Abstract
Background
Pre-eclampsia (PE) can be divided into 2 sub-groups: early-onset and late-onset PE. Although these sub-groups show overlapping molecular and cellular mechanisms and similar clinical manifestations, they are regarded as 2 different phenotypes with heterogeneous manifestations. The pathophysiological mechanisms underlying early-onset and late-onset PE still remain unclear. Therefore, the present study aimed to identify the key genes and pathways related to early-onset and late-onset PE, and to investigate the molecular mechanisms that are involved in gene regulation.
Material/Methods
Our analysis involved the Gene Expression Series (GSE) 74341 and GSE22526 from the National Center of Biotechnology Information (NCBI) Gene Expression Omnibus Database. These 2 microarray datasets included 15 patients with early-onset PE and 15 patients with late-onset PE.
Results
Our analyses identified 15 differentially expressed genes (DEGs), including CGA, EGR1, HBB, HBA2, LEP, and LHB. Gene Ontology (GO) functional annotation showed that the biological functions of these DEGs were mainly associated with steroid biosynthetic, oxidative stress, angiogenesis, and sex differentiation. Signaling pathway analyses showed that these DEGs were mainly involved in the prolactin signaling pathway, hormone metabolism, the AMPK signaling pathway, and the FoxO signaling pathway. Protein-protein interaction (PPI) network analysis identified 4 genes with the highest degree of interaction. The hub genes for this selection of DEGS were EGR1, LEP, and HBB.
Conclusions
Integrated bioinformatic analyses provide us with a new approach to further understand the pathophysiology and molecular mechanisms underlying early-onset and late-onset PE. The DEGs identified in this study represent potential biomarkers for the early diagnosis of PE and may provide significant options the treatment of these 2 subtypes of PE.
MeSH Keywords: Microarray Analysis, Pre-Eclampsia, Pregnant Women
Background
Pre-eclampsia (PE) is a pregnancy-related disorder. The characteristics of PE are elevated blood pressure, and proteinuria, both of which lead to a variety of pathophysiological processes, including endothelial dysfunction, systemic inflammation, and impaired implantation [1]. PE occurs in approximately 3% of all pregnant women, and it is the main cause of more than 60 000 global maternal deaths every year [2]. The prevalence of PE has been increasing relentlessly [3]. However, as yet, there are no effective methods for screening or managing PE. Following delivery, the clinical symptoms of this disorder disappear. Even without any fetal-derived tissue, patients with a hydatidiform mole can also develop PE. The general consensus of opinion is that the placenta is linked to the etiology of this complication.
Genome-wide profiling approaches, such as microarray analysis, provide researchers with an efficient way of investigating the etiology of placental disorders. PE has 2 subtypes: early-onset (<34 weeks) and late-onset (>34 weeks) [4]. The clinical features and biochemical markers of these 2 subtypes differ [5]. Early-onset PE is life-threatening, and the maternal mortality associated with this subgroup is 20 times higher than normal delivery [6]. Furthermore, previous studies that have analyzed the expression of serum cytokines, and leukocyte function, revealed that early-onset PE is a special subtype of PE compared to late-onset [7]. Women with a history of early-onset PE tend to have an increased risk of chronic hypertension, or ischemic heart disease. The latter could lead to earlier cardiovascular death [8]. Therefore, comparing the expression profiles of the 2 subtypes of PE is of critical clinical significance. By investigating the etiologies underlying these 2 subgroups of PE could help us to develop new therapies for the treatment of this pregnancy disorder.
In this study, we identified numerous differentially expressed genes (DEGs) by comparing the gene expression profiles of placental tissue between patients with the 2 subtypes of PE. Upon screening, we conducted Gene Ontology (GO) enrichment analysis, signaling pathways enrichment analysis, and protein-protein interaction (PPI) network analysis, in an attempt to identify the molecular interactions that are implicated in the two sub-classifications of PE.
Material and Methods
Microarray data
We acquired GSE74341 and GSE22526’s series matrix file(s) from the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo); these databases store microarray data that are accessible to all researchers. GSE74341 was generated on the GPL16699 platform (Agilent039494 SurePrintG3 HumanGEv2 Microarray039381). GSE22526 was generated on the GPL10579 platform (GynObsLU Human 800 PE-associated cDNA).
Identification of DEGs
In order to identify DEGs, we used the R software and annotation package to transfer the downloaded platform and series of matrix file(s). The probe number was then changed into a universal gene name (gene symbol) within a CSV document. We then identified DEGs in early-onset and late-onset PE samples in the GSE74341 and GSE22526 using R software, and the Limma package [9]. The threshold for differential expression genes was defined as a P-value <0.05 and |log2(fold change)| >1.
Enrichment analyses
To assess the function of DEGs in early-onset and late-onset PE, we performed GO term enrichment analyses using the ClusterProfiler package in R software [10], including biological process, cellular component, and molecular function; a P-value <0.05 was set as the threshold. We performed signaling pathway enrichment analyses, including KOBAS-Kyoto Encyclopedia of Genes and Genomes, KEGG), for all DEGs using a specific website (http://kobas.cbi.pku.edu.cn). The threshold was set to a P-value <0.05.
PPI network analyses
We used the STRING online tool (http://stringdb.org) to explore the relationships between the DEGs. Then, we attempted to reveal the biomolecular interactions that occur in the 2 different forms of PE. Hub genes were identified using Cytohubba, a plug-in of the Cytoscape software package [11].
