Abstract
AIMS:
Bradycardia contributes to tachy-brady arrhythmias or sinus arrest during heart failure (HF). Sinoatrial node (SAN) adenosine A1 receptors (ADO A1Rs) are upregulated in HF, and adenosine is known to exert negative chronotropic effects on the SAN. Here, we investigated the role of A1R signaling at physiologically relevant ADO concentrations on HF SAN pacemaker cells.
MAIN METHODS:
Dogs with tachypacing-induced chronic HF and normal controls (CTL) were studied. SAN tissue was collected for A1R and GIRK mRNA quantification. SAN cells were isolated for perforated patch clamp recordings and firing rate (bpm), slope of slow diastolic depolarization (SDD), and maximum diastolic potential (MDP) were measured. Action potentials (APs) and currents were recorded before and after addition of 1 and 10 μM ADO. To assess contributions of A1R and G protein-coupled Inward Rectifier Potassium Current (GIRK) to ADO effects, APs were measured after the addition of DPCPX (selective A1R antagonist) or TPQ (selective GIRK blocker).
KEY FINDINGS:
A1R and GIRK mRNA expression were significantly increased in HF. In addition, ADO induced greater rate slowing and membrane hyperpolarization in HF vs CTL (p<0.05). DPCPX prevented ADO-induced rate slowing in CTL and HF cells. The ADO-induced inward rectifying current, IKado, was observed significantly more frequently in HF than in CTL. TPQ prevented ADO-induced rate slowing in HF.
SIGNIFICANCE:
An increase in A1R and GIRK expression enhances IKAdo, causing hyperpolarization, and subsequent negative chronotropic effects in canine chronic HF at relevant [ADO]. GIRK blockade may be a useful strategy to mitigate bradycardia in HF.
Keywords: heart failure, adenosine, bradycardia, sinoatrial node, cellular electrophysiology
Introduction:
Heart failure (HF) results in over 300,000 deaths per year in the United States[1], with up to half of these deaths attributed to cardiac arrhythmia[2]. Patients with HF frequently have abnormalities of the pacemaker and conduction system[3], and sudden death in HF due to bradycardia accounts for approximately 40% of these deaths[4,5]. In addition, up to 50% of patients with sino-atrial node (SAN) dysfunction also experience tachy-brady syndrome with an increased risk of embolic stroke.[6] Unfortunately, pacemaker therapy has not been shown to improve survival in patients with bradycardia secondary to SAN dysfunction[7-9], and there remains an unmet need for identification of strategies to improve outcomes in patients with concomitant HF and SAN dysfunction.
Adenosine exerts its negative chronotropic actions through its effects on adenosine A1 receptors (A1Rs) in the SAN [10]; however there is a lack of consensus on the electrophysiological mechanism(s) that induce(s) rate slowing. A1Rs are G protein-coupled receptors (GPCRs), and upon activation activate G protein-coupled inward rectifying potassium channels (GIRK) by Gβγ subunits. The Gαi/o subunit, meanwhile, can inhibit adenylyl cyclase to reduce intracellular cAMP levels and PKA activity, putatively inhibiting funny current, If[11], and L-type Ca2+current, ICa,L[12], respectively. In rabbit SAN cells, the adenosine-induced G protein-coupled inward rectifying potassium (GIRK) current, IKAdo, has been suggested to be primarily responsible for hyperpolarization and rate slowing [13]. In the same study, If and ICa,L inhibition by adenosine was only observed when the currents were augmented with a β-agonist. However another study found that adenosine directly inhibits If [14]. The role of A1R signaling in SAN myocytes in HF has yet to be elucidated.
We have previously demonstrated that SAN A1R receptors are upregulated in a chronic model of canine HF [15,16]. Furthermore, dual-sided intramural optical mapping in this HF model demonstrated that pathophysiological concentrations of adenosine are associated with enhanced depression of SAN automaticity and conduction as well as facilitation of atrial fibrillation. The purpose of this study was to examine the role of A1R signaling in HF within pacemaker cells at both physiological and pathophysiological concentrations of adenosine. We hypothesized that HF SAN myocytes would demonstrate increased sensitivity to the negative chronotropic effects of adenosine due to upregulation of A1R receptors via enhanced GIRK-mediated IKAdo. To test this hypothesis we used the perforated patch clamp technique in isolated SAN myocytes from our canine HF model and measured action potentials and currents in the presence of adenosine. To assess A1R and GIRK contributions to adenosine effects, action potentials were also measured in the presence of 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), an A1-selective antagonist, or tertiapin-Q (TPQ), a selective GIRK antagonist.
Materials and Methods:
Heart failure canine model
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Ohio State University, conforming to the NIH Guide for the Care and Use of Laboratory animals. A total of 43 adult mixed breed dogs of either sex with normal cardiac function were used. Dogs were verified to have normal cardiac function by transthoracic two-dimensional and M-mode echocardiographic examinations during butorphanol tartrate (0.5 mg kg −1 intravenously) sedation. Dogs had an RV pacemaker lead implanted in the RV apex, and HF was induced (n=19, baseline weight 21.4±1.3 kg; mean±SEM) by tachypacing for four months as previously described[17] . Echocardiograms were measured at baseline and 4 months of pacing (with pacer temporarily turned off during the echocardiographic studies). Left atrial and left ventricular end-diastolic dimensions were significantly increased in HF dogs and left ventricular fractional shortening was reduced from 37.4±1.4% pre-pacing to 17.4±1.2% (p<0.01) post-tachypacing. An age matched group of 24 healthy dogs (weight, 21.7±1.2 kg) were used as controls (CTL) and studied in parallel.
