Abstract
Anxiety disorders and symptoms disproportionately impact women relative to men, but it is unclear what mechanism(s) contribute to this phenomenon. The present study examined sensitivity to unpredictable threat as a potential mechanism of gender differences in panic symptoms. The sample included 67 participants (35 women) who completed the no, predictable, and unpredictable threat (NPU-threat) startle paradigm with electric shocks as the aversive stimulus. Participants also completed the self-report Inventory of Depression and Anxiety Symptoms to assess current panic and depression symptoms. Results indicated that women, relative to men, reported greater panic symptoms and demonstrated increased startle potentiation in anticipation of predictable and unpredictable threat. Furthermore, across all participants increased startle potentiation in anticipation of unpredictable (but not predictable) threat was associated with greater panic symptoms, but there was no relationship with depression symptoms. Finally, the gender difference in panic symptoms was mediated by startle potentiation in anticipation of unpredictable (but not predictable) threat. The present study suggests that a heightened sensitivity to unpredictable threat might be a mechanism that contributes to increased anxiety in women.
Keywords: anxiety, gender, panic, startle, unpredictability
1. Introduction
Anxiety disorders are one of the most prevalent forms of mental illness in the United States (Kessler, Berglund, & Demler, 2005) and there are well-documented gender differences in prevalence, course, and symptom expression (McLean, Asnaani, Litz, & Hofmann, 2011). For example, women are twice as likely as men to experience panic disorder (Grant et al., 2006; Kessler et al., 2006). In addition, women, relative to men, with panic disorder are more likely to experience respiration-related difficulties during panic attacks (Sheikh, Leskin, & Klein, 2002), chronic and severe forms of the disorder (Hollifield et al., 1997; Yonkers et al., 1998), agoraphobic avoidance (Cameron & Hill, 1989; Turgeon, Marchand, & Dupuis, 1998), and comorbid anxiety disorders (Pigott, 1999; Turgeon et al., 1998). Several different etiological factors have been suggested to contribute to this gender difference (e.g., increased physiological reactivity, negative affectivity; see McLean & Anderson, 2009 for a review). However, there is still no consensus regarding what specific mechanism(s) contribute to increased anxiety in women.
A heightened sensitivity to the (un)predictability of threat is one potential mechanism that warrants consideration. The predictability of threat is an important characteristic that has been suggested to differentiate the emotional states of fear and anxiety (Barlow, 2000; Grillon, Baas, Lissek, Smith, & Milstein, 2004). Fear is elicited by imminent or predictable threat and is associated with behavioral responses of fight, flight, or immobilization, while anxiety is triggered by uncertain or unpredictable threat and is associated with avoidance, defensive preparedness, and hypervigilance. Fear and anxiety have also been shown to be mediated by distinction brain regions, specifically the central nucleus of the amygdala (CeA) for fear and the bed nucleus of the stria terminalis (BNST) for anxiety (Davis, 1998; Walker, Toufexis, & Davis, 2003).
Animal and human studies have provided initial evidence suggesting a gender difference in sensitivity to unpredictable threat. For example, one investigation found that female, compared to male, rats exhibited increased BNST-mediated startle potentiation, but male and female rats did not differ in CeA-mediated startle potentiation (Toufexis, 2007). Similarly, a separate investigation in humans found that women, relative to men, exhibited an increased sustained startle reflex in anticipation of unpredictable shocks, but there was no gender difference in phasic startle potentiation in anticipation of predictable shocks (Grillon, 2008). These results suggest that women, relative to men, demonstrate a greater sensitivity to the unpredictability of threat. The present study utilized data from a recent investigation (Nelson & Hajcak, 2017) for a secondary analysis to replicate and extend the Grillon (2008) gender difference finding. Consistent with Grillon (2008), we hypothesized that women, relative to men, would exhibit increased startle potentiation in anticipation of unpredictable (but not predictable) threat. This hypothesis would be supported by the presence of a Condition (predictable threat vs. unpredictable threat) × Gender (males vs. females) interaction.
The predictability of threat is particularly important for panic disorder, which is characterized by periods of both intense fear (i.e., panic attacks) and elevated anxious apprehension (Bouton, Mineka, & Barlow, 2001). A growing number of investigations have examined the association between panic-related phenomenology and startle potentiation in anticipation of predictable and unpredictable threat. For example, Grillon et al. (2008) found that individuals with panic disorder, relative to healthy controls, exhibited an increased sustained startle reflex in anticipation of unpredictable aversive sounds, but there were no group differences in phasic startle potentiation in anticipation of predictable aversive sounds. Shankman et al. (2013) found that individuals with panic disorder, relative to those with no lifetime history of an anxiety disorder, exhibited heightened startle potentiation in anticipation of both predicable and unpredictable shocks. However, Nelson et al. (2013) found that, in the same sample, unpredictable (but not predictable) threat-potentiated startle was associated with a family history of panic disorder, a well-established indicator of risk (Hettema, Neale, & Kendler, 2001), independent of concurrent anxiety disorders, suggesting that heightened startle to unpredictable threat may index risk for panic disorder. In addition to categorical measures, recent studies have indicated that continuous measures of panic-related phenomenology are associated with sensitivity to unpredictable threat. Specifically, Nelson et al. (2015) found that greater anxiety sensitivity physical concerns, a clinical trait that has been associated with panic symptoms (Olthuis, Watt, & Stewart, 2014), were associated with increased startle potentiation in anticipation of unpredictable (but not predictable) shocks. Furthermore, Lieberman et al. (2017) found that greater panic symptom severity was associated with increased startle potentiation in anticipation of unpredictable (but not predictable) shocks. Together, these studies suggest a consistent link between panic-related phenomenology and a heightened sensitivity to unpredictable threat. The present study examined the association between panic symptoms and startle potentiation in anticipation of predictable and unpredictable shocks. Consistent with the literature on panic and unpredictability, we hypothesized that greater panic symptoms would be associated with increased startle potentiation in anticipation of unpredictable (but not predictable) threat. This hypothesis would be supported by the presence of a Condition (predictable threat vs. unpredictable threat) × Panic Symptoms interaction.
Anxiety disorders and depression are two of the most highly comorbid conditions (Mineka, Watson, & Clark, 1998; Watson, 2009), but there is accumulating evidence that a heightened sensitivity to unpredictable threat may differentiate these conditions. Specifically, Shankman et al. (2013), Nelson et al. (2013), and Nelson et al. (2015) all found that panic-related phenomenology, but not depression, was associated with a heightened sensitivity to unpredictable threat (although see Grillon, Franco-Chaves, Mateus, Ionescu, & Zarate, 2013 for counterevidence). To further examine this issue, the present study also examined the association between depression symptoms and startle potentiation in anticipation of predictable and unpredictable threat.