Human participants
Ethical approval was granted by the Ethics Committee of The First Affiliated Hospital of China Medical University (Shenyang, China) and methods were carried out in accordance with the committee guidelines (Ethical Application Reference Number: AF-SOP-07-1.1-01). All participating patients signed informed consent. The diagnosis criteria for PE were published previously by Steegers et al. [12]. Patients enrolled in the PE group had no history of pre-existing or chronic hypertension, although they exhibited ≥140 mmHg systolic or ≥90 mmHg diastolic blood pressure on 2 occasions at least 4 hours apart after 20 weeks of gestation, and ≥300 mg per 24-hour urine collection after 20 weeks of gestation. Early-onset PE was defined as onset of the disease before 34 weeks of gestation (between 20 and 34 completed gestational weeks); late-onset was defined as onset of the disease after 34 weeks of gestation (between 34 and 41 completed gestational weeks). The clinical characteristics of the pregnant women enrolled in this study are given in Table 1.
Table 1.
Clinical characteristics of pregnant women enrolled in the study.
| Early-onset PE (n=5) | Late-onset PE (n=5) | P | |
|---|---|---|---|
| Maternal age (years) | 28 (23–34) | 30 (27–35) | 0.399 |
| BMI (kg/m2) | 20.4 (17.9–22.3) | 21.5 (19.5–23.1) | 0.347 |
| Systolic pressure (mmHg) | 153 (141–173) | 157 (142–177) | 0.465 |
| Diastolic pressure (mmHg) | 100 (78–115) | 97 (82–110) | 0.602 |
| 24h proteinuria (mg/L) | 4484 (319–8428) | 3420 (412–7361) | 0.754 |
| Gestational age at delivery (days) | 225 (215–235) | 262 (253–273) | 0.009 |
| Birthweight (grams) | 1256 (950–1790) | 2788 (2100–3590) | 0.009 |
| Placental weight (grams) | 338 (180–510) | 592 (490–750) | 0.016 |
BMI – body mass index; PE – pre-eclampsia.
Comparing early-onset PE group with late-onset PE group using independent-samples Mann-Whitney U test.
Validation of the mRNA expression of crucial genes
We selected 10 placental tissue samples for verification by quantitative reverse transcription polymerase chain reaction (PCR): 5 samples of early-onset PE (<34 weeks gestation), and 5 samples of late-onset PE (>36 weeks gestation). Chorionic tissues were obtained from 4 different parts of the placenta, from which the amniotic membrane and maternal decidual tissues were removed. Tissues were frozen and stored at −80°C until use. RNA isolation was performed with TRIzol (Invitrogen) in accordance with the manufacturer’s instructions. First-strand cDNA was reverse transcribed using the GoScript Reverse Transcription System (Promega). Quantitative PCR was then performed using GoTaq qPCR Master Mix (Promega), and amplification was quantified by the CFX-96 system (Bio-Rad). Expression levels were then normalized to Gapdh. At least 3 biological replicates were performed for each experiment. Primer sequences are given in Table 2.
Table 2.
Sequence of primers used for validation of expression levels of core DEGs.
| Genes | Genes ID | Sequence |
|---|---|---|
| CGA left primer | NM_000735.3 | tctccattccgctcctgat |
| CGA right primer | gggagaagaatgggttttcc | |
| EGR1 left primer | NM_001964.2 | tctgaacaacgagaaggtgct |
| EGR1 right primer | gggcagtcgagtggtttg | |
| HBA2 left primer | NM_000517.6 | GTCCCCACAGACTCAGAGA |
| HBA2 right primer | GGGAAGGACAGGAACATCC | |
| HBB left primer | NM_000518.4 | gcacgtggatcctgagaact |
| HBB right primer | cactggtggggtgaattctt | |
| LEP left primer | NM_000230.2 | tgacatttcacacacgcagtc |
| LEP right primer | atgaagtccaaaccggtgac | |
| LHB left primer | NM_000894.2 | atcctggctgtcgagaagg |
| LHB right primer | gtaggtgcacaccacctgag |
DEG – differentially expressed genes.
Results
Identification of DEGs
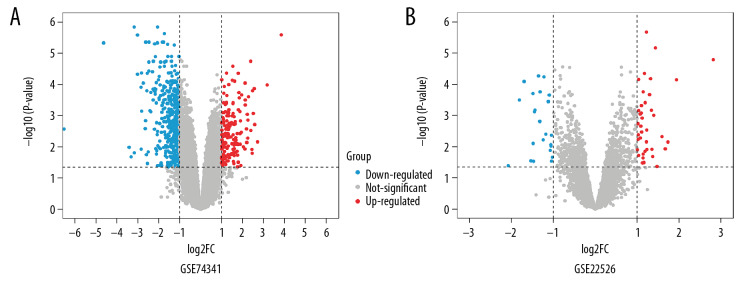
GSE74341 and GSE22526 data were downloaded from GEO, including 30 placenta samples (15 early-onset and 15 late-onset). We then used the Limma package to identify DEGs by comparing early-onset and late-onset PE. The Limma package identified 172 upregulated genes and 455 downregulated genes in the GSE74341 dataset (a total of 627 genes; Figure 1A). In total, 62 DEGs were acquired from the GSE22526 datasets, including 23 upregulated genes and 39 downregulated genes (Figure 1B).
Figure 1.
Volcano plot of gene expression profile data in early-onset pre-eclampsia and late-onset ones. (A) Volcano plot of GSE74341. (B) Volcano plot of GSE22526.