Cell Isolation
On the day of the terminal procedure, dogs were sedated with butorphonal tartrate (0.2 to 0.4 mg/kg intramuscularly) then given 10 mg diazepam. Dogs were intubated and maintained on 3-5% isoflurane to maintain a deep plane of anesthesia. The heart was rapidly removed and perfused with cold cardioplegia solution containing the following in mM: NaCl 110, CaCl2 1.2, KCl 16, MgCl2 16 and NaHCO3 10. The right atrium and surrounding tissue were removed and placed in ice-cold Tyrode’s solution (mM) containing NaCl 130, KCl 5.4, MgCl2 3.5, NaH2PO4 0.5, Glucose 10, HEPES 5 and taurine 20 with pH adjusted to 7.4. SAN tissue was excised along the borders of the SAN artery, and cut into perpendicular strips approximately 2 mm x 2 mm. For cell isolation, chunks were placed in Tyrode’s solution with 0.2 mM Ca2+ and gently shaken at 37°C for 5 minutes. The supernatant was replaced by enzyme solution: Tyrode’s solution containing 0.2 mM Ca2+, 250 U/mL collagenase (Type II, Worthington), 0.96 U/mL protease XIV (Sigma), 2.25 U/mL elastase (Worthington). The tissue was enzymatically digested while gently shaking for 20 minutes at 37°C. Supernatant was removed and chunks were transferred to 5 mL of KB solution containing (mM): Glutamic Acid 100, HEPES 5, Glucose 20, KCl 25, K Aspartate 10, MgSO4 2, KH2PO4 10, Taurine 20, Creatine 5, EGTA 0.5, BSA 1mg/mL, with pH adjusted to 7.2 with KOH. Chunks were gently triturated with a glass pipette for 3 minutes, and supernatant was examined under phase-contrast microscopy for spindle-shaped SAN cells. Enzymatic digestion was continued in intervals of 10 minutes until the presence of cells was confirmed. Cells were stored at 4° C until use. All cellular electrophysiology experiments were conducted within 12 hours of isolation.
Real-time PCR for gene expression
Total RNA was extracted from SAN tissue with Trizol reagent (Invitrogen), and 2 μg RNA was then converted to cDNA by using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Gene expression was quantified by real-time RT-PCR (Light Cycler 96, Roche Applied Science) using SYBR green assay reagent and gene-specific primer listed below. Relative amplification was quantified by normalizing the gene-specific amplification to that of 18s rRNA in each sample. Changes in mRNA abundance were calculated using 2(−ΔΔC T) method [18,19]. qPCR reactions were run in triplicates. Primers used were as follows (5’-3’): KCNJ3 (forward: CGTCCCCTTTAATAGCACCA; reverse: GGCAAATCTCCCAAGCTGTA), KCNJ5 (forward: GCTGGATCAAGAGGAGTTCG; reverse: TGTTGGTCTCGTAGGTGTCG), ADORA1 (forward: GTGATCTGGGCAGTGAAGGT; reverse: GAGCTCTGGGTGAGGATGAG)
Electrophysiological recordings
To assess SAN cell electrophysiology, amphotericin-B perforated patch clamp techniques with a bath temperature of 36 ± 0.5°C were used. The myocytes were placed in a laminin-coated cell chamber (Cell Microcontrols, Norfolk, VA) and superfused with bath solution containing (in mM): 135 NaCl, 5 MgCl2, 5 KCl, 10 glucose, 1.8 CaCl2, and 5 HEPES with pH adjusted to 7.40 with NaOH. For current measurements the calcium in the bath solution was reduced to 1.0 mM with the addition of 2 μM nifedipine to avoid contamination with L-type Ca2+ current. Borosilicate glass micropipettes with tip resistances of 2-5.5 MΩ, were filled with pipette solution containing the following (in mM): 100 K-aspartate, 40 KCl, 5 MgCl, 5 EGTA, 5 HEPES, pH adjusted to 7.2 with KOH. Action potentials (APs) were characterized by spontaneous firing rate (bpm), action potential duration at 50 and 90% repolarization (APD50 and APD90), maximal diastolic potential (MDP), action potential over shoot, maximum upstroke velocity (dV/dtmax), and slope of slow diastolic depolarization rate (SDD, measured as the slope of the linear portion of the diastolic rise at 10% and 50% between MDP and 50% of depolarization). Only stable baseline recordings with positive overshoots and MDP equal to or more negative than −48 mV were used for analysis[20]. Action potential parameters from 10 consecutive APs were averaged using a custom written MATLAB program. Cell capacitance was measured and compensated for using the MultiClamp auto whole cell compensation. Cellular capacitance was 50.5 ± 2.8 pF in control cells (n=46) and 52.1 ± 2.8 pF in HF (n=30, p=NS vs controls). For current recordings, only recordings with an access resistance <20 MΩ were included in the analysis. IKado was elicited with voltage steps of 1.5 seconds ranging between −130 mV and +50 mV from a holding potential of −40 mV in the presence of adenosine and digital subtraction of the baseline current. Data was collected with a low noise data acquisition system Digidata 1440A (Molecular devices, Sunnyvale, CA), Clampex software and a Multiclamp 700B amplifier (Axon Instruments, Sunnyvale, CA).
Chemicals
The GIRK blocker, Tertiapin Q was purchased from Tocris (Ellisville, MI). DPCPX was purchased from Sigma-Aldrich (St Louis, MO). All buffers and solutions were prepared daily.
Statistical analysis
Cellular electrophysiology data were analyzed using Clampfit 10.3 software (Axon Instruments, Sunnyvale, CA), MATLAB (Mathworks, Natick, MA), GraphPad Prism (GraphPad, La Jolla CA) and Origin 9.0 software (OriginLab, Northampton, MA, USA). Two sample student’s t-test was used to compare mRNA expression between CTL and HF. For action potentials with adenosine, two-way repeated measured ANOVA was used to analyze differences between and within groups, with post hoc least significant difference testing. . If two-way ANOVA was found to have an interaction, a paired two sample Student’s t test was used to determine significance between the two different doses of either HF or CTL. Effects of A1R or GIRK antagonists with adenosine were compared vs. adenosine alone with one way ANOVA One sample Student’s t-test was used to determine significance of the change from baseline characteristics of HF and CTL after addition of adenosine, A1R antagonist, or GIRK antagonist. . Nonparametric comparisons were made using a contingency table and Fisher’s Exact Test. All data are presented as mean ± SE and p<0.05 was the a priori criterion for statistical significance for all comparisons.
