Abstract
Objective
To evaluate the hypothesis that feeding volumes exceeding 100 mL/kg/day and exposure to cow’s milk formula pre-operatively increase the risk for pre-operative necrotizing enterocolitis (NEC) in infants with complex congenital heart disease.
Study Design
All infants, of any gestational age, with an isolated cardiac lesion at high risk for NEC (ductal- dependent lesions, transposition of the great arteries, truncus arteriosus, and aorto-pulmonary window) admitted to Texas Children’s Hospital from 2010–2016 were included. NEC was defined based on Modified Bell’s criteria. Feeding regimen information and relevant covariates were collected. Logistic regression was used to evaluate the association of feeding regimen and other potential risk factors with NEC.
Results
In this single center retrospective cohort of 546 infants, 3.3% developed Bell stage I-III NEC pre-operatively. An exclusive unfortified human milk diet was associated with a significantly lower risk of pre-operative NEC (OR 0.17, 95% CI 0.03–0.82, P=0.03) in a multivariable regression model controlling for cardiac lesion, race, feeding volume, birth weight small for gestational age, inotrope use pre-surgery/pre-NEC, and prematurity. Feeding volumes exceeding 100 mL/kg/day were associated with a significantly higher risk of pre-operative NEC (OR 3.05, 95% CI 1.19–7.90, P=0.02).
Conclusion
The findings suggest that an unfortified exclusive human milk diet may reduce the risk of pre-operative NEC in infants with complex congenital heart disease.
Keywords: Cohort Study, Ductal Dependent Cardiac Lesions
Necrotizing enterocolitis (NEC) is the most common gastrointestinal illness in infants. NEC is a complex illness with high morbidity and mortality.5–8 Most cases of NEC occur in premature, very-low and extremely-low birth weight infants; however, an estimated 10% of cases occur in full-term infants.1–3 Congenital heart disease (CHD) is a major risk factor for NEC and may account for as much as 33% of the cases seen in term infants.1,4 Depending on the study population, 1.5%−11% of infants with CHD develop NEC in the neonatal period.5–11 Among infants with CHD, it is theorized that those with ductal dependent cardiac lesions may have the highest risk of developing NEC. This is a result of diastolic steal from a patent ductus arteriosus leading to mucosal damage from decreased intestinal perfusion, predisposing the infant to NEC.12
Infants with ductal dependent cardiac lesions often have feeds held or severely limited during the pre-operative period due to concern for NEC,13 despite a lack of evidence for this practice. Although the incidence of pre-operative NEC is largely unknown, a systematic review of infants with CHD reported that, of those who developed NEC in a cohort of 6,683 patients, 48% of cases occurred before cardiac surgery14. Feeding infants during the pre-operative period may be beneficial by improving overall fluid status and stability in the post-operative period,18 and may improve overall growth and nutrition. Prolonged periods of fasting result in intestinal villus atrophy as well as derangement of the intestinal microbiota,13,15 which may increase the risk for NEC post-operatively and have a long-term impact on growth. As such, there is increasing recognition of the need to develop standardized feeding protocols that optimize nutrition and minimize the risk for NEC during the pre-operative period.16–19
In this retrospective cohort study of infants with complex CHD, we evaluated multiple potential feeding risk factors for NEC in the pre-operative period. We hypothesized that exposure to cow’s milk formula during the pre-operative period and feeding volumes exceeding 100 mL/kg/day would increase the risk for NEC in this population.
Methods
This was a retrospective cohort study of infants with complex CHD admitted to Texas Children’s Hospital from January 1, 2010-January 1, 2016. Infants of all gestational ages were enrolled into the study if they had one of the following cardiac physiologies: bi-ventricular lesion with ductal dependent pulmonary perfusion (BiV dd-PBF), bi-ventricular lesion with ductal dependent systemic perfusion (BiV dd-SBF), single ventricle lesion with ductal dependent pulmonary perfusion (SV dd-PBF), single ventricle lesion with ductal dependent systemic perfusion (SV dd-SBF), single ventricle without ductal dependent pulmonary or systemic perfusion (SV), d-transposition of the great arteries (d-TGA), truncus arteriosus, aorto-pulmonary window, severe Ebstein anomaly requiring prostaglandin, or Tetralogy of Fallot absent pulmonary valve.
Infants were excluded if they were admitted at >72 hours of age or had heterotaxy, omphalocele, gastroschisis, bowel atresia, Hirschsprung disease, imperforate anus, diaphragmatic hernia or hypoxic ischemic encephalopathy. Infants with cardiac anomalies that did not require intervention were excluded. There were no restrictions on the type of intervention needed, including transcatheter procedures. Infants were identified for possible inclusion in the study via pharmacological records or echocardiogram results. The hospital’s pharmacological database was used to identify patients admitted during the time frame of interest who received prostaglandin E1. Further, infants with the above congenital heart lesions were identified from the hospital’s echocardiogram database. These two lists were combined, duplications were removed, and each resulting medical record was reviewed for possible inclusion.