Finally, the aforementioned studies examining gender differences in the expression and prevalence of panic disorder (Grant et al., 2006; Kessler et al., 2005, 2006; McLean et al., 2011) and sensitivity to unpredictable threat (Grillon, 2008; Toufexis, 2007), as well as the association between sensitivity to unpredictable threat and panic disorder (Grillon et al., 2008; Nelson et al., 2013, 2015; Shankman et al., 2013) have been conducted independent of each other. Nonetheless, these findings suggest that a heightened sensitivity to unpredictable threat may be a potential mechanism of gender differences in anxiety. Therefore, as a final aim the present study examined whether gender differences in panic symptoms were mediated by startle potentiation in anticipation of unpredictable threat.
2. Methods
2.1. Participants
The sample included 76 Introduction to Psychology students from Stony Brook University who participated for course credit. Exclusion criteria were an inability to read or write English. All participants provided written informed consent, and the research protocol was approved by the local Institutional Review Board.
2.2. Measures
2.2.1. Inventory of Depression and Anxiety Symptoms -
Expanded Version (IDAS-II) The IDAS-II (Watson et al., 2012; Watson et al., 2007) is a 99-item factor-analytically derived self-report inventory of empirically distinct dimensions of depression and anxiety symptoms. Each item assesses symptoms over the past two weeks on a five-point Likert scale ranging from 1 (not at all) to 5 (extremely). The IDAS has demonstrated good internal consistency, test-retest reliability, and convergent and discriminant validity with diagnoses and other self-report measures (Watson et al., 2007, 2008). The present study focused on the panic subscale (8 items) and the dysphoria subscale (10 items), which is the most discriminative symptom dimension of depression.
2.3. Stimuli
Stimuli were administered using PSYLAB (Contact Precision Instruments, London, UK). Acoustic startle probes were 40-ms duration, 103-dB bursts of white noise with near instantaneous rise time presented binaurally through headphones. Electric shocks were 400-ms in duration and administered to the wrist of the participant’s non-dominant hand. Shock intensity was determined ideographically using a work-up procedure (see below).
2.4. Procedure
After electrode placement,1 participants were seated in a chair approximately 2-ft from a 19-in computer monitor. Participants first completed a 150-s baseline habituation task during which four acoustic startle probes were administered.
The NPU-threat task was a variant of that used by Grillon and colleagues (Schmitz & Grillon, 2012) and has been described elsewhere (see Nelson & Hajcak, 2017)2. Prior to completing task, shock intensity was determined using a work-up procedure where participants received increasing levels of shock, until they reached a level they described as “highly annoying but not painful” (maximum shock level was 5-mA). The mean shock level across the entire sample was 2.20 mA (SD = 0.88). The shock electrodes were attached to the participant’s wrist during the task and not during the baseline habituation phase. Instructions were provided prior to the beginning of the task.
The task included three within-subject conditions: no shock, predictable shock, and unpredictable shock. Text at the bottom of the screen informed participants of the current condition by displaying “no shock”, “shock at 1”, or “shock at any time”. Each condition lasted 75-s, during which a 5-s visual countdown was presented four times. The interstimulus interval (i.e., time between countdowns during the 75-s condition) ranged from 9 to 15-s during which only the text describing the condition was on the screen. In the no threat condition, no shocks were delivered. In the predictable threat condition, participants received a shock every time the countdown reached 1. In the unpredictable threat condition, shocks were administered at any time (i.e., shocks could be administered either during the interstimulus interval or the countdown). Startle probes were presented both during the countdown (1 to 4-s following countdown onset) and interstimulus interval (5 to 12-s following interstimulus interval onset). The time intervals between shocks and subsequent startle probes were always greater than 10-s to ensure that subsequent probes were not affected by prior shocks.
There were two presentations of each 75-s condition (no threat, predictable threat, unpredictable threat), during which the countdown appeared four times (see Nelson & Hajcak, 2017 for a schematic of the task). Participants received startle probes during three out of the four countdown and interstimulus interval presentations. Conditions were presented in one of the following orders (counterbalanced between-subjects): PNUPNU or UNPUNP. All participants received 16 electric shocks (8 during predictable threat and 8 during unpredictable threat), and heard 36 startle probes (12 during no shocks, 12 during predictable shocks, 12 during unpredictable shocks during the countdown and interstimulus interval (with an equal number of startle probes occurring during the countdown and interstimulus interval).
2.5. EMG Recording and Processing
Startle eye blink electromyography (EMG) was recorded using PSYLAB (Contact Precision Instruments, London, UK) and measured from two 4-mm sintered Ag/AgCl electrodes placed over the orbicularis oculi muscle beneath the left eye. EMG activity was sampled at 1000 Hz and filtered between 30 and 500 Hz. Offline, EMG activity was rectified in a 200 ms window, beginning 50 ms before the onset of the startle probe, and a 6-point running average was applied to the rectified data to smooth out sharp peaks. Peak amplitude of the startle reflex was determined in the 20 to 150-ms time frame following the startle probe onset relative to baseline (i.e., average EMG activity in the 50 ms preceding the startle probe onset). Blinks were scored as non-responses if EMG activity during the 20 to 150-ms post-probe time frame did not produce a blink peak that was visually differentiated from baseline activity. Blinks were scored as missing if the baseline period was contaminated with noise, movement artifact, or if a spontaneous or voluntary blink began before minimal onset latency and thus interfered with the probe-elicited blink response. The present study examined blink magnitude (i.e., averages include values of 0 for non-response trials) as this is a more conservative estimate of the startle response (Blumenthal et al., 2005).
2.6. Data Analysis
Five participants were excluded from analyses for having startle data with less than 50% useable trials, and 4 participants were excluded for taking current psychotropic medication, leaving a final sample of 67 participants. One-way analysis of variance (ANOVA) and χ2 analyses were conducted to test for gender differences in continuous and categorical, respectively, demographics and clinical characteristics. Male and female participants did not differ in their startle reflex during the no threat countdown or interstimulus interval (ps > .25). Similarly, IDAS panic and dysphoria symptoms were not correlated with the startle reflex during the no threat countdown or interstimulus interval (ps > .48) Therefore, startle potentiation was computed for the predictable and unpredictable threat relative to no threat conditions (i.e., predictable threat – no threat, unpredictable threat – no threat).
To test for gender differences in startle potentiation, we conducted a Condition (predictable threat vs. unpredictable threat) × Cue (countdown vs. interstimulus interval) × Gender (males vs. females) mixed-measures ANOVA, with condition and cue as within-subject factors and gender as a between-subjects factor. To test for associations between startle potentiation and symptoms, we conducted a Condition (predictable threat vs. unpredictable threat) × Cue (countdown vs. interstimulus interval) mixed-measures analysis of covariance (ANCOVA), with condition and cue as within-subject factors and the panic and dysphoria symptoms as continuous covariate.