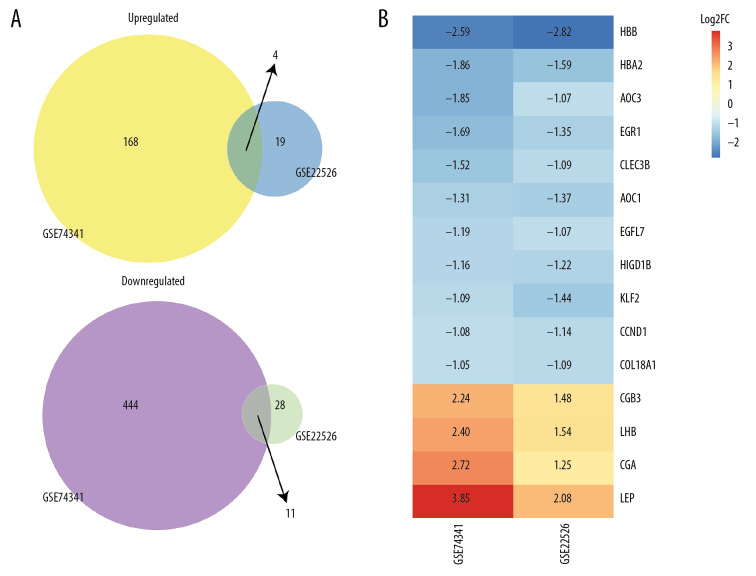
The expression levels of the top 100 DEGs in GSE74341, and all the DEGs in GSE22526, are presented in a heatmap (Figure 2). After merging all DEGs in GSE74341 and GSE22526, 15 DEGs were revealed, including 4 upregulated genes and 11 downregulated genes: HBB, HBA2, AOC3, EGR1, CLEC3B, AOC1, EGFL7, HIGD1B, KLF2, CCND1, COL18A1, CGB3, LHB, CGA, and LEP (Figure 3). Of these, HBB, HBA2, AOC3, EGR1, CLEC3B, AOC1, HIGD1B, KLF2, CCND1, and COL18A1, were novel and provided us with a new direction with which to investigate the etiopathogenetic mechanisms underlying PE.
Figure 2.
Heat map of DEGs. (A) Top 100 of GSE74341. (B) GSE22526, blue represents a lower expression level, red represents higher expression levels,and white represents that there is no different expression amongst the genes. Each column represents one dataset and each row represents one gene. The gradual color ranged from blue to red represents the changing process from downregulation to upregulation. DEG – differentially expressed gene.
Figure 3.
DEGs after merged. (A) Venn diagram shows that 4 upregulated and 11 downregulated genes. (B) Heat map of DEGs after merged GSE74341 and GSE22526. The number in each rectangle represents the normalized gene expression level. DEG – differentially expressed gene.
Enrichment analyses
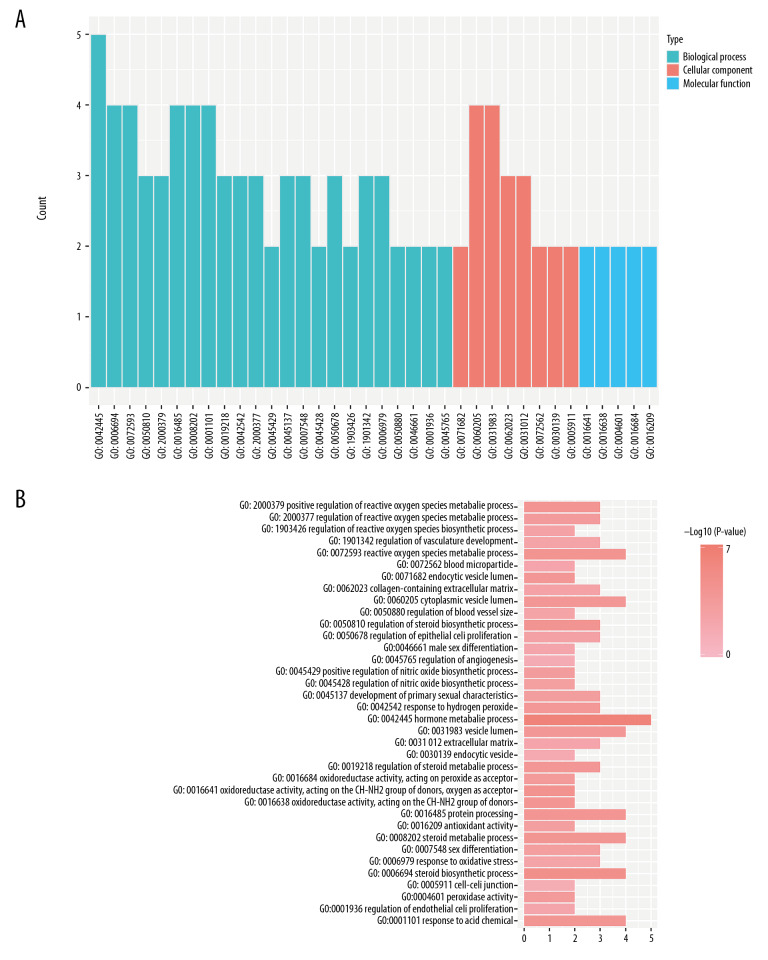
In terms of biological processes, our analyses showed that DEGs were significantly enriched in oxidative stress, the biosynthesis of steroids, the regulation of nitric oxide biosynthesis, angiogenesis, and sex differentiation. With regards to cellular components, DEGs were significantly enriched in the endocytic vesicles, blood microparticles, and extracellular matrix. With regards to molecular function, DEGs were significantly enriched in peroxidase activity, oxidoreductase activity, and antioxidant activity (Figure 4). Enrichment analyses suggested that the DEGs were mainly implicated in oxidative stress and related function.
Figure 4.