For the infants who met inclusion criteria, the data were retrospectively collected starting from admission until the first cardiac surgery, first episode of NEC, or discharge, whichever came first. The following data were collected for every enrolled infant: race; sex; gestational age; birth weight; cardiac lesion; days to first surgery; need for respiratory support, antibiotics, and inotropes; date feeds were initiated; type of feeds provided; largest volume of feeds (mL/kg/day) provided; and the feeding route that was used (oral vs nasogastric).
Cardiac lesions were categorized based on physiology. Each infant’s echocardiogram results were reviewed along with surgical records. Infants were defined to have single ventricle (SV) or bi-ventricle (BiV) disease and determined to have ductal dependent pulmonary or systemic perfusion based on the initial echocardiogram. Infants with d-transposition of the great arteries, truncus arteriosus, and aorto-pulmonary window were defined as separate categories as they have slightly different physiologies than those of the other groups.
Pre-operatively these infants were either admitted to the Cardiovascular Intensive Care Unit or the level 4 Neonatal Intensive Care Unit at Texas Children’s Hospital. At the time of this study, there were no institutional guidelines addressing the feeding of these infants. Feeds were started pre-operatively if an infant was deemed stable by the care team. Each provider, in conjunction with a multi-disciplinary team (consisting of cardiologists, neonatologists, and neonatal dietitians) could make decisions about the timing of feeding initiation, feeding type, feeding initiation volume, feeding advancement rate, and the feeding route. Typically, infants with complex CHD were fed pre-operatively in our hospital if they were hemodynamically stable and not requiring respiratory support, regardless of the use of a prostaglandin infusion. In term infants, feedings were usually initiated with mother’s own milk. If mother’s own milk was not available, unfortified formula or donor human milk was offered depending on family and physician preference. Feeds were started at a trophic volume of 20 mL/kg/day and advanced by 20 mL/kg/day with a total fluid restriction of 120–140 mL/kg/day, though there were no institutional guidelines at the time of this study. The maximum enteral feeding volume was also at the discretion of the provider. Feeds in term infants were not fortified until feeding volumes exceeded 100 mL/kg/day and the infant failed to achieve appropriate weight gain.
A case of NEC was defined based on Bell’s Modified Criteria (see Figure 1, available online).19 Each case of NEC was reviewed by two neonatologists and a radiologist to determine staging (Bell’s Ia-IIIb). Discrepancies were first addressed by mutual discussion with third party involvement if necessary. In the case of a perforation, surgical and pathological reports were used to differentiate between NEC and spontaneous perforation.
Figure 1.
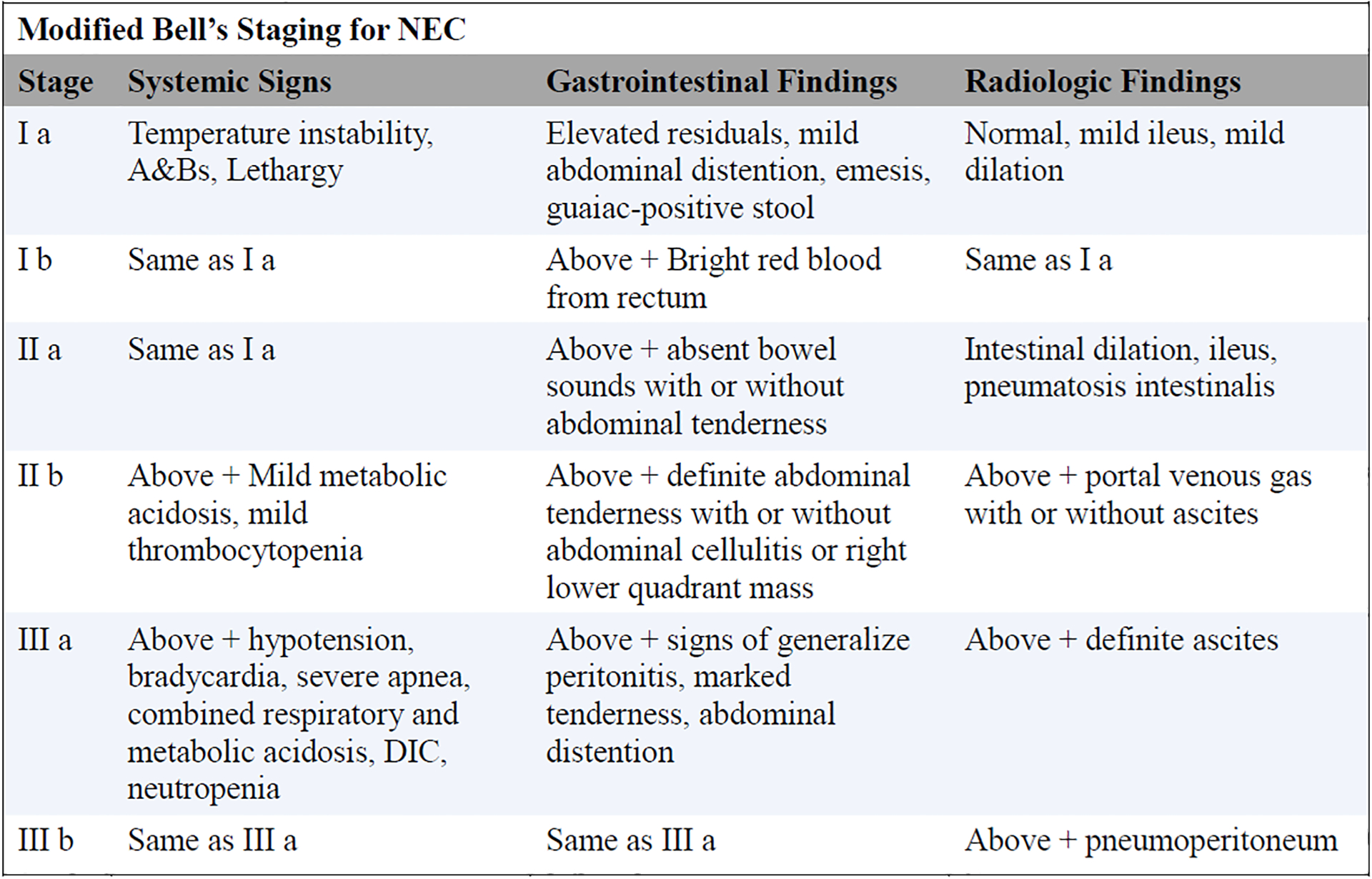
Clinical criteria for diagnosis of NEC by Modified Bell’s Staging.