To test for mediation, we used a nonparametric bootstrapping method (MacKinnon et al., 2004), which is statistically more powerful than other tests of mediation (MacKinnon et al., 2002). To this end, we used the SPSS PROCESS macro model 4 (Hayes, 2013; Preacher & Hayes, 2004), which provides a bootstrap estimate of the indirect effect between the independent variable and dependent variable, an estimated standard error, and 95% confidence intervals (CI) for the population value of the indirect effect. CIs that do not include zero indicate a significant indirect effect at the p < .05 significance level. Analyses were conducted using 5,000 bootstrap samples. Prior to conducting mediational analyses, all variables were z-scored to produce standardized β weights. All analyses were conducted in IBM SPSS Statistics, Version 24.0 (Armonk, NY, USA).
3. Results
3.1. Post-Hoc Power Analyses
Post-hoc power analyses for the ANOVA and ANCOVA models were calculated using G*Power software with a sample size of N = 67, significance of p < .05, and Cohen’s f = 0.25. The ANOVA model achieved a power of 72.2% and the ANCOVA achieved a power of 52.2%. For the mediational analyses, the PROCESS macro uses bootstrapping to construct confidence intervals, and the present study utilized 5,000 bootstrap samples. Research has demonstrated that the PROCESS macro model 4 indirect effect achieves power > 99% (Preacher, Rucker, & Hayes, 2007).
3.2. Gender Differences
Table 1 displays demographics and clinical characteristics. Male and female participants did not differ in demographics or depression symptoms. However, female participants had greater panic symptoms compared to male participants.
Table 1.
Demographics and Clinical Characteristics
| Males (n =32) | Females (n = 35) | F or χ2 | P | |||
|---|---|---|---|---|---|---|
| M or % | SD | M or % | SD | |||
| Age (years) | 19.78 | 1.54 | 19.26 | 2.23 | F = 1.23 | .27 |
| Education (years) | 13.66 | 1.43 | 13.11 | 1.35 | F = 2.56 | .12 |
| Ethnicity/Race | χ2 = 2.33 | .68 | ||||
| Asian | 62.5% | 51.4% | ||||
| Black | 6.3% | 14.3% | ||||
| Caucasian | 21.9% | 22.9% | ||||
| Latino | 9.4% | 8.6% | ||||
| Other | 0.0% | 2.9% | ||||
| IDAS | ||||||
| Dysphoria | 39.22 | 9.05 | 38.43 | 10.43 | F = 0.11 | .74 |
| Panic | 9.88 | 2.04 | 11.77 | 3.73 | F = 6.50 | .01 |
Note. IDAS = Inventory of Depression and Anxiety Symptoms, M = mean, SD = standard deviation.
ANOVA analyses indicated a main effect of gender, F(1, 65) = 5.05, p = .03, ηp2 = .07; there were no other main effects or interactions involving gender (ps > .20). As shown in Figure 1, female, relative to male, participants demonstrated greater startle potentiation across both predictable and unpredictable threat conditions. Given that female participants reported greater panic symptoms compared to male participants, we conducted additional analyses where panic symptoms were included as a covariate. Results indicated that, even after controlling for panic symptoms, female participants continued to demonstrate greater startle potentiation, F(1, 64) = 4.30, p = .04, ηp2 = .06.
Figure 1.
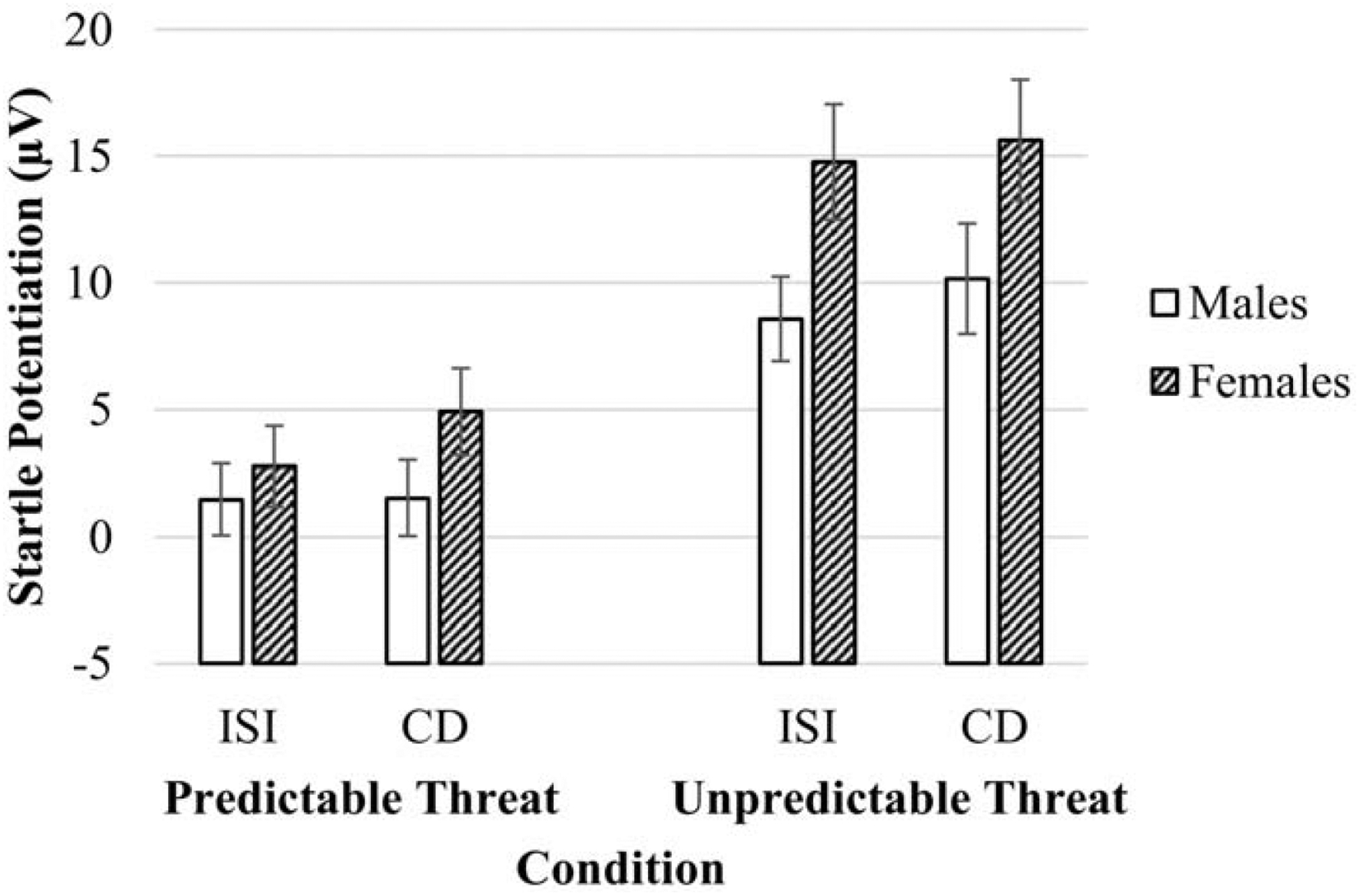
Startle potentiation in anticipation of predictable and unpredictable threat (relative to no threat) for males and females. CD = countdown, ISI = interstimulus interval
3.3. Panic Symptoms
The IDAS-II panic (M = 10.87, SD = 3.17) and dysphoria (M = 18.81, SD = 5.77) symptom scores were consistent with student populations (Watson et al., 2008). Across all participants, 31.3% of participants had panic symptom scores that were in the top quartile, and 9.0% of participants had panic symptom scores that exceeded the clinical cut-off (Nelson, O’Hara, & Watson, 2018; Stasik-O’Brien et al., 2019). Startle reflex analyses indicated a Condition × Panic Symptoms interaction, F(1, 64) = 7.88, p = .007, ηp2 = .11; there were no other main effects or interactions involving panic or dysphoria symptoms (ps > .10). As shown in Figure 2, greater panic symptoms were associated with greater startle potentiation during the unpredictable threat condition, r(67) = .26, p = .04, but panic symptoms were not associated with startle potentiation during the predictable threat condition, r(67) = −.20, p = .10.3 A comparison of the correlation between startle potentiation to unpredictable threat and panic symptoms and the correlation between startle potentiation to predictable threat and dysphoria symptoms indicated that they statistically differed, z = 1.94, p = .03, one-tailed (Lee & Preacher, 2013).