(A, B) GO functional annotation of DEGs between early-onset and late-onset pre-eclampsia. GO – Gene Ontology; DEG – differentially expressed gene.
Signaling pathway analyses
Several enriched pathways of DEGs were identified between early-onset and late-onset PE (Table 3). The top 10 P-values included the prolactin signaling pathway, peptide hormone metabolism, the take up of oxygen by erythrocytes and the subsequent release of carbon dioxide, glycoprotein hormones, hormone ligand-binding receptors, peptide hormone biosynthesis, the metabolism of steroid hormones, the AMP-activated protein kinase (AMPK) signaling pathway, and the FoxO signaling pathway. Hormone metabolism was identified as the most predominant signaling pathway when compared between early-onset and late-onset PE.
Table 3.
Signaling pathway enrichment.
| Term | Database | ID | Gene number | P-value | Corrected P-value | Genes |
|---|---|---|---|---|---|---|
| Prolactin signaling pathway | KEGG pathway | hsa04917 | 3 | 1.82E-06 | 0.000278974 | LHB, CCND1, CGA |
| Peptide hormone metabolism | Reactome | R-HSA-2980736 | 3 | 2.56E-06 | 0.000278974 | LEP, LHB, CGA |
| Erythrocytes take up oxygen and release carbon dioxide | Reactome | R-HSA-1247673 | 2 | 5.42E-06 | 0.000361262 | HBA2, HBB |
| Glycoprotein hormones | Reactome | R-HSA-209822 | 2 | 5.42E-06 | 0.000361262 | LHB, CGA |
| Hormone ligand-binding receptors | Reactome | R-HSA-375281 | 2 | 6.50E-06 | 0.000383313 | LHB, CGA |
| Peptide hormone biosynthesis | Reactome | R-HSA-209952 | 2 | 7.68E-06 | 0.000395435 | LHB, CGA |
| Metabolism of steroid hormones | Reactome | R-HSA-196071 | 2 | 5.18E-05 | 0.000964628 | LHB, CGA |
| Metabolism | Reactome | R-HSA-1430728 | 5 | 0.000279689 | 0.003168314 | HBA2, HBB, LHB, CGA, AOC3 |
| AMPK signaling pathway | KEGG pathway | hsa04152 | 2 | 0.000771072 | 0.006385017 | LEP, CCND1 |
| FoxO signaling pathway | KEGG pathway | hsa04068 | 2 | 0.000883228 | 0.007065826 | KLF2, CCND1 |
| Signal Transduction | Reactome | R-HSA-162582 | 4 | 0.006567989 | 0.021934604 | LEP, LHB, CCND1, CGA |
| Metabolism of proteins | Reactome | R-HSA-392499 | 3 | 0.009201322 | 0.025954137 | LEP, LHB, CGA |
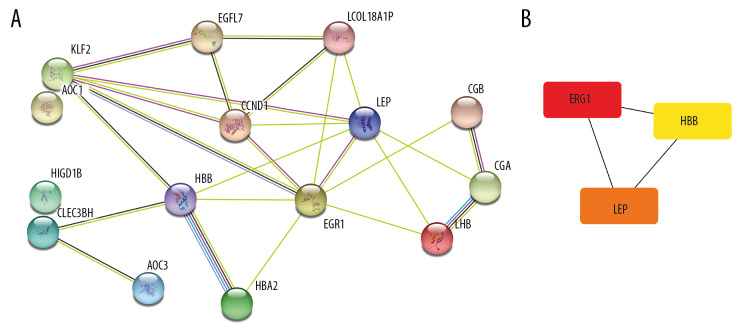
PPI network analysis and hub genes
All 15 DEGs were entered into the STRING online database. PPI networks were then constructed (Figure 5). The PPI enrichment P-value was 4.13×10−6. The top 5 combined scores were 0.995 (between HBB and HBA2), 0.981 (between LHB and CGA), 0.775 (between LEP and CCND1), 0.737 (between CGB3 and CGA), and 0.715 (between EGR1 and CCND1). Three genes (EGR1, LEP and HBB) were identified as hub genes (Figure 5B).
Figure 5.
The PPI network and the hub genes of DEGs. (A) The PPI network was analyzed by String online website. (B) The 3 hub genes of DEGs. DEG – differentially expressed gene; PPI – protein–protein interaction.
The validation of hub gene expression in clinical samples
To further determine the expression of hub genes in placental tissue from early-onset PE and late-onset PE, we used real-time quantitative PCR to detect the expression of the 3 hub genes in placental tissue, and 2 pairs of proteins showing the highest interaction. Placentas were collected from patients with early-onset PE and late-onset PE. We confirmed that EGR1, HBB, and HBA2, were significantly downregulated in early-onset PE when compared with late-onset PE. Furthermore, LHB, CGA, and LEP were significantly upregulated in early-onset PE compared with late-onset PE (Figure 6). These results were consistent with our bioinformatic results.
Figure 6.
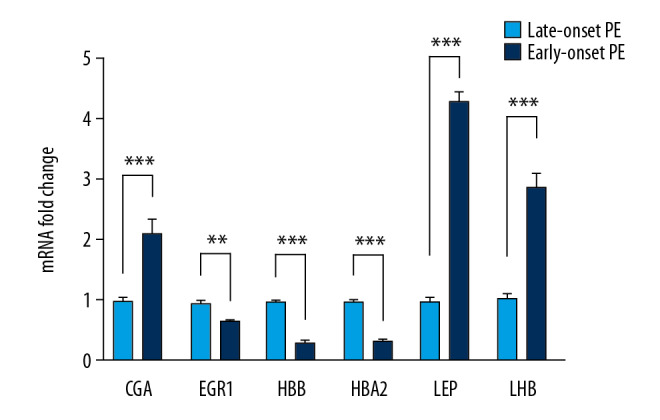
The validation of hub genes expression in clinical samples. Real-time quantitative PCR revealed that EGR1, HBB, and HBA2 were downregulated in early-onset pre-eclampsia group compared with late-onset pre-eclampsia. While LHB, CGA, and LEP were significantly upregulated.