We limited the cohort to the last 6 years as pasteurized donor human milk has only been available for use in infants of any gestational age at Texas Children’s Hospital since 2009. In the remainder of this paper, human milk diet (HMD) will refer to feedings of either mother’s own milk or donor human milk.
Statistical Analysis
Quantitative variables were summarized using the median and interquartile range separately for the pre-operative NEC versus no NEC groups. Maximum feeding volume and gestational age were compared for patients with and without NEC using the Wilcoxon rank sum test. Bivariate associations with NEC were examined by fitting logistic regression models separately for each hypothesized risk factor. A multivariable logistic regression model was built by including all risk factors found to be significantly associated with NEC (p=0.05) and those that were considered to be clinically relevant covariables. SAS version 9.4 (SAS Institute, Inc.; Cary, NC) was used for all data analysis.
Results
We identified 878 infants for possible inclusion in the study, and 546 patients were enrolled (Figure 2, available online). The demographics of the final cohort are summarized in Table 1. The cohort was predominately male, term, and Caucasian. Forty-four percent of the cohort had left-sided obstructive cardiac lesions. During their first hospitalization, 3.3% of patients developed NEC before their first cardiac surgery. Patients who developed NEC in the pre-operative period did so at a median age of 8.5 days, interquartile range (IQR) 5–11; the median age at surgery was 16 days (IQR 13–34). The median age at which feeds were started was 3 days (IQR 2–5). NEC developed a median of 5 days (IQR 3–10.5) after starting feeds. Two infants with NEC received packed red blood cell transfusions pre-operatively.
Figure 2.
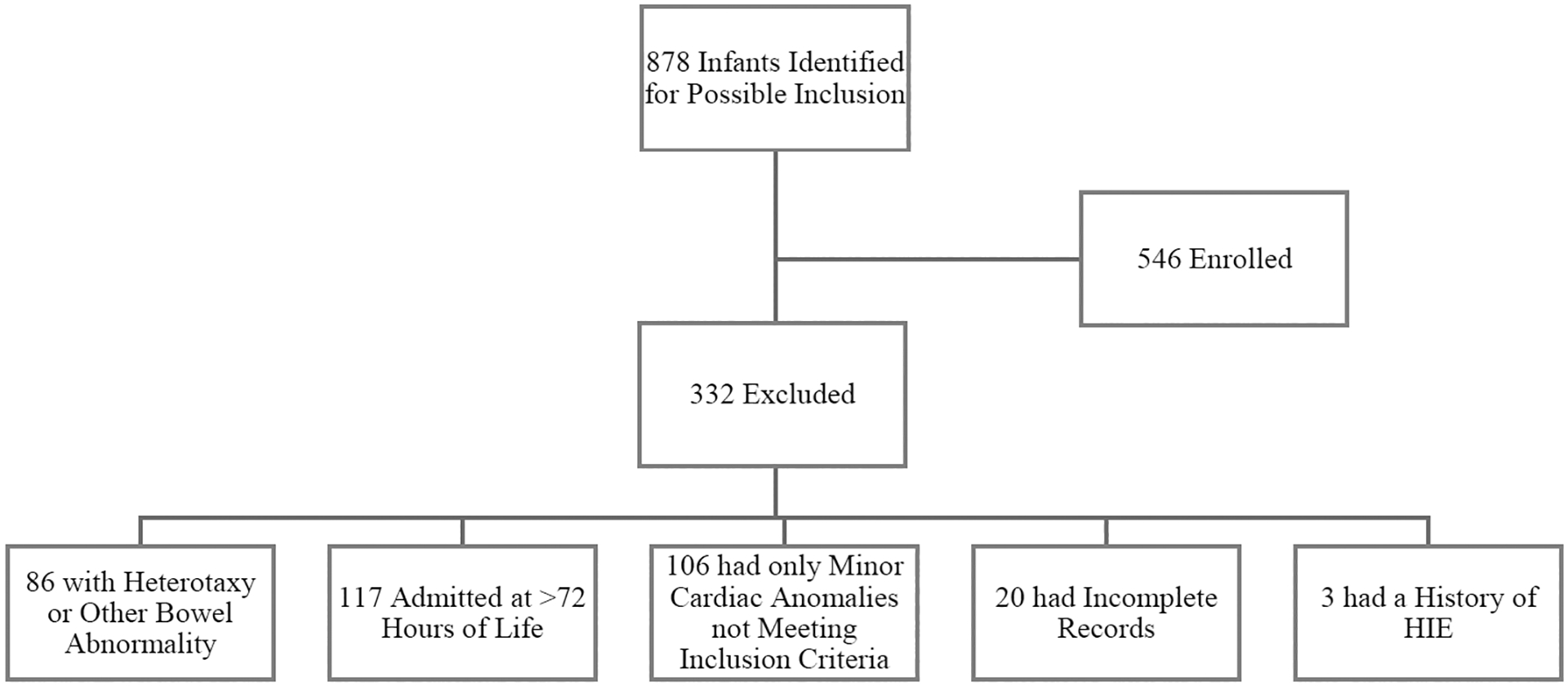
Flowchart of infants included in the study and reasons for exclusion.