Results
HF increases SAN tissue A1R and GIRK subunit mRNA expression
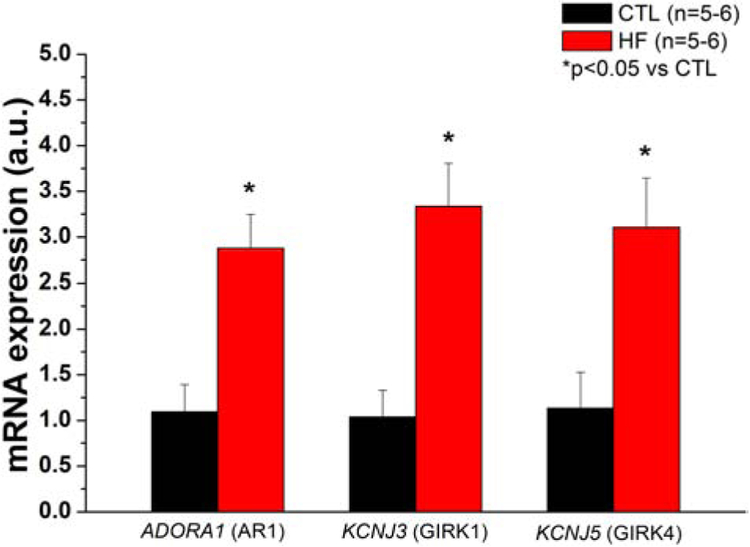
In the SAN, GIRK channels form heterotetrameric complexes between Kir3.4 (GIRK4) and Kir 3.1 (GIRK1)[21], although there is evidence that Kir3.4 can form homotetramers[22] To determine if major A1R-GIRK signaling pathway elements that activate IKAdo are enhanced in HF, mRNA expression levels of A1R, GIRK1, and GIRK4 were measured in SAN tissue from control and failing hearts. We found that A1R, GIRK1, and GIRK4 mRNA were all upregulated approximately threefold in HF compared to controls (Fig. 1).
Fig. 1.
A1R and GIRK subunit mRNA are increased in the SAN in HF
Expression of major IKado- dependent signaling elements in control and failing canine SAN tissue. mRNA levels were compared using a two sample Student’s t-test. SAN mRNA levels for each target was quantified by normalizing the gene-specific amplification to that of 18s rRNA in each sample. N= 5-6 per h group.
HF increases SAN cell sensitivity to negative chronotropic effects of adenosine
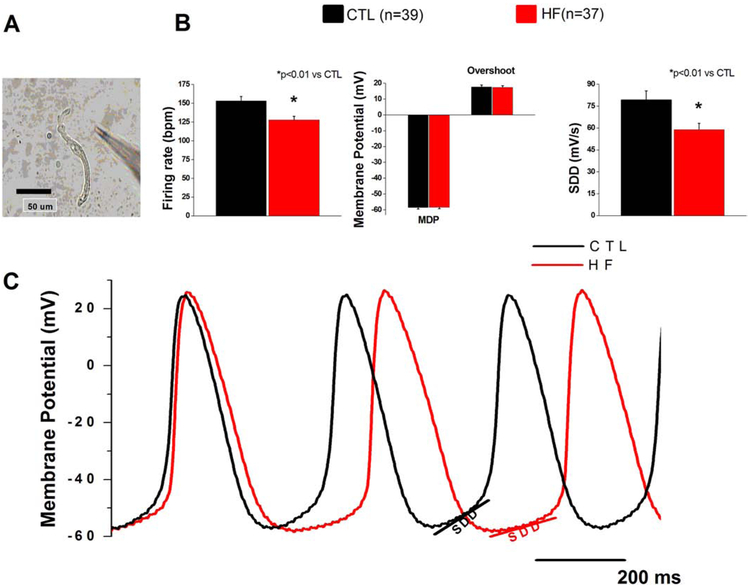
To determine if altered A1R expression modulates the response of HF SAN cells to adenosine, we measured spontaneous activity of isolated HF and control SAN cells at baseline and in response to 1 and 10 μM adenosine (Fig. 2 and 3). HF SAN cells showed a slower intrinsic firing rate and reduction of SDD compared to control cells (Fig. 2b,c). Notably, with the exception of significant reductions in SDD and intrinsic firing rate, all other action potential characteristics were unchanged between HF and controls (Fig. 2b, Supplemental Fig. 1a,b).
Fig. 2.
Intrinsic firing is decreased in SAN HF cells
(a) Representative SAN cell (b) Action potential parameters of CTL and HF SAN cells. (c) Representative CTL and HF SAN action potentials. SAN cells were isolated from 14 dogs in each group.
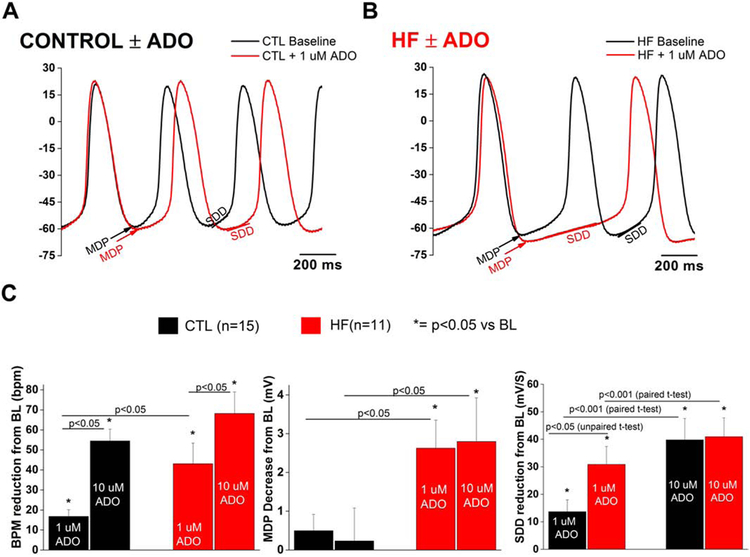
Fig. 3.