Figure 2.
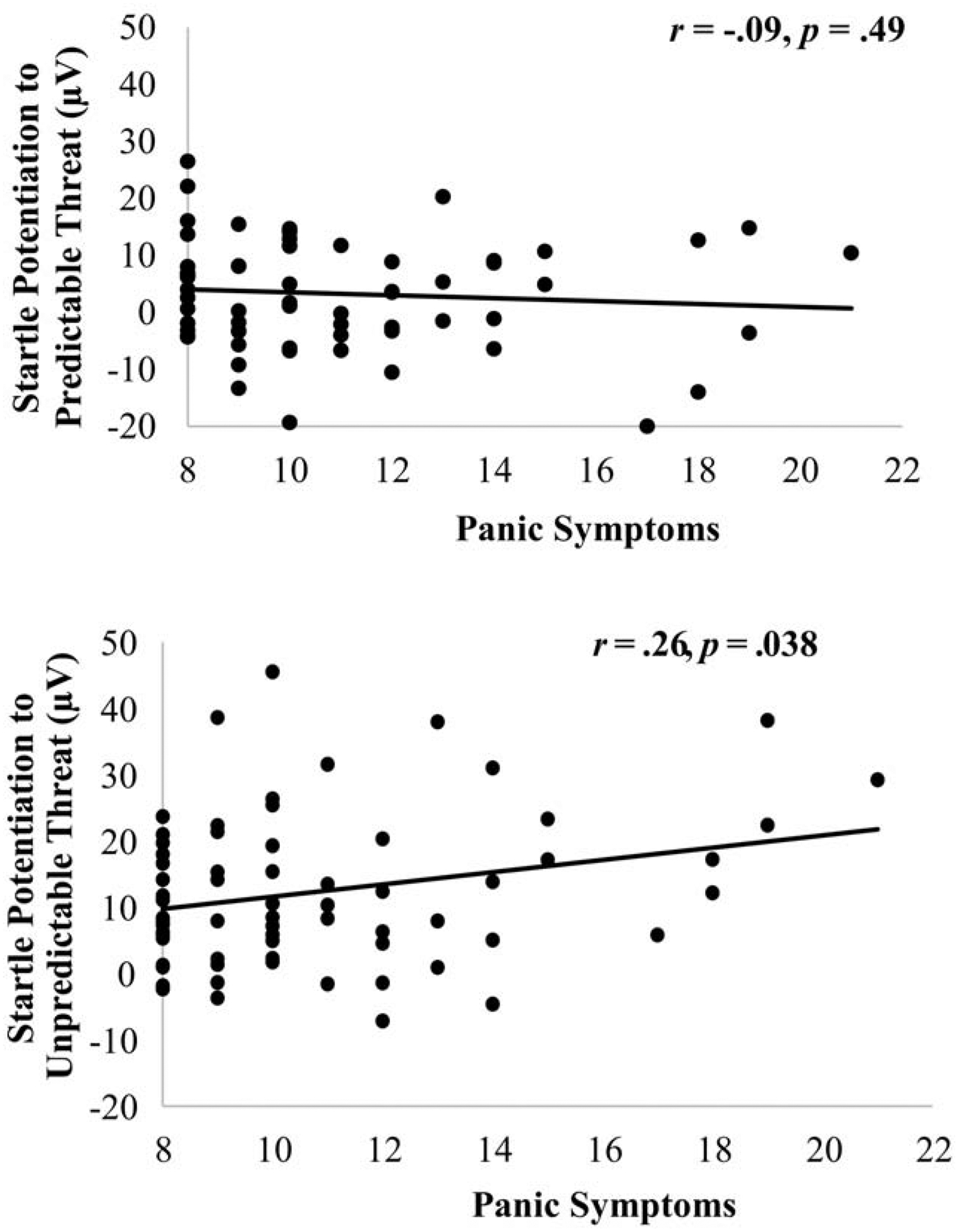
Scatterplots depicting the association between panic symptoms and startle potentiation in anticipation of predictable threat (top row) and unpredictable threat (bottom row) relative to no threat across all participants. Data were collapsed across cue (countdown vs. interstimulus interval) for the unpredictable threat startle potentiation scatterplots, and data are only show for the countdown for the predictable threat startle potentiation scatterplots.
3.4. Mediational Model
Finally, we examined whether startle potentiation during the predictable and/or unpredictable threat condition mediated the gender difference in panic symptoms. To this end, we examined startle potentiation during the predictable threat countdown (as this was the only phase during which participants were in danger) and the unpredictable threat countdown and interstimulus interval (as during both phases participants were in danger).4 As shown in Figure 3, female, compared to male, participants demonstrated greater startle potentiation during the unpredictable threat condition and reported greater panic symptoms. In turn, greater startle potentiation during the unpredictable threat condition was associated with increased panic symptoms. Importantly, there was an indirect effect of gender, mediated through startle potentiation during the unpredictable threat condition, on panic symptoms. In contrast, there was no indirect effect of gender, mediated through startle potentiation during the predictable threat condition, on panic symptoms.