Table 1.
Demographics
| Attribute | N=546 (%) |
|---|---|
| Sex | |
| Male | 319 (58.4) |
| Race | |
| African American | 67 (12.3) |
| Caucasian | 453 (83) |
| Asian | 16 (2.9) |
| Native American/Pacific Islander | 1 (0.2) |
| Unknown | 9 (1.6) |
| Ethnicity | |
| Hispanic | 197 (36.1) |
| APGAR Scores | |
| Median 1 Min. APGAR | 8 |
| Median 5 Min. APGAR | 9 |
| Gestational Age | |
| Gestational Age in Weeks: Mean(SD) | 38 (1.9) |
| % Born at <37 Weeks | 105 (19.2) |
| Birth Weight | |
| <1000g | 0 (0) |
| 1000–1500g | 7 (1.3) |
| 1501–2000g | 29 (5.3) |
| >2000g | 510 (93.4) |
| Growth Restriction1 | |
| <10% | 76 (13.9) |
| Cardiac Lesions | |
| SV w/ductal dep Sys. | 126 (23.0) |
| BV w/ductal dep Sys. | 119 (21.8) |
| D-TGA | 89 (16.3) |
| BV w/ductal dep Lung | 85 (15.6) |
| SV w/ductal dep Lung | 61 (11.2) |
| SV w/o ductal dep perf. | 31 (5.7) |
| Truncus | 28 (5.1) |
| Other2 | 7 (1.3) |
| Necrotizing Enterocolitis | |
| NEC Pre-Surgery | 18 (3.3) |
| NEC Stage | |
| Stage I | 8 (44.4) |
| Stage II | 6 (33.3) |
| Stage III | 4 (22.2) |
Based on the 2013 age specific Fenton Charts
A-P Window, Severe Ebstein’s requiring PGE, Absent Pulmonary Valve
Approximately two thirds of the cohort were fed prior to their first cardiac surgery or first episode of NEC (Table 2). An exclusive unfortified HMD was received by 54.5% of infants in the study and 25.5% attained a maximum feeding volume greater than 100 mL/kg/day. In bivariate analyses, an exclusive HMD and Caucasian race were associated with a lower risk for NEC, whereas birth at <37 weeks of gestation, feeding volumes >100 ml/kg/day and BiV dd-PBF cardiac lesions were associated with increased risk for NEC (Table 3).
Table 2.
Pre-Operative Feeding Exposures
| Risk Factor | N (%) |
|---|---|
| Any Feeds | 363 (66.5) |
| Receiving > 100 ml/kg/day | 139 (25.5) |
| Received feed by N1 | 139 (38.3) |
| Exclusive Unfortified BMD1 | 198 (54.5) |
| Received Fortification1 | 63 (17.4) |
| Received any Formula1 | 151 (41.6) |
| Feeds started with UAC in place1 | 184 (50.7) |
% of those patients receiving any feeds (N=363)
Table 3.