SAN HF cells demonstrate increased sensitivity to negative chronotropic effects of adenosine
Representative SAN cell action potential with 1 μM ADO in (a) CTL and (b) HF (c) Change in action potential parameters from baseline (BL) in CTL and HF SAN cells. Action potentials parameters for CTL and HF grouped were compared via two-way repeated measures ANOVA. SDD was determined to have a significant interaction, and comparisons for this parameter were performed via paired t-test. SAN cells were isolated from 9 CTL dogs and 6 HF dogs.
Adenosine reversibly slowed the intrinsic firing rate in control and HF cells at both tested concentrations (Fig 3, Supplemental Fig. 2a). Compared to controls, HF cells demonstrated an increased negative chronotropic response to 1 μM adenosine, with increased hyperpolarization and greater reduction of SDD (Fig. 3a-c). For this set of experiments, only cells exposed to both concentrations of adenosine were included in the analysis. Absolute values of firing rate at baseline were 143.5±11.1 bpm in CTL and 140.3±9.1 in HF (p>0.05, NS). Absolute MDP values at baseline were −58.0±1.8 mV in CTL and −58.6±1.6 mV in HF (p>0.05, NS). Compared to baseline, the spontaneous rate of control SAN cells were reduced by 16.8±3.4 bpm with 1 μM adenosine compared to 43.5±10.1 bpm in HF cells (p<0.05 vs CTL, Fig. 2c). In HF, this rate slowing was associated with similar increases in MDP hyperpolarization as well as SDD reduction relative to controls (Fig. 2c). Exposure to 10 μM adenosine caused further rate slowing in both HF and controls, although the response was similar in both groups. Furthermore, there was no further MDP hyperpolarization compared to 1 μM in either group. SDD reduction between groups and adenosine concentration showed statistically significant (p<0.05) between factor-interactions. Thus, within each group there was a significant adenosine dose effect, and at 1 μM the SDD reduction is greater in HF than control – indicating greater sensitivity (Supplemental Fig. 2b). However, there was no difference in SDD reduction between groups at the higher concentration, likely due to a saturating effect at the 10 μM dose. Absolute values of overshoot were decreased after 10 μM adenosine exposure in CTL (15.4±2.5 mV vs 11.7±2.3 mV, p<0.05) and in HF (15.6±2.0 mV vs 13.4±2.0 mV, p=0.22, NS).
Negative chronotropic effects of adenosine on SAN cells in both control and HF are mediated by A1R
To evaluate the role of A1Rs in the negative chronotropic effects of adenosine, we treated SAN cells with DPCPX (an A1R-selective antagonist) prior to exposing cells to 1 and 10 μM adenosine. We compared action potential parameters obtained with exposure to adenosine alone to the cells pretreated with DPCPX. In CTL, pretreatment with DPCPX had no significant effect on absolute values for firing rate (157.1±10.2 bpm vs pretreated 156.0±11.3 bpm), MDP (−60.5±2.3 mV vs pretreated −60.4±2.7 mV), or SDD (72.1±10.1 mV/s vs pretreated 70.3±11.1 mV/s). Similarly, in HF, absolute values for firing rate (113.8±12.9 bpm vs pretreated 115.9±13.0 bpm), MDP (−58.2±1.4 mV vs pretreated −58.7±1.7 mV), and SDD (46.4±8.4 mV/s vs pretreated 48.4±8.8 mV/s) were not significantly altered by DPCPX. A1R antagonism prevented adenosine-induced rate slowing and action potential properties in both controls and HF (Fig. 4a-d). Pretreatment with DPCPX attenuated 10 μM adenosine-induced overshoot reduction in both CTL (17.1±2.2 vs 16.5±3.7, p=0.65) and HF (16.5±4.8 vs 14.5±4.7, p=0.71) cells pretreated with DPCPX, suggesting that A1Rs mediate this effect as well.
Fig. 4.
Adenosine-induced negative chronotropic effects on SAN cells in both CTL and HF are mediated by A1R
(a) Representative SAN action potential in CTL (top) and HF (bottom) exposed to ADO in the presence of A1R antagonist, DPCPX (b) Firing rate reduction, (c) SDD reduction, and (d) MDP reduction from BL compared in presence of ADO+ DPCPX compared to ADO alone. Action potential parameters from Fig. 2 were compared with their respective group (HF or control) + DPCPX via one-way ANOVA. SAN cells were isolated from 4 dogs in each group.
IKAdo is activated predominately in SAN HF cells
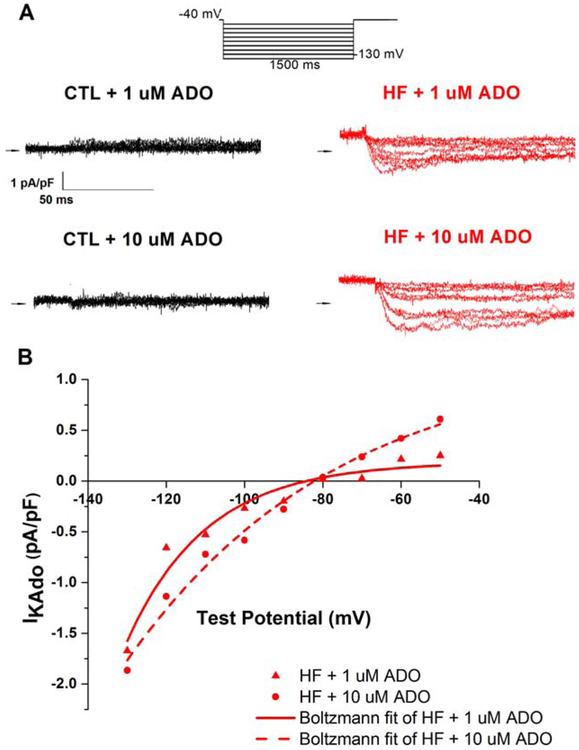
To evaluate whether HF was associated with increased GIRK-mediated IKado, we assayed IKado in SAN cells via a patch clamp step protocol (Fig. 5a). IKAdo was evident as an adenosine-induced, desensitizing, inwardly rectifying current characteristic of GIRK activation [23] measured by digital subtraction of the untreated baseline from each cell (Fig. 5a,b) IKAdo was present in one of eight CTL and six of eight HF SAN cells (p<0.05, contingency table with Fisher’s exact test). After addition of 1 μM adenosine, this current peaked at 63.3±3.0 ms, well before the APD50for these cells (Supplemental Fig. 1a). Importantly, in HF, an outward current was generated at voltages positive to −80 mV at both 1 μM and 10 μM adenosine (Fig. 5 b).