Discussion
Early-onset and late-onset PE should be viewed as distinct nosological entities rather than different clinical forms of the same illness. The differences between these conditions are not limited only to the time that clinical symptoms emerge; and it should be noted that this issue is still debated [13]. Previous studies performed gene expression analyses of early-onset and late-onset PE to identify DEGs and investigate their potential for exploring the role of genetic heterogeneity in the pathogenesis of the 2 sub-classifications of PE [14–16]. These genome-wide expression profiling techniques were valuable given the significant availability of complementary data. Genome-wide approaches have also been used to explore differential expression in independent placental tissues of early-onset and late-onset PE. However, the underlying molecular mechanisms associated with early-onset and late-onset PE have not been investigated; many of the previous studies have focused on a single gene, or results were derived from a single-cohort study. In the current study, we acquired placental gene expression data from early-onset and late-onset patients, and used bioinformatic analysis to explore the importance of key genes. Using 2 genome-wide transcriptomic datasets, we identified several DEGs between early-onset and late-onset PE, including 4 upregulated genes and 11 downregulated genes. These genes may represent crucial biomarkers, with mechanistic associations, for early-onset pathogenesis and prediction. Five of these 15 DEGs, EGFL7, CGB3, LHB, CGA, and LEP, were differentially expressed when compared between early-onset PE and healthy controls [17].
GO functional annotation showed that several biological processes were associated with the enriched genes, including steroid biosynthesis, oxidative stress, angiogenesis, and sex differentiation. Steroids, such as estradiol (E2), can modulate the synthesis of stress factors and both angiogenic and vascular endothelium functions. Furthermore, E2 increases the synthesis of angiogenic factors and nitric oxide (NO) [18], thus mediating vascular tone and increasing uterine blood flow in normal pregnancies [19]. Previous work demonstrated that CGA is an estrogen receptor-responsive gene [20]. Further research is necessary to determine if the association between CGA and estrogen is involved in the pathogenetic mechanisms underlying early-onset PE. Oxidative stress is one of the most important humoral factors to consider when dealing with a poorly perfused placenta. Previous studies have indicated that oxidative stress is more significant in early-onset PE compared to late-onset PE [21]. Furthermore, oxidative stress in the syncytiotrophoblast is a particular hallmark of PE, particularly early-onset PE [22]. A complex blend of factors released from a stressed syncytiotrophoblast can lead to a systemic inflammatory response, the predominant clinical feature of PE. Recent studies have suggested that fetal gender might act as a critical risk factor for PE. It was suggested that nearly half of the cases of PE are X-linked and arise due to problems associated with X-inactivation [23,24]. Moreover, women carrying male fetuses show higher levels of vascular resistance in terms of uterine artery pulsatility index [25]. Therefore, the male fetus may be less adaptable to an unfavorable environment due to the fact that they are more sensitive to suboptimal placentation [26]. The existence of a more “pro-inflammatory environment” in the male fetus could also explain the clear bias of early-onset PE towards female fetuses [27]. Above all, our GO analysis of DEGs indicated that early-onset PE is primarily due to a serious defective placentation and poor uterine blood flow, and shares common pathophysiology with other disorders of placentation; these findings were similar to those reported previously [28].
Furthermore, the enriched DEGs were associated with the prolactin signaling pathway, hormone metabolism, the AMPK signaling pathway, and the FoxO signaling pathway. Prolactin is released from multiple extra-pituitary sites during pregnancy, including adipose tissue, lymphocytes, myometrium, prostate, breast, and the decidua [29]. Prolactin generated from decidua can regulate placental angiogenesis and immunological functions at the feto-maternal interface [29]. The full-length, 23 kiloDalton (kDa) prolactin protein can promote placenta angiogenesis, while the proteolytic 16 kDa fragment can suppress placenta angiogenesis [30]. Disfunction associated with the 23 kDa and 16 kDa fragments could explain both impaired early placentation, and subsequent endothelial dysfunction; this represents the core pathophysiology of PE. The elevation of levels of the 16 kDa prolactin fragment in the circulation, amniotic fluid, and urine of PE patients also fits this model [31]. AMPK is a one of the central regulators of cellular and organismal metabolism in eukaryotes. During pregnancy, AMPK plays an important role in placental differentiation, maternal and fetal energy homeostasis, nutrient transportation, and fetal membrane protection [32]. In a previous paper, Nadiye et al. showed that the levels of AMPK were significantly elevated in patients with severe PE compared to controls and those with mild PE. Importantly, there was a positive relationship between the levels of AMPK and blood pressures, and there was a negative relationship between the levels of AMPK and the gestational week at birth and fetal weight [33]. These findings indicate that AMPK might act as a novel marker to predict disease severity. FoxO1 is a transcription factor expressed in syncytiotrophoblasts; its phosphorylated form (p-FoxO1), and acetylated form (ac-FoxO1) are expressed in cytotrophoblasts and syncytiotrophoblasts [34]. In addition to regulating apoptosis and oxidative stress, FoxO1 regulates various other cellular processes that are important for the placenta, including cellular proliferation and differentiation, cell cycle regulation, DNA repair, and metabolism. A previous study found that the number of FoxO1-negative syncytiotrophoblast nuclei were increased, and FoxO1-positive syncytiotrophoblast nuclei were significantly decreased, in mild PE compared to controls [35]. The abnormal expression of FoxO1 may be involved in the impaired placental cellular morphogenesis observed in mild PE. Activation of FoxO1 could repress the expression of CCND1 [36,37], a novel DEG identified in the present study. The downregulation of CCND1 suggests that the activation of FoxO1 might be more serious in early-onset PE.