Bivariate Associations with NEC in the Pre-Operative Period
| Risk Factor | Odds Ratio | 95% CI | P-Value |
|---|---|---|---|
| Patient Gender Female | 0.56 | 0.22–1.43 | 0.22 |
| Caucasian | 0.36 | 0.13–0.99 | 0.049 |
| <37 weeks gestation | 2.79 | 1.06–7.39 | 0.04 |
| PGE dose > 0.0125 | 0.82 | 0.31–2.20 | 0.68 |
| Patient was Growth Restricted | 1.79 | 0.57–5.60 | 0.32 |
| Nasal CPAP | 1.48 | 0.32–6.95 | 0.62 |
| Mechanical Ventilation | 0.93 | 0.28–3.10 | 0.91 |
| Patient Received Inotropes | 1.14 | 0.37–3.52 | 0.83 |
| Patient Received Antibiotics | 2.40 | 0.87–6.4 | 0.09 |
| Patient had culture + bacteremia | 1.74 | 0.21–14.6 | 0.61 |
| Patient received any feeds | 2.59 | 0.74–9.05 | 0.14 |
| Feeds > 100 ml/kg/day | 3.05 | 1.19–7.90 | 0.02 |
| Received Feeds while on PGE | 1.46 | 0.57–3.73 | 0.43 |
| Received any Feeds via Ng | 1.89 | 0.67–5.34 | 0.23 |
| Exclusively unfortified Human Milk Diet | 0.12 | 0.03–0.54 | 0.006 |
| Formula Fed | 2.94 | 0.98–8.77 | 0.054 |
| Fortified Feeds | 2.49 | 0.82–7.56 | 0.11 |
| Feeds started while UAC in place | 0.64 | 0.22–1.84 | 0.41 |
| Cardiac Lesion | |||
| Patient with SV w/o ductal dep lung or sys perfusion | 0.98 | 0.13–7.59 | 0.98 |
| Patient with SV w/ductal dep pulmonary perfusion | 0.99 | 0.22–4.43 | 0.99 |
| Patient with SV w/ductal dep systemic perfusion | 0.41 | 0.09–1.80 | 0.24 |
| Patient with BV w/ductal dep pulmonary perfusion | 5.95 | 2.29–15.46 | 0.0003 |
| Patient with BV w/ductal dep systemic perfusion | 0.2 | 0.03–1.55 | 0.13 |
| Patient with dTGA | 0.29 | 0.04–2.24 | 0.24 |
| Truncus Arteriosus | 1.09 | 0.14–8.51 | 0.93 |
| Tetralogy of Fallot with absent pulmonary valve | 5.12 | 0.58–44.89 | 0.14 |
A larger maximum volume of feeds during the pre-operative period was associated with an increased risk for NEC (median 100 mL/kg/day (IQR 40–140) for the NEC group versus median 20 mL/kg/day (IQR 0–100) for the non-NEC group, P=0.04).
In the multivariable regression model (Table 4), an exclusive unfortified HMD was associated with a significantly lower risk for NEC after controlling for feeding volume, birth weight small for gestational age, race, inotropic support pre-operatively/pre-NEC, prematurity (gestational age <37 weeks), and cardiac lesion. Infants with cardiac lesions characterized as BiV dd-PBF had an increased risk of NEC. Compared with infants with other types of ductal dependent lesions, patients with BiV dd-PBF were younger (37.5 weeks vs 38.2 weeks, p-value 0.01), waited longer for surgery (19 days vs 9.2 days, p-value 0.008), reached larger maximum feeding volumes (103.2 mL/kg/day vs 45.6 ml/kg/day, p-value <0.0001), and were less likely to be maintained on an exclusive unfortified HMD (31.9% vs 60.1%, p-value <0.001).
Table 4.
Multivariate Regression Model
| Risk Factor | Odds Ratio | 95% CI | P-Value |
|---|---|---|---|
| Exclusively Unfortified BM Diet | 0.17 | 0.04–0.84 | 0.03 |
| Cardiac Lesion: BV w/ductal Dep L | 3.27 | 1.07–9.96 | 0.04 |
| Feeds >100ml/kg/day | 1.02 | 0.32–3.28 | 0.97 |
| SGA Infant | 1.20 | 0.30–4.73 | 0.80 |
| Premature Infant | 1.45 | 0.43–4.90 | 0.55 |
| Caucasian | 0.37 | 0.11–1.21 | 0.10 |
| Needed Inotropic Support Pre-Surgery/Pre-NEC | 1.05 | 0.26–4.26 | 0.95 |
Additional analyses were conducted in infants who developed stage II or stage III NEC during the pre-operative period. Bivariate associations are summarized in Table 5 (available online). No risk factor for stage II or III NEC remained statistically significant when included in a multivariable regression model that controlled for the covariates previously described.
Table 5.
Bivariate Associations with Stage II/III NEC in the Pre-Operative Period
| Risk Factor | Odds Ratio | 95% CI | P-Value |
|---|---|---|---|
| Caucasian | 0.28 | 0.08–0.99 | 0.05 |
| <37 weeks gestation | 6.62 | 1.80–23.91 | 0.004 |
| SGA | 1.55 | 0.32–7.43 | 0.59 |
| Feeds > 100 ml/kg/day | 4.53 | 1.26–16.31 | 0.02 |
| Exclusively unfortified Human Milk Diet1 | 0.23 | 0.05–1.12 | 0.07 |
| Received any fortified feeds2 | 3.99 | 1.04–15.3 | 0.04 |
| Patient with BV w/ductal dep pulmonary blood flow | 3.75 | 1.03–13.56 | 0.04 |
Unfortified Human Milk Vs Unfortified Formula, Fortified Human Milk, Fortified Formula
Unfortified Human Milk, Unfortified Formula Vs. Fortified Human Milk, Fortified Formula
Evaluating the risk for NEC during the entire first hospital admission, including both the pre-operative and post-operative periods, infants with SV dd-SBF lesion were at the highest risk (unadjusted OR 4.30, 95% CI 2.39–7.73, p-value <0.0001).