Fig. 5.
Adenosine generates GIRK-mediated IKAdo in HF SAN cells
(a) Representative raw current traces of IKAdo in CTL (left) and HF (right) SAN cells activated by 1 μM and 10 μM ADO. Current was elicited using a 1500 ms step protocol (above) and measured after digitally subtracting the paired untreated baseline for each cell (b) I-V relationship of IKado in HF activated by 1 μM and 10 μM ADO. SAN cells were isolated from 5 CTL dogs and 4 HF dogs.
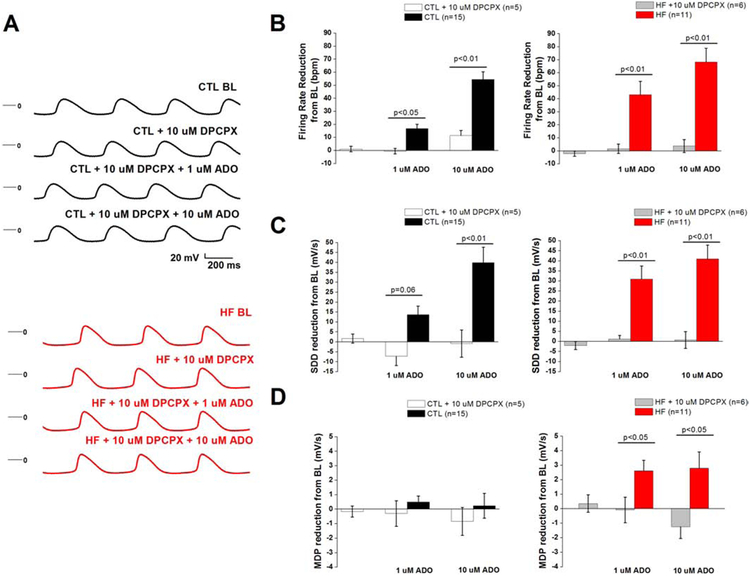
GIRK inhibition by Tertiapin Q: increased in HF SAN cells
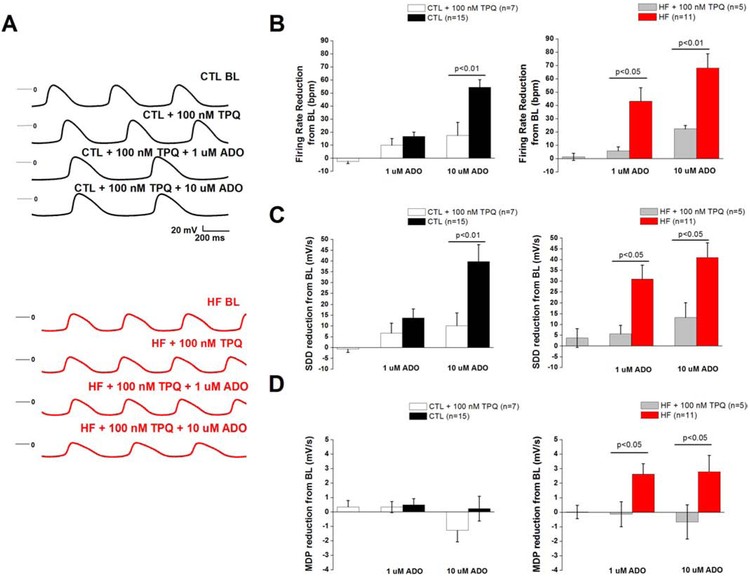
To test the hypothesis that altered responsiveness of GIRK-mediated IKAdo plays an important role in adenosine-induced negative chronotropy in HF, we measured SAN action potentials in the presence of TPQ and adenosine (Fig. 6a-d). We compared action potential parameters obtained from exposure to adenosine alone to the cells pretreated with TPQ. In CTL, pretreatment with TPQ had no significant effect on absolute values for firing rate (132.0±8.8 bpm vs pretreated 134.3±8.5 bpm), MDP (−58.9±1.6 mV vs pretreated −59.2±1.6 mV), or SDD (55.1±8.7 mV/s vs pretreated 55.8±9.6 mV/s). Similarly, in HF, absolute values for firing rate (139.4±7.7 bpm vs pretreated 138.0±8.4 bpm), MDP (−59.7±3.5 mV vs pretreated −59.7±2.5 mV), and SDD (62.3±9.7 mV/s vs pretreated 58.5±7.9 mV/s) were not significantly altered by DPCPX. TPQ abolished negative chronotropy when 1 μM adenosine was applied to the cells (4.3±2.0% HF + TPQ (n=5) vs. 30.7±6.7% HF (n=11), p<0.05), and antagonized rate slowing induced by 10 μM adenosine (16.6±2.8% HF + TPQ (n=5) vs. 48.9±6.4% HF (n=11), p<0.05). In control cells, TPQ only prevented rate slowing at 10 μM (12.2±6.5% CTL+TPQ (n=7) vs 38.9±4.6% (n=15), p<0.05). In both groups, TPQ prevented rate reduction by preventing hyperpolarization and SDD reduction.
Fig. 6.
GIRK blockade prevents adenosine-induced rate slowing
(a) Representative action SAN potential in CTL (top) and HF (bottom) exposed to ADO in the presence of GIRK antagonist TPQ (b) Firing rate reduction, (c) SDD reduction, and (d) MDP reduction from BL compared in presence of ADO+TPQ compared to ADO alone. Action potential parameters from Fig. 2 were compared with their respective group (HF or control) + TPQ via one-way ANOVA. SAN cells were isolated from 4 CTL dogs and 2 HF dogs.