The PPI network of DEGs was constructed by STRING. In this study, the pairing of CGA with LHB, and the pairing of HBB with HBA2, showed the highest levels of interaction in the PPI network. Human chorionic gonadotropin (hCG) is associated with placental disorders [38]. This glycoprotein consists of 2 dissimilar peptide subunits, designated alpha (α) and beta (β). CGA encodes the alpha subunit. Previous studies have found that α-hCG is correlated with PE [38,39]. These studies indicated that hCG is a reliable trophoblastic marker, and that α-hCG could reflect chronic placental hypoxia [38]. One of the DEGs identified in the present study was CGA; this indicates that α-hCG could represent a potential serum biomarker with which to make a differential diagnosis between early-onset and late-onset PE. LHB is a member of the glycoprotein hormone beta chain family and encodes the beta subunit of luteinizing hormone (LH). Many studies have found that LHB was unregulated in early-onset PE or PE, largely due to the abnormal physiology of trophoblasts [17,40]. LH is primarily expressed in syncytiotrophoblasts, thus indicating that altered pathways are related to placental oxidative stress or uteroplacental vascular insufficiency [41]. As LH is a characteristic for PE in the maternal circulation, it could also be used as a novel screening biomarker for early-onset PE via serum testing [42]. Hemoglobin subunit alpha 2 (HBA2) is a protein-coding gene and was one of the novel DEGs identified in the present study. Although previous research has not identified a role for HBA2 in PE, the diseases most associated with HBA2 include hemoglobin H disease, and alpha-thalassemia; these are also significantly related to certain pregnancy complications, including fetal growth restriction, automatic miscarriages, and perinatal mortality [43,44]. Oxidative stress as a result of iron overload, and placental hypoxia caused by maternal anemia, appear to provoke such complications. It is possible that the oxidative stress associated with HBA2 is implicated in the pathogenetic mechanisms underlying PE. This concept is worth exploring in future research.
Three hub genes were identified in our analyses. Earlier studies showed that EGR1 protein plays a key role in implantation [45,46]; while LEP plays a major role in the regulation of energy homeostasis and can regulate the cytokine pathway by connecting the inflammatory and immune systems [47]. Recently, LEP was also shown to have vital roles in various physiological processes, including angiogenesis and the regulation of arterial blood pressure [48]. The level of serum LEP may therefore act as a biomarker to differentiate between early-onset and late-onset PE [49]. Moreover, LEP could activate AMPK [50], which is also elevated in PE and associated with blood pressure. Blocking the activation of AMPK stimulated by LEP could also be proposed as a promising drug target for PE. HBB’s basic function is oxygen transportation. Placental hypoxia has been suggested to play a key role in the mechanisms underlying PE. Alternations in gene expression in the placenta have further confirmed that upregulated pathways are related to hypoxia in PE [51]. The expression of HBB was shown to be altered because of its responses to oxidative or nitrosative stress, and/or hypoxia, in the rhesus macaques fetal/neonatal brain [52]. Owing to its ability to reversibly bind O2 and other gaseous ligands in erythrocytes, HBB could also be used as a therapeutic target to improve the clinical manifestations caused by hypoxia.
Conclusions
We conducted comprehensive bioinformatics analysis of gene expression profiles in placental tissue from early-onset and late-onset PE, and identified 15 DEGs, including 3 hub genes. Abnormal placentation and hypoxia are more serious in early-onset PE, where placental and fetal tissues are subjected to adverse exposure from early pregnancy onwards. Defective placentation appears to play a much more critical role in the pathophysiology of early-onset PE than late-onset PE, with more adverse consequences for the fetus, such as fetal growth restriction, thus supporting the current results. Integrated bioinformatics analyses therefore yielded several promising serum biomarkers, such as LEP, LH, and α-hCG encoded by CGA, for the early diagnosis of the 2 different subtypes of PE. Furthermore, blocking the activation of AMPK stimulated by LEP, and using the ability of HBB to transport oxygen, could represent new therapeutic targets for early-onset PE.