Figure 3.
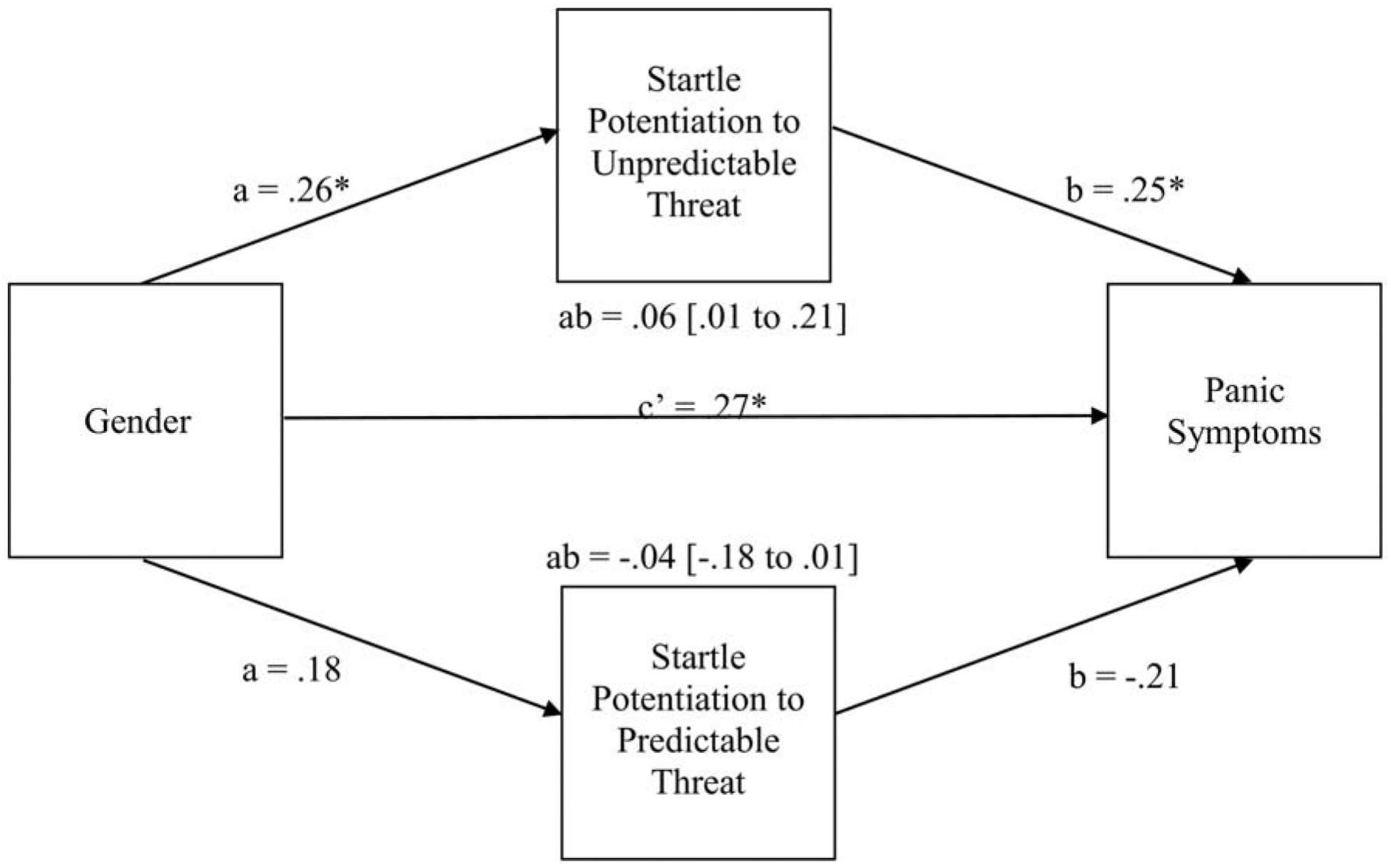
Mediational analysis for gender, startle potentiation during the unpredictable threat condition (collapsed across cue [countdown vs. interstimulus interval]) and predictable threat condition (during the countdown only), and panic symptoms. Coefficients are standardized regression weights. a = association between the independent variable and mediator; b = association between mediator and dependent variable; ab = indirect effect; c’ = direct effect of independent variable on dependent variable. ** p < .01, *p < .05.
4. Discussion
The present study examined sensitivity to unpredictable threat as a potential mechanism of gender differences in panic symptoms. Results indicated that women, relative to men, reported greater panic symptoms and demonstrated greater startle potentiation in anticipation of predictable and unpredictable threat. In addition, greater startle potentiation in anticipation of unpredictable (but not predictable) threat was associated with increased panic symptoms, but there was no relationship with depressive symptoms. Finally, the gender difference in panic symptoms was mediated by startle potentiation in anticipation of unpredictable threat.
The present study replicates a previous investigation indicating the presence of gender differences in sensitivity to unpredictable threat (Grillon, 2008). The anticipation of unpredictable and uncertain threat has been shown to involve a neural network that spans multiple brain regions, including the amygdala, anterior insula, cingulate cortex, orbitofrontal cortex, prefrontal cortex, and ventral striatum (Alvarez, Chen, Bodurka, Kaplan, & Grillon, 2011; Grupe & Nitschke, 2013; Shankman et al., 2014). Research has indicated gender differences in brain activation across several of these regions (Sacher, Neumann, Okon-Singer, Gotowiec, & Villringer, 2013). For example, a meta-analysis of neuroimaging studies indicated that women, relative to men, demonstrate greater activation in the amygdala, cingulate cortex, and orbitofrontal cortex during negative emotional states (Stevens & Hamann, 2012). In addition, in a separate investigation, women, relative to men, showed a greater association between momentary arousal ratings and neural responses in the anterior insula cortex, which has been implicated in interoceptive awareness (Moriguchi, Touroutoglou, Dickerson, & Barrett, 2014). Therefore, it is possible that gender differences in brain function across these regions contribute to increased sensitivity to unpredictable threat and the development of anxiety in women. There are also important gender differences in endogenous hormones that have been shown to impact sensitivity to threat. For example, testosterone has been suggested to possess anxiolytic effects (Maeng & Milad, 2015; McHenry, Carrier, Hull, & Kabbaj, 2014), and a single dose of testosterone has been shown to attenuate threat-potentiated startle response in women (Hermans, Putman, Baas, Koppeschaar, & van Honk, 2006). In addition, puberty, a developmental period characterized by acute changes in hormones, physiology, and behavior, has been shown to impact threat sensitivity. Specifically, one investigation found that 10 to 13-year-old girls demonstrated increased threat-potentiated startle across pubertal development, whereas boys did not display the same increase (Schmitz, Grillon, Avenevoli, Cui, & Merikangas, 2014). Similarly, a separate investigation found that in a group of 7 to 17-year-olds, girls demonstrated an increased startle potentiation to unpredictable (but not predictable) threat compared to boys (Schmitz et al., 2011). It is important to note that the present study did not assess endogenous hormones and their contribution to sensitivity to unpredictable threat remains speculative. Future studies should examine endogenous hormones in the context of research on sex differences in sensitivity to unpredictability and anxiety.