Discussion
While upregulation of SAN A1Rs in HF has been implicated in the induction of bradyarrhythmia[15], the effects of adenosine on altered A1R signaling in pacemaker cells from failing hearts have remained poorly elucidated. To our knowledge, this is the first study to report action potentials of individual SAN cells from a large animal model of HF. In our canine chronic model of HF, physiological levels of adenosine produced an exaggerated rate slowing response and hyperpolarization of SAN cells, relative to controls, through activation of IKAdo. Through pharmacological blockade of GIRK channels, we were able to abolish rate slowing at physiological concentrations of adenosine in the canine chronic HF model. Thus, the primary effect of adenosine on SAN cells in HF is GIRK-mediated rate slowing via hyperpolarization resulting from activation of IKAdo.
HF increases SAN pacemaker cell sensitivity to the negative chronotropic effects of adenosine. The normal physiological level of adenosine in the pericardial fluid is approximately 1 μM[24,25] and was used as one of the tested concentrations in this study. To test effects of dose-dependence, as well as mimic the reported increased extracellular adenosine (in some patients up to seven-fold[26]) we used 10 μM adenosine, to bracket the expected range of in vivo concentrations. In addition to A1R upregulation, GIRK4 protein has been reported to be upregulated in the SAN our HF model[15]. An increase in both A1R and GIRK subunit expression in HF would be expected to amplify downstream IKAdo and its rate-slowing hyperpolarization effects on the SAN action potential. Consistent with this notion, there was a clear, exaggerated rate slowing response to 1 μM adenosine in HF, with concurrent membrane hyperpolarization that was absent in controls. Furthermore, IKAdo was almost exclusively present in HF cells. The outward current generated at 1 μM and 10 μM adenosine were at voltages positive to the MDPs for SAN cells observed in this study, providing a mechanistic rationale for the observed hyperpolarization.
In CTL cells exposed to 1 μM adenosine, there was a small but significant reduction in firing rate and SDD that occurred in the absence of observed hyperpolarization and IKAdo. One explanation may be that adenosine inhibits If, as demonstrated in the reduction of firing rate in healthy rabbit sinoatrial node cells by similar concentrations of adenosine [14]. In the absence of membrane hyperpolarization, the slope of diastolic depolarization was reduced in these cells, where If is active. We showed a similar reduction of SDD in CTL cells exposed to adenosine in our study. However, pharmacological reduction of SAN intrinsic rate by direct If inhibition is modest. Microelectrode recordings of canine sinoatrial node preparations showed a 15 bpm firing rate reduction after superfusion with 3 μM ivabradine, a specific inhibitor of If [27].
Although there was an exaggerated firing rate response to 1 μM adenosine in HF versus CTL, the response was only incrementally increased when increased from1 μM to 10 uM. This pattern was also observed in MDP and SDD action potential parameters, as well as IKAdo. Furthermore, a similar firing rate reduction response was observed between CTL and HF at 10 μM adenosine, despite the paucity of IKAdo or demonstrated hyperpolarization in CTL cells in the study. These results suggest higher concentrations of may promote chronotropy through inhibition of other adenosine targets, such as ICa,L or If. Although we did not directly measure ICa,L in this study, ICa,L is the primary determinant of the upstroke of the SAN action potential [11]. In a study of human atrial myocytes, 10 μM adenosine inhibited approximately 30% of basal ICa,L [28]. At 10 μM adenosine, in this study, overshoots were decreased in both CTL and HF SAN cells, but not at 1 μM. Calcium channel antagonism, such as with nifedipine, has been demonstrated to slow firing rate and reduce the overshoot in pacemaker cells [29]. Even in HF, at the higher doses of adenosine used in this study, approximately 30% of total rate reduction appears to be independent of IKAdo It is therefore possible that at higher doses of adenosine (10 μM), a reduction of ICa,L contributes to negative chronotropic effects.
In clinical trials, nonselective antagonism of the AR receptors with theophylline has been demonstrated to prevent deterioration to HF in patients with sick sinus syndrome[30]. In addition, antagonism of A1Rs prevented adenosine-induced SAN dysfunction and subsequent development of atrial fibrillation in right atrial preparations from chronic HF dogs [15]. Unfortunately, the expression and critical role of A1Rs in the central nervous system[31], as well as its cardioprotective effects [32]makes global A1R inhibition undesirable. In the present study, we report that blockage of GIRK channels may be an alternative strategy to prevent adenosine-induced SAN dysfunction and/or arrhythmia. Recently, a gain of function mutation of human GIRK4 has been identified to cause familial sinus node dysfunction including bradycardia[33]. Because GIRK channels are also present in a variety of tissues [34], targeted local modulation of these channels may not only prevent bradycardia in HF, but may have other effects to modulate HF.
Limitations:
The spontaneous firing rate for CTL SAN cells in this study is comparable to the intrinsic firing rate of normal dogs of similar weight and age measured in vivo [35] and in coronary perfused sinoatrial preparations[36]. Our HF SAN cells had increased firing rates compared to the HF sinoatrial preparations [37] of dogs of similar weight and age; however both were significantly slower than their respective controls, indicating sinus node impairment [38]. Differences between the two studies may represent individual differences in severity of HF-induced sinus node impairment or the effects of uncoupled isolated cells vs. an intact preparation.
We did not directly measure ICA,L (Cav1.2) or If but used action potential morphology changes to describe their changes. In the case of SDD, it is possible that other elements of diastolic depolarization, such as ICa,L (Cav 1.3)[39] or T-type calcium current (ICa,T)[40] may have been modulated by adenosine as well. Furthermore, there are several elements of cardiac pacemaking that were not investigated in this study such as modulation by Ca2+/calmodulin-dependent protein kinase II [41] or alterations in the calcium clock [42]. However, it is evident from this study that blockage of the GIRK channel prevents rate slowing in heart failure, exclusively implicating the A1R-GIRK signaling pathway and IKAdo. Our current findings of upregulated mRNA levels for GIRK4 and AR1 are consistent with our previous report on protein expression in the SAN in HF. However, high levels of GIRK1 did not result in corresponding translation to protein, and the mechanism is not well-understood. This finding could reflect the complexity of the system which can be affected by post-transcriptional, translational, and protein degradation regulation [43] More studies would be needed to determine the effect of HF on GIRK1 protein expression. Moreover, we did not address the effects of chronic modulation of adenosine signaling. Studies are also needed to determine if any other elements of this signaling pathway (e.g. RGS proteins [44]) are altered in HF. Finally, given the exaggerated effects on rate slowing by 1 μM adenosine in HF, it is possible that lower concentrations of adenosine may also have a significant chronotropic effect in HF.