Footnotes
Source of support: Departmental sources
References
- 1.Guo L, Tsai SQ, Hardison NE, et al. Differentially expressed microRNAs and affected biological pathways revealed by modulated modularity clustering (MMC) analysis of human preeclamptic and IUGR placentas. Placenta. 2013;34(7):599–605. doi: 10.1016/j.placenta.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vigil-De Gracia P. Maternal deaths due to eclampsia and HELLP syndrome. Int J Gynaecol Obstet. 2009;104(2):90–94. doi: 10.1016/j.ijgo.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens. 2008;21(5):521–26. doi: 10.1038/ajh.2008.20. [DOI] [PubMed] [Google Scholar]
- 4.von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnancy. 2003;22(2):143–48. doi: 10.1081/PRG-120021060. [DOI] [PubMed] [Google Scholar]
- 5.Johnson A, Federico C, Martinez M, et al. [192-POS]: Term and preterm preeclampsia: Are there two distinct phenotypes? Pregnancy Hypertension. 2015;5(1):97. [Google Scholar]
- 6.MacKay AP, Berg CJ, Atrash HK. Pregnancy-related mortality from preeclampsia and eclampsia. Obstet Gynecol. 2001;97(4):533–38. doi: 10.1016/s0029-7844(00)01223-0. [DOI] [PubMed] [Google Scholar]
- 7.von Dadelszen P, Magee LA, Lee SK, et al. Activated protein C in normal human pregnancy and pregnancies complicated by severe preeclampsia: A therapeutic opportunity? Crit Care Med. 2002;30(8):1883–92. doi: 10.1097/00003246-200208000-00035. [DOI] [PubMed] [Google Scholar]
- 8.Smith GC, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: A retrospective cohort study of 129,290 births. Lancet. 2001;357(9273):2002–6. doi: 10.1016/S0140-6736(00)05112-6. [DOI] [PubMed] [Google Scholar]
- 9.Ritchie ME, Phipson B, Wu D, et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu G, Wang LG, Han Y, He QY. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284–87. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chin CH, Chen SH, Wu HH, et al. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst Biol. 2014;8(Suppl 4):S11. doi: 10.1186/1752-0509-8-S4-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376(9741):631–44. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 13.American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy: Report of the American College of Obstetricians and Gynecologists’ task force on hypertension in pregnancy. Obstet Gynecol. 2013;122(5):1122–23. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 14.Blair JD, Yuen RK, Lim BK, et al. Widespread DNA hypomethylation at gene enhancer regions in placentas associated with early-onset pre-eclampsia. Mol Hum Reprod. 2013;19(10):697–708. doi: 10.1093/molehr/gat044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Junus K, Centlow M, Wikstrom AK, et al. Gene expression profiling of placentae from women with early- and late-onset pre-eclampsia: down-regulation of the angiogenesis-related genes ACVRL1 and EGFL7 in early-onset disease. Mol Hum Reprod. 2012;18(3):146–55. doi: 10.1093/molehr/gar067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang M, Niu J, Zhang L, et al. Gene expression profiling reveals different molecular patterns in G-protein coupled receptor signaling pathways between early- and late-onset preeclampsia. Placenta. 2016;40:52–59. doi: 10.1016/j.placenta.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 17.Varkonyi T, Nagy B, Fule T, et al. Microarray profiling reveals that placental transcriptomes of early-onset HELLP syndrome and preeclampsia are similar. Placenta. 2011;32(Suppl):S21–29. doi: 10.1016/j.placenta.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simoncini T, Genazzani AR, Liao JK. Nongenomic mechanisms of endothelial nitric oxide synthase activation by the selective estrogen receptor modulator raloxifene. Circulation. 2002;105(11):1368–73. doi: 10.1161/hc1102.105267. [DOI] [PubMed] [Google Scholar]
- 19.Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. Am J Physiol. 1997;272(2 Pt 2):R441–63. doi: 10.1152/ajpregu.1997.272.2.R441. [DOI] [PubMed] [Google Scholar]
- 20.Bieche I, Parfait B, Le Doussal V, et al. Identification of CGA as a novel estrogen receptor-responsive gene in breast cancer: An outstanding candidate marker to predict the response to endocrine therapy. Cancer Res. 2001;61(4):1652–58. [PubMed] [Google Scholar]
- 21.Yung HW, Atkinson D, Campion-Smith T, et al. Differential activation of placental unfolded protein response pathways implies heterogeneity in causation of early- and late-onset pre-eclampsia. J Pathol. 2014;234(2):262–76. doi: 10.1002/path.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myatt L, Cui X. Oxidative stress in the placenta. Histochem Cell Biol. 2004;122(4):369–82. doi: 10.1007/s00418-004-0677-x. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez TL, Sun T, Koeppel AF, et al. Sex differences in the late first trimester human placenta transcriptome. Biol Sex Differ. 2018;9(1):4. doi: 10.1186/s13293-018-0165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong S, Johnson MD, Dopierala J, et al. Genome-wide oxidative bisulfite sequencing identifies sex-specific methylation differences in the human placenta. Epigenetics. 2018;13(3):228–39. doi: 10.1080/15592294.2018.1429857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broere-Brown ZA, Schalekamp-Timmermans S, Hofman A, et al. Fetal sex dependency of maternal vascular adaptation to pregnancy: A prospective population-based cohort study. BJOG. 2016;123(7):1087–95. doi: 10.1111/1471-0528.13519. [DOI] [PubMed] [Google Scholar]
