J Clin Lab Anal 1996; 10:110-117. doi: org/10.1002/(SICI)1098-2825(1996)10:2<110::AID-JCLA9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 19.Alimonti A, Bocca B, Mannella E, Petrucci F, Zennaro F, Cotichini R, et al. Assessment of reference values for selected elements in a healthy urban population. Ann 1st Super Sanita 2005;41(2):181-187. [PubMed] [Google Scholar]
- 20.Forrer R, Gautschi K, Lutz H. Simultaneous measurement of the trace elements Al, As, B, Be, Cd, Co, Cu, Fe, Li, Mn, Mo, Ni, Rb, Se, Sr, and Zn in human serum and their reference ranges by ICP-MS. Biol Trace Elem Res 2001;80(1):77-93. doi: 10.1385/BTER:80:1:77. [DOI] [PubMed] [Google Scholar]
- 21.Rahil-Khazen R, Bolann BJ, Ulvik RJ. Trace element reference values in serum determined by inductively coupled plasma atomic emission spectrometry. Clin Chem Lab Med 2000;38(8):765-772. [DOI] [PubMed] [Google Scholar]
- 22.McMaster D, McCrum E, Patterson CC, Kerr MM, O’Reilly D, Evans AE, et al. Serum copper and zinc in random samples of the population of Northern Ireland. Am J Clin Nutr 1992; 56:440-446. doi: 1093/ajcn/56.2.440. [DOI] [PubMed] [Google Scholar]
- 23.Hussain W, Mumtaz A, Yasmeen F, Khan SQ, Butt T. Reference range of zinc in adult population (20-29 years) of Lahore, Pakistan. Pak J Med Sci 2014;30(3):545-548. doi: 10.12669/pjms.303.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abiaka C, Olusi S, Al-Awadh A. Reference Ranges of Copper and Zinc and the Prevalence of Their Deficiencies in an Arab Population Aged 15–80 Years. Biol Trace Elem Res 2003; 91: 33-43. doi.10.1385/BTER:91:1:33. [DOI] [PubMed] [Google Scholar]
- 25.Rükgauer M, Klein J, Kruse-Jarres JD. Reference values for the trace elements copper, manganese, selenium, and zinc in the serum/plasma of children, adolescents, and adults. J Trace Elem Med Biol 1997;11(2):92-98. doi:org/10.1016/S0946-672X(97)80032-6. [DOI] [PubMed] [Google Scholar]
- 26.Grandjean P, Nielsen GD, Jorgensen PJ, Horder M. Reference intervals for trace elements in blood: significance of risk factors. Scan J Clin Lab Invest 1992;52: 321-337. doi:10.1080/00365519209088366. [DOI] [PubMed] [Google Scholar]
- 27.Lewis-Jones DI, Aird IA, Biljan MM, Kingsland CR. Effects of sperm activity on zinc and fructose concentrations in seminal plasma. Hum Reprod 1996;11:2465–2467. [DOI] [PubMed] [Google Scholar]
- 28.WHO. Environmental Health Criteria 221. Zinc. Geneva: World Health Organization; 2001. Available at http://whqlibdoc.who.int/ehc/who_ehc_221.pdf; Accessed 5th June 2018. [Google Scholar]
- 29.Schuhmacher M, Domingo JL, Corbella J. Zinc and copper levels in serum and urine: relationship to biological, habitual and environmental factors. Sci Total Environ 1994; 148:67–72. doi: 10.1016/0048-9697(94)90376-X. [DOI] [PubMed] [Google Scholar]
- 30.Li Yu-yan, Wei-jin Zhou, Jun-qing Wu. Contrast of serum trace elements in different age groups of male adults. Chin J Public Health Mar 2006; 22(3):277–279. [Google Scholar]
- 31.Zhang HQ, Li N, Zhang Z, Gao S, Yin HY, Guo DM, Gao X. Serum zinc, copper, and zinc/copper in healthy residents of Jinan. Biol Trace Elem Res 2009;131: 25-32. doi:10.1007/s12011-009-8350-9. [DOI] [PubMed] [Google Scholar]