In contrast to Grillon (2008), the present study also found that found that women, relative to men, demonstrated greater startle potentiation in anticipation of predictable threat. There is an important task difference between the Grillon investigation and the present study that might explain this discrepant result. In the Grillon investigation, the NPU-threat task utilized geometric shapes as cues, and participants received a shock during one of the four predictable threat cues and one of the four unpredictable threat interstimulus intervals. Thus, in the predictable threat condition, there was uncertainty during the interstimulus interval whether the next cue would be associated with a shock. As a result, participants demonstrated increased startle magnitude during the predictable interstimulus interval, a period during which they were safe. In the Grillon investigation, phasic startle potentiation was examined by subtracting startle magnitude during the predictable interstimulus interval from that during the predicable cue, but since both periods contained an element of uncertainty/unpredictability this might have eliminated any potential Gender difference. In the present study, the NPU-threat task utilized a countdown and administered a shock every time the predictable condition countdown reached 1. This variant of the NPU-threat task resulted in startle potentiation during the predictable countdown, but not the predictable interstimulus interval or the no threat countdown or interstimulus interval, when participants were safe. This variant of the NPU-threat task better distinguishes predictable vs. unpredictable threat and resulted in a gender difference in sensitivity to both predictable and unpredictable threat (although, importantly, only sensitivity to unpredictable threat was associated with panic symptoms). Future studies should continue to consider important task details and analytic strategies when examining gender differences in sensitivity to threat. The present study adds additional evidence to a growing literature on panic-related phenomenology and sensitivity to unpredictable threat (Grillon et al., 2008; Lieberman et al., 2017; Nelson et al., 2015; Shankman et al., 2013). Specifically, we found that greater panic symptoms were associated with heightened startle potentiation in anticipation of physical threat. Moreover, this relationship was independent of depression, which was not associated with sensitivity to predictable or unpredictable threat. An important feature of panic disorder is increased anxious apprehension regarding potential future panic attacks, which can increase the probability and intensity of the next panic attack (Bouton et al., 2001). The results of this investigation suggest that a heightened sensitivity to unpredictable threat is associated with greater panic symptoms in a non-clinical sample.
Finally, the results of the present study suggest that gender differences in panic symptoms are mediated by heightened sensitivity to unpredictable, but not predictable, threat. To date, several different etiological factors have been suggested to contribute to the gender difference in anxiety, including increased physiological reactivity and negative affectivity (McLean & Anderson, 2009). It is possible that sensitivity to uncertainty and unpredictability might transcend these broader constructs and represent a more refined manifestation of this potential mechanisms. Future studies should include measures of multiple proposed constructs to better determine whether they index overlapping or unique mechanisms of gender differences in anxiety. Moreover, the present study only provides cross-sectional evidence of mediation, and future studies should use longitudinal designs to determine whether gender differences in sensitivity to unpredictable threat prospectively predict changes in anxiety symptoms and disorders.
The present study had several important limitations that should be taken into consideration. First, the sample consisted of college students who, on average, had relatively low levels of panic symptoms, and this might limit the generalizability of the findings to other populations (i.e., clinical samples). Future studies in clinical samples that contain greater variability in psychopathology are needed to corroborate the results. Second, the present study utilized a cross-sectional design, which limits our ability to make casual interpretations regarding what contributes to increased sensitivity to threat and panic symptoms. Third, menstrual cycle phase was not assessed in the female participants. It is possible that normative changes in endogenous hormone levels might have influenced the pattern of results or may entirely account for the results. Thus, the interpretation of the current results is limited without an assessment of the menstrual cycle. Future longitudinal studies are needed that include assessments of endogenous hormone levels as well as other factors (e.g., stress) that might contribute to gender differences in sensitivity to predictable and/or unpredictable threat and the development of anxiety disorders and symptoms. Fourth, the present study was a secondary analysis of a previous investigation (Nelson & Hajcak, 2017), and future studies should attempt to replicate these results in a pre-registered, independent sample to increase confidence in the reliability of the results. Fifth, predictable threat startle potentiation was limited due to task order effects (counterbalanced between-subjects, see Nelson & Hajcak, 2017) and might have impacted the magnitude of the results. However, it is important to note that task order was not systematically related to any individual difference measure (i.e., gender or symptoms). Finally, the present study focused on the (un)predictability of physical threat (i.e., electric shocks). However, recent evidence suggests that temporal unpredictability enhances defensive motivation in anticipation of aversive and appetitive stimuli (Parisi, Hajcak, Aneziris, & Nelson, 2017). It is possible that sensitivity to unpredictability more broadly (rather than just unpredictable threat) is a core mechanism of gender differences in psychopathology. Future studies should attempt to replicate these findings using appetitive stimuli and additional forms of threat.
In conclusion, the present study found that women, relative to men, reported greater panic symptoms and increased startle potentiation in anticipation of predictable and unpredictable threat, but only sensitivity to unpredictable threat mediated the relationship between gender and greater panic symptoms. These results suggest that sensitivity to unpredictable threat might be a potential mechanism of gender differences in anxiety symptoms and disorders. Future studies are needed to better understand how threat systems develop across critical periods during which anxiety symptoms and disorders begin to emerge (e.g., childhood and adolescence) to better understand the etiology of gender differences in psychopathology.
Acknowledgements
This project was funded by National Institute of Mental Health grant K01MH107808 award to B.D.N. The authors have no conflicts of interest to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Electroencephalography was also measured to examine startle probe event-related potential and retrospective self-report emotions ratings were also collected after completion of the NPU-threat task. However, we did not have a priori hypotheses for measures and they were not included in this investigation.
As described in Nelson and Hajcak (2017), participants also completed a variant of the NPU-threat task with unpleasant pictures as the aversive stimulus (shock and unpleasant picture variants were administered in a counterbalanced order). In the unpleasant picture variant of the NPU-threat task, participants demonstrated startle potentiation during both the countdown and interstimulus interval of the predicable threat condition (in the shock variant startle is only enhanced during the predictable countdown and not the predictable interstimulus interval). The present study only included startle data from the shock variant of the NPU-threat task due to the abnormal pattern of results obtained during the unpleasant pictures variant of the NPU-threat task. However, it should be noted that when Stimulus (shocks vs. unpleasant pictures) was included as a within-subject factor (i.e., Stimulus × Condition × Cue × Gender × Panic Symptoms × Dysphoria Symptoms), the gender main effect (p = .01) and Condition × Panic Symptoms interaction (p < .001) remained significant.