- 26.Kalisch-Smith JI, Simmons DG, Dickinson H, Moritz KM. Review: Sexual dimorphism in the formation, function and adaptation of the placenta. Placenta. 2017;54:10–16. doi: 10.1016/j.placenta.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Yeganegi M, Watson CS, Martins A, et al. Effect of Lactobacillus rhamnosus GR-1 supernatant and fetal sex on lipopolysaccharide-induced cytokine and prostaglandin-regulating enzymes in human placental trophoblast cells: Implications for treatment of bacterial vaginosis and prevention of preterm labor. Am J Obstet Gynecol. 2009;200(5):532.e1–8. doi: 10.1016/j.ajog.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 28.Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: Pathophysiology and clinical implications. BMJ. 2019;366:l2381. doi: 10.1136/bmj.l2381. [DOI] [PubMed] [Google Scholar]
- 29.Ben-Jonathan N, LaPensee CR, LaPensee EW. What can we learn from rodents about prolactin in humans? Endocr Rev. 2008;29(1):1–41. doi: 10.1210/er.2007-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clapp C, Gonzalez C, Macotela Y, et al. Vasoinhibins: A family of N-terminal prolactin fragments that inhibit angiogenesis and vascular function. Front Horm Res. 2006;35:64–73. doi: 10.1159/000094309. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez C, Parra A, Ramirez-Peredo J, et al. Elevated vasoinhibins may contribute to endothelial cell dysfunction and low birth weight in preeclampsia. Lab Invest. 2007;87(10):1009–17. doi: 10.1038/labinvest.3700662. [DOI] [PubMed] [Google Scholar]
- 32.Carey EA, Albers RE, Doliboa SR, et al. AMPK knockdown in placental trophoblast cells results in altered morphology and function. Stem Cells Dev. 2014;23(23):2921–30. doi: 10.1089/scd.2014.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koroglu N, Tola E, Temel Yuksel I, et al. Maternal serum AMP-activated protein kinase levels in mild and severe preeclampsia. J Matern Fetal Neonatal Med. 2019;32(16):2735–40. doi: 10.1080/14767058.2018.1448774. [DOI] [PubMed] [Google Scholar]
- 34.Lappas M, Lim R, Riley C, et al. Localisation and expression of FoxO1 proteins in human gestational tissues. Placenta. 2009;30(3):256–62. doi: 10.1016/j.placenta.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Sheridan R, Belludi C, Khoury J, et al. FOXO1 expression in villous trophoblast of preeclampsia and fetal growth restriction placentas. Histol Histopathol. 2015;30(2):213–22. doi: 10.14670/hh-30.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lam EW, Francis RE, Petkovic M. FOXO transcription factors: Key regulators of cell fate. Biochem Soc Trans. 2006;34(Pt 5):722–26. doi: 10.1042/BST0340722. [DOI] [PubMed] [Google Scholar]
- 37.Furukawa-Hibi Y, Kobayashi Y, Chen C, Motoyama N. FOXO transcription factors in cell-cycle regulation and the response to oxidative stress. Antioxid Redox Signal. 2005;7(5–6):752–60. doi: 10.1089/ars.2005.7.752. [DOI] [PubMed] [Google Scholar]
- 38.Huang SC, Hwang JL, Hsieh CY, et al. Human chorionic gonadotropin and its subunits in pre-eclampsia. Taiwan Yi Xue Hui Za Zhi. 1989;88(2):132–36. [PubMed] [Google Scholar]
- 39.Moodley D, Moodley J, Buck R, et al. Free alpha-subunits of human chorionic gonadotropin in preeclampsia. Int J Gynaecol Obstet. 1995;49(3):283–87. doi: 10.1016/0020-7292(95)02375-m. [DOI] [PubMed] [Google Scholar]
- 40.Lapaire O, Grill S, Lalevee S, et al. Microarray screening for novel preeclampsia biomarker candidates. Fetal Diagn Ther. 2012;31(3):147–53. doi: 10.1159/000337325. [DOI] [PubMed] [Google Scholar]
- 41.Kang JH, Song H, Yoon JA, et al. Preeclampsia leads to dysregulation of various signaling pathways in placenta. J Hypertens. 2011;29(5):928–36. doi: 10.1097/HJH.0b013e328344a82c. [DOI] [PubMed] [Google Scholar]
- 42.Baig M, Azhar A, Rehman R, et al. Relationship of serum leptin and reproductive hormones in unexplained infertile and fertile females. Cureus. 2019;11(12):e6524. doi: 10.7759/cureus.6524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rabiee M, Shams JA, Zafargandie N. The adverse effects of pregnancies complicated by hemoglobin H (HBH) disease. Iran J Pathol. 2015;10(4):318–21. [PMC free article] [PubMed] [Google Scholar]
- 44.Petrakos G, Andriopoulos P, Tsironi M. Pregnancy in women with thalassemia: Challenges and solutions. Int J Womens Health. 2016;8:441–51. doi: 10.2147/IJWH.S89308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang XH, Deng WB, Li M, et al. EGR1 protein acts downstream of estrogen-leukemia inhibitory factor (LIF)-STAT3 pathway and plays a role during implantation through targeting Wnt4. J Biol Chem. 2014;289(34):23534–45. doi: 10.1074/jbc.M114.588897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo B, Tian XC, Li DD, et al. Expression, regulation and function of EGR1 during implantation and decidualization in mice. Cell Cycle. 2014;13(16):2626–40. doi: 10.4161/15384101.2014.943581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doster Y, Cetinkaya Demir B, Atalay MA, et al. The possible role of serum leptin in preeclampsia. Clin Exp Obstet Gynecol. 2016;43(1):98–102. [PubMed] [Google Scholar]
- 48.Kalinderis M, Papanikolaou A, Kalinderi K, et al. Serum levels of leptin and IP-10 in preeclampsia compared to controls. Arch Gynecol Obstet. 2015;292(2):343–47. doi: 10.1007/s00404-015-3659-4. [DOI] [PubMed] [Google Scholar]
- 49.Salimi S, Farajian-Mashhadi F, Naghavi A, et al. Different profile of serum leptin between early onset and late onset preeclampsia. Disease Markers. 2014;2014 doi: 10.1155/2014/628476. 628476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Minokoshi Y, Kim YB, Peroni OD, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415(6869):339–43. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 51.Soleymanlou N, Jurisica I, Nevo O, et al. Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinol Metab. 2005;90(7):4299–308. doi: 10.1210/jc.2005-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitsunaga F, Umezawa M, Takeda K, Nakamura S. Maternal administration of nanomaterials elicits hemoglobin upregulation in the neonatal brain of non-human primates. J Toxicol Sci. 2016;41(2):265–71. doi: 10.2131/jts.41.265. [DOI] [PubMed] [Google Scholar]