Discussion
Our study evaluated the association between an exclusive HMD and pre-operative NEC in a population of infants with complex CHD and found that an unfortified HMD was associated with a statistically significant reduction in the risk for pre-operative NEC within this population after controlling for multiple covariables. We also found, contrary to past studies,5–11 that infants with BiV dd-PBF physiology were at an increased risk of NEC in the pre-operative period compared with those with other types of ductal dependent lesions. It is unlikely that infants with BiV dd-PBF lesions are truly at increased risk for NEC compared with infants with other types of ductal dependent lesions, but rather, this association may reflect current practices at Texas Children’s Hospital. We are more willing to offer aggressive medical therapy for younger infants with this physiology. This creates a unique situation, increasing their risk for NEC in the pre-operative period, simply because they have a longer duration of exposure to risk factors during this period. Although statistical methodology may help control for confounders in a retrospective cohort study, these methods often do not completely control for all biases. We recognize this limitation and acknowledge that the results of this study may be biased by the large group of infants with BiV dd-PBF lesions.
We found that unfortified HMD was associated with a statistically significant reduction in the risk for pre-operative NEC Stage I-III. When infants with Bell’s Stage I NEC were excluded from the analysis, the association with human milk was no longer statistically significant. However, we chose to include infants with Stage I NEC for the primary outcome because, at our institution, any condition that warrants cessation of feeds and antibiotics could delay surgical repair and subsequently affect the clinical course of the infant. We note that two infants received packed red blood cell transfusions pre-operatively, though based on the timing of the transfusions, it does not appear that the transfusions were related to the development of NEC.
It is noteworthy that when we considered the risk for NEC during the entire first hospital admission, including both the pre-operative and post-operative periods, infants with SV dd-SBF lesion were at the highest risk. Although the primary purpose of our study was to evaluate pre-operative NEC, post-operative NEC in infants with congenital heart disease deserves further investigation. This is especially true for those at-risk infants with single ventricle physiology given the high overall mortality rate during the inter-stage periods. As a result, a number of studies have focused on post-operative gastrointestinal morbidity in this population.16, 20–21 Our results are consistent with this observation.
Though it has not been shown previously that an exclusive HMD is protective against NEC in infants with complex CHD, the use of exclusive human milk diets (mother’s own milk, and/or pasteurized donor human milk) has been extensively studied in premature infants, and there is mounting evidence to support a possible biological explanation for protective effects in infants with complex CHD. The consensus in premature infants is that an exclusive HMD reduces the risk of medical NEC by 60% and surgical NEC by 90%.2, 22–25 In the premature infant, there are a wide variety of initial insults that can result in mucosal damage in the intestines, including decreased blood flow secondary to a patent ductus arteriosus, medications, hypotension, inflammation due to milk components in the feeds themselves, or abnormal intestinal flora.26–31 Current theory proposes that the initial response of the immune system in the immature gut is dysregulated, and once activated, triggers a cascade of events resulting in NEC. This dysregulated immune response leads to mucosal breakdown and ultimately bowel wall necrosis. Human milk appears to play an important role in lessening the dysregulated response and may have a protective impact on the infant’s microbiota, though further studies of this are needed.29–31 Though outside the scope of this paper, extensive research on the role of specific immune factors in the development of NEC is ongoing with new receptors and modulators being discovered regularly.26–31
In term infants, the immune system is typically more developed, thereby decreasing the risk for NEC. However, in infants with congenital heart disease, ischemia from hypoperfusion may serve as the trigger for dysregulation of the immune system. Similar to the preterm population, the numerous immune protective factors in human milk may provide a protective mechanism against NEC in infants with congenital heart disease who are at risk for similar inflammatory insults.
The protective effects of human milk against NEC in this population appear to be multifactorial. There are many beneficial factors of human milk including immunoglobulin A, lactoferrin, growth factors, and cytokines. In addition, the use of human milk has been thought to impact the intestinal microbiota of both preterm and term infants32. As a particularly vulnerable population, infants with congenital heart disease are subject to a number of inflammatory processes that could cause an alteration of the microbiota. These include hypoxia, infection, hospitalization, and exposure to antibiotics. Further, the absence of enteral feeding can lead to intestinal mucosal atrophy causing a loss of important cell wall barriers. This can result in bacterial overgrowth and trigger an inflammatory response33, particularly in those infants who are already at risk for poor splanchnic perfusion. When these risk factors are combined, the risk of NEC may be compounded. We hypothesize that the unique components of human milk may dampen the inflammatory process and serve as a protective mechanism against NEC in this population. The association of lower volume feeds with a decreased risk of NEC also supports the concept of priming the gut with the protective factors of human milk. However, it is important to appreciate that the splanchnic perfusion is in a vulnerable state and could be burdened by high volume feeding.