We also examined the predictable threat countdown and interstimulus interval separately. Results indicate that panic symptoms were not associated with startle potentiation during the predictable threat countdown, r(67) = −.09, p = .49, or interstimulus interval, r(67) = −.23, p = .07.
Results remained identical when startle potentiation to predictable threat, collapsed across the countdown and interstimulus interval, were included in the mediation model.
References
- Alvarez RP, Chen G, Bodurka J, Kaplan R, & Grillon C (2011). Phasic and sustained fear in humans elicits distinct patterns of brain activity. NeuroImage, 55, 389–400. 10.1016/j.neuroimage.2010.11.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow DH (2000). Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. American Psychologist, 55, 1247–1263. 10.1037/0003-066X.55.11.1247 [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Manual for the Beck depression inventory-II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, & Van Boxtel A (2005). Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology, 42, 1–15. 10.1111/j.1469-8986.2005.00271.x [DOI] [PubMed] [Google Scholar]
- Bouton ME, Mineka S, & Barlow DH (2001). A modern learning theory perspective on the etiology of panic disorder. Psychological Review, 108, 4–32. 10.1037//0033-295X.108.1.4 [DOI] [PubMed] [Google Scholar]
- Cameron OG, & Hill EM (1989). Women and anxiety. The Psychiatric Clinics of North America, 12, 175–186. [PubMed] [Google Scholar]
- Davis M (1998). Are different parts of the extended amygdala involved in fear versus anxiety? Biological Psychiatry, 44, 1239–1247. 10.1016/S0006-3223(98)00288-1 [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Stinson FS, Dawson DA, Goldstein RB, Smith S, … Saha TD (2006). The epidemiology of DSM-IV panic disorder and agoraphobia in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry, 67, 363–374. 10.4088/JCP.v67n0305 [DOI] [PubMed] [Google Scholar]
- Grillon C (2008). Greater sustained anxiety but not phasic fear in women compared to men. Emotion, 8, 410–413. 10.1037/1528-3542.8.3.410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Baas JP, Lissek S, Smith K, & Milstein J (2004). Anxious responses to predictable and unpredictable aversive events. Behavioral Neuroscience, 118, 916–924. 10.1037/0735-7044.118.5.916 [DOI] [PubMed] [Google Scholar]
- Grillon C, Franco-Chaves JA, Mateus CF, Ionescu DF, & Zarate C. a. (2013). Major depression is not associated with blunting of aversive responses; evidence for enhanced anxious anticipation. PloS One, 8, e70969 10.1371/journal.pone.0070969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, & Nitschke JB (2013). Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nature Reviews. Neuroscience, 14, 488–501. 10.1038/nrn3524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A (2013). Introduction to mediation, moderation, and conditional process analysis. New York, NY: Guilford; http://doi.org/978-1-60918-230-4 [Google Scholar]
- Hermans EJ, Putman P, Baas JM, Koppeschaar HP, & van Honk J (2006). A single administration of testosterone reduces fear-potentiated startle in humans. Biological Psychiatry, 59, 872–874. 10.1016/j.biopsych.2005.11.015 [DOI] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, & Kendler KS (2001). A review and meta-analysis of the genetic epidemiology of anxiety disorders. American Journal of Psychiatry, 158, 1568–1578. 10.1176/appi.ajp.158.10.1568 [DOI] [PubMed] [Google Scholar]
- Hollifield M, Katon W, Skipper B, Chapman T, Ballenger JC, Mannuzza S, & Fyer AJ (1997). Panic disorder and quality of life: Variables predictive of functional impairment. American Journal of Psychiatry, 154, 766–772. 10.1176/ajp.154.6.766 [DOI] [PubMed] [Google Scholar]
- Kessler R, Berglund P, & Demler O (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62, 593–602. 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Jin R, Ruscio AM, Shear K, & Walters EE (2006). The epidemiology of panic attacks, panic disorder, and agoraphobia in the National Comorbidity Survey Replication. Archives of General Psychiatry, 141, 415–424. 10.1016/j.surg.2006.10.010.Use [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P, Bradley M, & Cuthbert B (2008). International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. [Google Scholar]
- Lee IA, & Preacher KJ (2013). Calculation for the test of the difference between two dependent correlations with one variable in common. Retrieved from http://quantpsy.org
- Lieberman L, Gorka S, Shankman S, & Phan KL (2017). Impact of panic on psychophysiological and neural reactivity to unpredictable threat in depression and anxiety. Clinical Psychological Science, 5, 52–63. 10.1177/2167702616666507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, & Sheets V (2002). A comparison of methods to test mediation and other intervening variable effects. Psychological Methods, 7, 83–104. 10.1037/1082-989X.7.1.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, & Williams J (2004). Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivariate Behavioral Research, 39, 99–128. 10.1207/s15327906mbr3901_4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng LY, & Milad MR (2015). Sex differences in anxiety disorders: Interactions between fear, stress, and gonadal hormones. Hormones and Behavior, 76, 106–117. 10.1016/j.yhbeh.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry J, Carrier N, Hull E, & Kabbaj M (2014). Sex differences in anxiety and depression: Role of testosterone. Frontiers in Neuroendocrinology, 35, 42–57. 10.1016/j.yfrne.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CP, & Anderson ER (2009). Brave men and timid women? A review of the gender differences in fear and anxiety. Clinical Psychology Review, 29, 496–505. 10.1016/j.cpr.2009.05.003 [DOI] [PubMed] [Google Scholar]
- McLean CP, Asnaani A, Litz BT, & Hofmann SG (2011). Gender differences in anxiety disorders: Prevalence, course of illness, comorbidity and burden of illness. Journal of Psychiatric Research, 45, 1027–1035. 10.1016/j.jpsychires.2011.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineka S, Watson D, & Clark LA (1998). Comorbidity of anxiety and unipolar mood disorders. Annual Review of Psychology, 49, 377–412. 10.1146/annurev.psych.49.1.377 [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Touroutoglou A, Dickerson BC, & Barrett LF (2014). Sex differences in the neural correlates of affective experience. Social Cognitive and Affective Neuroscience, 9, 591–600. 10.1093/scan/nst030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, & Hajcak G (2017). Defensive motivation and attention in anticipation of different types of predictable and unpredictable threat: A startle and event-related potential investigation. Psychophysiology, 54, 1180–1194. 10.111/psyp.12869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, Hajcak G, & Shankman SA (2015). Event-related potentials to acoustic startle probes during the anticipation of predictable and unpredictable threat. Psychophysiology, 52, 887–894. 10.1111/psyp.12418 [DOI] [PubMed] [Google Scholar]