Conclusion
Adenosine-induced hyperpolarization and SAN rate slowing are greater in HF than in CTLs, and can be prevented by GIRK inhibition. Thus, GIRK blockade may be a useful strategy to mitigate bradycardia in HF.
Supplementary Material
Acknowledgements:
The authors thank Jeanne Green, RVT for technical assistance. Pacing devices and leads provided as a gift of St. Jude Medical.
Grants:
This work was supported by the National Institutes of Health [HL115580 to CAC, VVF; HL074045 to SG; HL084583, HL083422 to PJM; HL114893 to TJH]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de FS, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB (2015) Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation [DOI] [PubMed] [Google Scholar]
- 2.Stevenson WG, Sweeney MO (1997) Arrhythmias and sudden death in heart failure. Jpn Circ J 61:727–740 [DOI] [PubMed] [Google Scholar]
- 3.Sanders P, Kistler PM, Morton JB, Spence SJ, Kalman JM (2004) Remodeling of sinus node function in patients with congestive heart failure: reduction in sinus node reserve. Circulation 110:897–903 [DOI] [PubMed] [Google Scholar]
- 4.Luu M, Stevenson WG, Stevenson LW, Baron K, Walden J (1989) Diverse mechanisms of unexpected cardiac arrest in advanced heart failure. Circulation 80:1675–1680 [DOI] [PubMed] [Google Scholar]
- 5.Faggiano P, d'Aloia A, Gualeni A, Gardini A, Giordano A (2001) Mechanisms and immediate outcome of in-hospital cardiac arrest in patients with advanced heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 87:655–1 [DOI] [PubMed] [Google Scholar]
- 6.Semelka M, Gera J, Usman S (2013) Sick sinus syndrome: a review. Am Fam Physician 87:691–696 [PubMed] [Google Scholar]
- 7.Simon AB, Janz N (1982) Symptomatic bradyarrhythmias in the adult: natural history following ventricular pacemaker implantation. Pacing Clin Electrophysiol 5:372–383 [DOI] [PubMed] [Google Scholar]
- 8.Lamas GA, Lee KL, Sweeney MO, Silverman R, Leon A, Yee R, Marinchak RA, Flaker G, Schron E, Orav EJ, Hellkamp AS, Greer S, McAnulty J, Ellenbogen K, Ehlert F, Freedman RA, Estes NA III, Greenspon A, Goldman L (2002) Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N Engl J Med 346:1854–1862 [DOI] [PubMed] [Google Scholar]
- 9.Brandt NH, Kirkfeldt RE, Nielsen JC, Mortensen LS, Jensen GVH, Johansen JB, Haugan K (2017) Single lead atrial vs. dual chamber pacing in sick sinus syndrome: extended register-based follow-up in the DANPACE trial. Europace 19:1981–1987 [DOI] [PubMed] [Google Scholar]
- 10.Koeppen M, Eckle T, Eltzschig HK (2009) Selective deletion of the A1 adenosine receptor abolishes heart-rate slowing effects of intravascular adenosine in vivo. PLoS One 4:e6784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiFrancesco D (1993) Pacemaker mechanisms in cardiac tissue. Annu Rev Physiol 55:455–472 [DOI] [PubMed] [Google Scholar]
- 12.Mery PF, Abi-Gerges N, Vandecasteele G, Jurevicius J, Eschenhagen T, Fischmeister R (1997) Muscarinic regulation of the L-type calcium current in isolated cardiac myocytes. Life Sci 60:1113–1120 [DOI] [PubMed] [Google Scholar]
- 13.Belardinelli L, Giles WR, West A (1988) Ionic mechanisms of adenosine actions in pacemaker cells from rabbit heart. J Physiol 405:615–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaza A, Rocchetti M, DiFrancesco D (1996) Modulation of the hyperpolarization-activated current (I(f)) by adenosine in rabbit sinoatrial myocytes. Circulation 94:734–741 [DOI] [PubMed] [Google Scholar]
- 15.Lou Q, Hansen BJ, Fedorenko O, Csepe TA, Kalyanasundaram A, Li N, Hage LT, Glukhov AV, Billman GE, Weiss R, Mohler PJ, Gyorke S, Biesiadecki BJ, Carnes CA, Fedorov VV (2014) Upregulation of adenosine A1 receptors facilitates sinoatrial node dysfunction in chronic canine heart failure by exacerbating nodal conduction abnormalities revealed by novel dual-sided intramural optical mapping. Circulation 130:315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lou Q, Glukhov AV, Hansen B, Hage L, Vargas-Pinto P, Billman GE, Carnes CA, Fedorov VV (2013) Tachy-brady arrhythmias: the critical role of adenosine-induced sinoatrial conduction block in post-tachycardia pauses. Heart Rhythm 10:110–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sridhar A, Nishijima Y, Terentyev D, Khan M, Terentyeva R, Hamlin RL, Nakayama T, Gyorke S, Cardounel AJ, Carnes CA (2009) Chronic heart failure and the substrate for atrial fibrillation. Cardiovasc Res 84:227–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 19.Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108 [DOI] [PubMed] [Google Scholar]
- 20.Woods WT, Urthaler F, James TN (1976) Spontaneous action potentials of cells in the canine sinus node. Circ Res 39:76–82 [DOI] [PubMed] [Google Scholar]
- 21.Krapivinsky G, Gordon EA, Wickman K, Velimirovic B, Krapivinsky L, Clapham DE (1995) The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K(+)-channel proteins. Nature 374:135–141 [DOI] [PubMed] [Google Scholar]
- 22.Bender K, Wellner-Kienitz MC, Inanobe A, Meyer T, Kurachi Y, Pott L (2001) Overexpression of monomeric and multimeric GIRK4 subunits in rat atrial myocytes removes fast desensitization and reduces inward rectification of muscarinic K(+) current (I(K(ACh))). Evidence for functional homomeric GIRK4 channels. J Biol Chem 276:28873–28880 [DOI] [PubMed] [Google Scholar]