This study was a retrospective cohort study at a single pediatric center and the associations described here may not be generalizable to all infants with similar lesions. The study was not powered to fully evaluate risk factors associated with stage II and stage III NEC in the pre-operative period. As the symptoms of stage I NEC are notoriously non-specific there is an increased risk for bias in this data set. However, every attempt was made to faithfully identify cases of NEC. We strictly adhered to Bell’s Modified criteria for stage I NEC and each case of NEC was reviewed by research staff and met specific pre-designated criteria for inclusion. The fact that the study period was defined by the use of donor breast milk at our center may introduce selection bias. Although our findings suggest that an unfortified, exclusive human milk diet may reduce the risk for pre-operative NEC in infants with complex congenital heart disease, this study should be primarily viewed as a hypothesis-generating study and further prospective randomized controlled studies are needed.
Funding Source:
Evie Whitlock Grant - Texas Children’s Hospital DRR is supported by the National Institutes of Health (1K23HLI130522)
Abbreviations
- NEC
Necrotizing Enterocolitis
- CHD
Congenital Heart Disease
- BiV
Bi-ventricular
- BiV dd-PBF
Bi-Ventricular Lesion with Ductal-Dependent Pulmonary Blood Flow
- BiV dd-SBF
Bi-Ventricular Lesion with Ductal-Dependent Systemic Blood Flow
- SV dd-PBF
Single Ventricle Lesion with Ductal-Dependent Pulmonary Blood Flow
- SV dd-SBF
Single Ventricle Lesion with Ductal-Dependent Systemic Blood Flow
- SV
Single Ventricle without Ductal-Dependent Pulmonary or Systemic Blood Flow
- HMD
Human Milk Diet
Footnotes
Conflict of Interest: No authors have any conflicts of interest to disclose.
References
- 1.Al Tawil K, Sumaily H, Ahmed IA, Sallam A, Al Zaben A, Al Namshan M, et al. Risk factors, characteristics and outcomes of necrotizing enterocolitis in late preterm and term infants. J Neonatal Perinatal Med 2013;6:125–130. [DOI] [PubMed] [Google Scholar]
- 2.Lambert DK, Christensen RD, Henry E, Besner GE, Baer VL, Wiedmeier SE, et al. Necrotizing enterocolitis in term neonates: data from a multihospital health-care system. Journal of Perinatology 2007;27:437–443. [DOI] [PubMed] [Google Scholar]
- 3.Ostlie DJ, Spilde TL, St Peter SD, Sexton N, Miller KA, Sharp RJ, et al. Necrotizing enterocolitis in full-term infants. Journal of Pediatric Surgery 2003;38:1039–1042. [DOI] [PubMed] [Google Scholar]
- 4.Motta C, Scott W, Mahony L, Koch J, Wyckoff M, Reisch J, et al. The association of congenital heart disease with necrotizing enterocolitis in preterm infants: a birth cohort study. J Perinatol 2015;35:949–953. [DOI] [PubMed] [Google Scholar]
- 5.Becker KCKC. Necrotizing enterocolitis in infants with ductal-dependent congenital heart disease. American journal of perinatology 2015;32:633–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luce WA, Schwartz RM, Beauseau W, Giannone PJ, Boettner BL, Cheatham JP, et al. Necrotizing enterocolitis in neonates undergoing the hybrid approach to complex congenital heart disease. Pediatr Crit Care Med 2011;12:46–51. [DOI] [PubMed] [Google Scholar]
- 7.McElhinney DB, Hedrick HL, Bush DM, Pereira GR, Stafford PW, Gaynor JW, et al. Necrotizing Enterocolitis in Neonates with Congenital Heart Disease: Risk Factors and Outcomes. Pediatrics 2000;106:1080–1087. [DOI] [PubMed] [Google Scholar]
- 8.Cheng W, Leung MP, Tam PKH. Surgical intervention in necrotzing enterocolitis in neonates with symptomatic congenital heart disease. Pediatr Surg Int 1999;15:492–495. [DOI] [PubMed] [Google Scholar]
- 9.Leung MP, Chau K, Hui P, Tam AYC, Chan FL, Lai C, et al. Necrotizing enterocolitis in neonates with symptomatic congenital heart disease. The Journal of Pediatrics 1988;113:1044–1046. [DOI] [PubMed] [Google Scholar]
- 10.Mukherjee D, Zhang YY, Chang DC, Vircella LA, Brenner JI, Abdullah F. Outcomes Analysis of Necrotizing Enterocolitis within 11 958 Neonates Undergoing Cardiac Surgical Procedures. Arch Surg 2010;145:389–392. [DOI] [PubMed] [Google Scholar]
- 11.Natarajan G, Anne SR, Aggarwal S. Outcomes of congenital heart disease in late preterm infants: double jeopardy? Acta Paediatr 2011;100:1104–1107. [DOI] [PubMed] [Google Scholar]
- 12.Carlo WF, Kimball TR, Michelfelder EC, Border WL. Persistent diastolic flow reversal in abdominal aortic Doppler-flow profiles is associated with an increased risk of necrotizing enterocolitis in term infants with congenital heart disease. Pediatrics 2007;119:330–335. [DOI] [PubMed] [Google Scholar]