- Nelson BD, Hodges A, Hajcak G, & Shankman SA (2015). Anxiety sensitivity and the anticipation of predictable and unpredictable threat: Evidence from the startle response and event-related potentials. Journal of Anxiety Disorders, 33, 62–71. 10.1016/j.janxdis.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, McGowan SK, Sarapas C, Robison-Andrew EJ, Altman SE, Campbell ML, … Shankman SA (2013). Biomarkers of threat and reward sensitivity demonstrate unique associations with risk for psychopathology. Journal of Abnormal Psychology, 122, 662–671. 10.1037/a0033982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson GH, O’Hara MW, & Watson D (2018). National norms for the expanded version of the inventory of depression and anxiety symptoms (IDAS-II). Journal of Clinical Psychology, 74, 953–968. 10.1002/jclp.22560 [DOI] [PubMed] [Google Scholar]
- Olthuis JV, Watt MC, & Stewart SH (2014). Anxiety Sensitivity Index (ASI-3) subscales predict unique variance in anxiety and depressive symptoms. Journal of Anxiety Disorders, 28, 115–24. 10.1016/j.janxdis.2013.04.009 [DOI] [PubMed] [Google Scholar]
- Parisi EA, Hajcak G, Aneziris E, & Nelson BD (2017). Effects of anticipated emotional category and temporal predictability on the startle reflex. International Journal of Psychophysiology, 119, 67–72. 10.1016/j.ijpsycho.2017.03.003 [DOI] [PubMed] [Google Scholar]
- Pigott TA (1999). Gender differences in the epidemiology and treatment of anxiety disorders. The Journal of Clinical Psychiatry, 60, 4–15. [PubMed] [Google Scholar]
- Preacher KJ, & Hayes AF (2004). SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments, & Computers, 36, 717–731. 10.3758/BF03206553 [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Rucker DD, & Hayes AF (2007). Addressing moderated mediation hypotheses: Theory, methods, and prescriptions. Multivariate Behavioral Research, 42, 185–227. 10.1080/00273170701341316 [DOI] [PubMed] [Google Scholar]
- Sacher J, Neumann J, Okon-Singer H, Gotowiec S, & Villringer A (2013). Sexual dimorphism in the human brain: Evidence from neuroimaging. Magnetic Resonance Imaging, 31, 366–375. 10.1016/j.mri.2012.06.007 [DOI] [PubMed] [Google Scholar]
- Schmitz A, & Grillon C (2012). Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test). Nature Protocols, 7, 527–532. 10.1038/nprot.2012.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz A, Grillon C, Avenevoli S, Cui L, & Merikangas KR (2014). Developmental investigation of fear-potentiated startle across puberty. Biological Psychology, 97, 15–21. 10.1016/j.biopsycho.2013.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz A, Merikangas K, Swendsen H, Cui L, Heaton L, & Grillon C (2011). Measuring anxious responses to predictable and unpredictable threat in children and adolescents. Journal of Experimental Child Psychology, 110, 159–170. 10.1016/j.jecp.2011.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Gorka SM, Nelson BD, Fitzgerald D. a, Phan KL, & O’Daly O (2014). Anterior insula responds to temporally unpredictable aversiveness: an fMRI study. Neuroreport, 25, 596–600. 10.1097/WNR.0000000000000144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Nelson BD, Sarapas C, Robison-Andrew EJ, Campbell ML, Altman SE, … Gorka SM (2013). A psychophysiological investigation of threat and reward sensitivity in individuals with panic disorder and/or major depressive disorder. Journal of Abnormal Psychology, 122, 322–338. 10.1037/a0030747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh JI, Leskin GA, & Klein DF (2002). Gender differences in panic disorder: Findings from the national comorbidity survey. American Journal of Psychiatry, 159, 55–58. [DOI] [PubMed] [Google Scholar]
- Stasik-O’Brien SM, Brock RL, Chmielewski M, Naragon-Gainey K, Koffel E, McDade-Montez E, … Watson D (2019). Clinical utility of the Inventory of Depression and Anxiety Symptoms (IDAS). Assessment, 26, 944–960. 10.1177/1073191118790036 [DOI] [PubMed] [Google Scholar]
- Stevens JS, & Hamann S (2012). Sex differences in brain activation to emotional stimuli: A meta-analysis of neuroimaging studies. Neuropsychologia, 50, 1578–1593. 10.1016/j.neuropsychologia.2012.03.011 [DOI] [PubMed] [Google Scholar]
- Toufexis D (2007). Region- and sex-specific modulation of anxiety behaviours in the rat. Journal of Neuroendocrinology, 19, 461–473. 10.1111/j.1365-2826.2007.01552.x [DOI] [PubMed] [Google Scholar]
- Turgeon L, Marchand A, & Dupuis G (1998). Clinical features in panic disorder with agoraphobia: a comparison of men and women. Journal of Anxiety Disorders, 12, 539–553. 10.1016/S0887-6185(98)00031-0 [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, & Davis M (2003). Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. European Journal of Pharmacology, 463, 199–216. 10.1016/S0014-2999(03)01282-2 [DOI] [PubMed] [Google Scholar]
- Watson D (2009). Differentiating the mood and anxiety disorders: A quadripartite model. Annual Review of Clinical Psychology, 5, 221–247. 10.1146/annurev.clinpsy.032408.153510 [DOI] [PubMed] [Google Scholar]
- Watson D, O’Hara MW, Chmielewski M, McDade-Montez EA, Koffel E, Naragon K, & Stuart S (2008). Further validation of the IDAS: Evidence of convergent, discriminant, criterion, and incremental validity. Psychological Assessment, 20, 248–259. 10.1037/a0012570 [DOI] [PubMed] [Google Scholar]
- Watson D, O’Hara MW, Naragon-Gainey K, Koffel E, Chmielewski M, Kotov R, … Ruggero CJ (2012). Development and validation of new anxiety and bipolar symptom scales for an expanded version of the IDAS (the IDAS-II). Assessment, 19, 399–420. 10.1177/1073191112449857 [DOI] [PubMed] [Google Scholar]
- Watson D, O’Hara MW, Simms LJ, Kotov R, Chmielewski M, McDade-Montez EA, … Stuart S (2007). Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS). Psychological Assessment, 19, 253–268. 10.1037/1040-3590.19.3.253 [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Zlotnick C, Allsworth J, Warshaw M, Shea T, & Keller MB (1998). Is the course of panic disorder the same in women and men? American Journal of Psychiatry, 155, 596–602. 10.1176/ajp.155.5.596 [DOI] [PubMed] [Google Scholar]