- 23.Shi H, Wang H, Wang Z (1999) Identification and characterization of multiple subtypes of muscarinic acetylcholine receptors and their physiological functions in canine hearts. Mol Pharmacol 55:497–507 [PubMed] [Google Scholar]
- 24.Rubio R, Berne RM (1969) Release of adenosine by the normal myocardium in dogs and its relationship to the regulation of coronary resistance. Circ Res 25:407–415 [DOI] [PubMed] [Google Scholar]
- 25.Driver AG, Kukoly CA, Spence PA, Chitwood WR Jr., Mustafa SJ (1995) Pericardial fluid adenosine in ischemic and valvular heart disease. Chest 107:346–351 [DOI] [PubMed] [Google Scholar]
- 26.Funaya H, Kitakaze M, Node K, Minamino T, Komamura K, Hori M (1997) Plasma adenosine levels increase in patients with chronic heart failure. Circulation 95:1363–1365 [DOI] [PubMed] [Google Scholar]
- 27.Sosunov EA, Anyukhovsky EP (2012) Differential effects of ivabradine and ryanodine on pacemaker activity in canine sinus node and purkinje fibers. J Cardiovasc Electrophysiol 23:650–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pelzmann B, Schaffer P, Machler H, Rigler B, Koidl B (1995) Adenosine inhibits the L-type calcium current in human atrial myocytes. Naunyn Schmiedebergs Arch Pharmacol 351:293–297 [DOI] [PubMed] [Google Scholar]
- 29.Ning W, Wit AL (1983) Comparison of the direct effects of nifedipine and verapamil on the electrical activity of the sinoatrial and atrioventricular nodes of the rabbit heart. Am Heart J 106:345–355 [DOI] [PubMed] [Google Scholar]
- 30.Alboni P, Menozzi C, Brignole M, Paparella N, Gaggioli G, Lolli G, Cappato R (1997) Effects of permanent pacemaker and oral theophylline in sick sinus syndrome the THEOPACE study: a randomized controlled trial. Circulation 96:260–266 [DOI] [PubMed] [Google Scholar]
- 31.Sebastiao AM, Ribeiro JA (2009) Adenosine receptors and the central nervous system. Handb Exp Pharmacol 471–534 [DOI] [PubMed] [Google Scholar]
- 32.Slawsky MT, Givertz MM (2009) Rolofylline: a selective adenosine 1 receptor antagonist for the treatment of heart failure. Expert Opin Pharmacother 10:311–322 [DOI] [PubMed] [Google Scholar]
- 33.Kuss J, Stallmeyer B, Goldstein M, Rinne S, Pees C, Zumhagen S, Seebohm G, Decher N, Pott L, Kienitz MC, Schulze-Bahr E (2019) Familial Sinus Node Disease Caused by a Gain of GIRK (G-Protein Activated Inwardly Rectifying K(+) Channel) Channel Function. Circ Genom Precis Med 12:e002238. [DOI] [PubMed] [Google Scholar]
- 34.Lujan R, Marron Fd V, Aguado C, Wickman K (2014) New insights into the therapeutic potential of Girk channels. Trends Neurosci 37:20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evans JM, Randall DC, Funk JN, Knapp CF (1990) Influence of cardiac innervation on intrinsic heart rate in dogs. Am J Physiol 258:H1132–H1137 [DOI] [PubMed] [Google Scholar]
- 36.Lou Q, Glukhov AV, Hansen B, Hage L, Vargas-Pinto P, Billman GE, Carnes CA, Fedorov VV (2013) Tachy-brady arrhythmias: the critical role of adenosine-induced sinoatrial conduction block in post-tachycardia pauses. Heart Rhythm 10:110–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lou Q, Hansen BJ, Fedorenko O, Csepe TA, Kalyanasundaram A, Li N, Hage LT, Glukhov AV, Billman GE, Weiss R, Mohler PJ, Gyorke S, Biesiadecki BJ, Carnes CA, Fedorov VV (2014) Upregulation of adenosine A1 receptors facilitates sinoatrial node dysfunction in chronic canine heart failure by exacerbating nodal conduction abnormalities revealed by novel dual-sided intramural optical mapping. Circulation 130:315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan CS, Lin YK, Chen YC, Lu YY, Chen SA, Chen YJ (2019) Heart Failure Differentially Modulates Natural (Sinoatrial Node) and Ectopic (Pulmonary Veins) Pacemakers: Mechanism and Therapeutic Implication for Atrial Fibrillation. Int J Mol Sci 20: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Z, Xu Y, Song H, Rodriguez J, Tuteja D, Namkung Y, Shin HS, Chiamvimonvat N (2002) Functional Roles of Ca(v)1.3 (alpha(1D)) calcium channel in sinoatrial nodes: insight gained using gene-targeted null mutant mice. Circ Res 90:981–987 [DOI] [PubMed] [Google Scholar]
- 40.Hagiwara N, Irisawa H, Kameyama M (1988) Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J Physiol 395:233–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vinogradova TM, Zhou YY, Bogdanov KY, Yang D, Kuschel M, Cheng H, Xiao RP (2000) Sinoatrial node pacemaker activity requires Ca(2+)/calmodulin-dependent protein kinase II activation. Circ Res 87:760–767 [DOI] [PubMed] [Google Scholar]
- 42.Lakatta EG, Maltsev VA, Vinogradova TM (2010) A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart's pacemaker. Circ Res 106:659–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vogel C, Marcotte EM (2012) Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet 13:227–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fu Y, Huang X, Zhong H, Mortensen RM, D'Alecy LG, Neubig RR (2006) Endogenous RGS proteins and Galpha subtypes differentially control muscarinic and adenosine-mediated chronotropic effects. Circ Res 98:659–666 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.