- 13.Kasiraj AC, Harmoinen J, Isaiah A, Westermarck E, Steiner JM, Spillmann T, et al. The effects of feeding and withholding food on the canine small intestinal microbiota. FEMS Microbiol Ecol 2016;92. [DOI] [PubMed] [Google Scholar]
- 14.Siano E, Lauriti G, Ceccanti S, Zani A. Cardiogenic Necrotizing Enterocolitis: A Clinically Distinct Entity from Classical Necrotizing Enterocolitis. Eur J Pediatr Surg 2019;29(1):14–22. [DOI] [PubMed] [Google Scholar]
- 15.Remely M, Hippe B, Geretschlaeger I, Stegmayer S, Hoefinger I, Haslberger A. Increased gut microbiota diversity and abundance of Faecalibacterium prausnitzii and Akkermansia after fasting: a pilot study. Wien Klin Wochenschr 2015;127:394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.del Castillo SL, McCulley ME, Khemani RG, Jeffries HE, Thomas DW, Peregrine J, et al. Reducing the incidence of necrotizing enterocolitis in neonates with hypoplastic left heart syndrome with the introduction of an enteral feed protocol. Pediatr Crit Care Med 2010;11:373–377. [DOI] [PubMed] [Google Scholar]
- 17.Gephart SM, Hanson CK. Preventing necrotizing enterocolitis with standardized feeding protocols: not only possible, but imperative. Adv Neonatal Care 2013;13:48–54. [DOI] [PubMed] [Google Scholar]
- 18.Natarajan GG. Enteral feeding of neonates with congenital heart disease. Neonatology (Basel, Switzerland) 2010;98:330–336. [DOI] [PubMed] [Google Scholar]
- 19.Willis L, Thureen P, Kaufman J, Wymore E, Skillman H, da Cruz E. Enteral feeding in prostaglandin-dependent neonates: is it a safe practice? J Pediatr 2008;153:867–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toms R, Jackson KW, Dabal RJ, Reebals CH, Alten JA. Preoperative trophic feeds in neonates with hypoplastic left heart syndrome. Congenital Heart Disease, 2015. 10(1): 36–42. [DOI] [PubMed] [Google Scholar]
- 21.Slicker J, Sables-Baus S, Lambert LM, et al. Perioperative Feeding Approaches in Single Ventricle Infants: A Survey of 46 Centers. Congenital Heart Disease, 2016. 11(6): p. 707–715. [DOI] [PubMed] [Google Scholar]
- 22.Boyd CA, Quigley MA, Brocklehurst P. Donor breast milk versus infant formula for preterm infants: systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 2007;92:F169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hair AB, Peluso AM, Hawthorne KM, Perez J, Smith DP, Khan JY, et al. Beyond Necrotizing Enterocolitis Prevention: Improving Outcomes with an Exclusive Human Milk-Based Diet. Breastfeed Med 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGuire QM. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database of Systematic Reviews 2014(4). [DOI] [PubMed] [Google Scholar]
- 25.Sullivan S, Schanler RJ, Kim JH, Patel AL, Trawoger R, Kiechl-Kohlendorfer U, et al. An Exclusively Human Milk-Based Diet Is Associated with a Lower Rate of Necrotizing Enterocolitis than a Diet of Human Milk and Bovine Milk-Based Products. The Journal of Pediatrics 2010;156:562–567. [DOI] [PubMed] [Google Scholar]
- 26.Anderson JB, Beekman RH 3rd, Border WL, Kalkwarf HJ, Khoury PR, Uzark K, et al. Lower weight-for-age z score adversely affects hospital length of stay after the bidirectional Glenn procedure in 100 infants with a single ventricle. J Thorac Cardiovasc Surg 2009;138:397–404 e391. [DOI] [PubMed] [Google Scholar]
- 27.Anderson JB, Kalkwarf HJ, Kehl JE, Eghtesady P, Marino BS. Low weight-for-age z-score and infection risk after the Fontan procedure. Ann Thorac Surg 2011;91:1460–1466. [DOI] [PubMed] [Google Scholar]
- 28.Wallace MC, Jaggers J, Li JS, Jacobs ML, Jacobs JP, Benjamin DK, et al. Center variation in patient age and weight at Fontan operation and impact on postoperative outcomes. Ann Thorac Surg 2011;91:1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho S, Berger P, Nold-Petry C, Nold M. The immunological landscape in necrotising enterocolitis. Expert Reviews in Molecular Medicine 2016;18:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Good M, Sodhi C, Egan C, Afrazi A, Jia H, Yamaguchi Y, et al. Breast milk protects against the development of necrotizing enterocolitis through inhibition of Toll Like Receptor 4 in the intestinal epithelium via activation of the epiderma growth factor receptor. Mucosal Immunol. 2015;8:1166–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ellis C, Rutledge J, Underwood M. Intestinal microbiota and blue baby syndrome: Probiotic therapy for term neonates with cyanotic congenital heart disease. Gut Microbes. 2010;1: 359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Underwood MA. Human mik for the premature infant. Pediatr Clin North Am. 2013;1: 189–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. 2006;368: 1271–1283. [DOI] [PubMed] [Google Scholar]


