Abstract
Purpose:
American Academy of Pediatric Dentistry (AAPD) guidelines recommend treatment of primary teeth with 38% silver diamine fluoride (SDF) as a non-invasive option to arrest active dental caries lesions. A significant outcome of SDF treatment are lesions that clinically harden and become more resistant to further decay. Many practicing dentists believe that this increased hardening is due to the reaction of silver and fluoride with carious dentin. In this study, we focus on the structural and chemical effects of SDF treatment on the native tooth.
Methods:
In SDF-treated cavitated dentin lesions in teeth subsequently extracted for orthodontic reasons, we observed continuous, filamentous silver densities formed in situ from 50–2,100 µm in length and 0.25–7.0 µm in diameter using high resolution synchrotron X-ray microCT and field emission scanning electron microscopy. These “microwires” fill voids in the lesion caused by disease and permeate through surrounding dentinal tubules.
Results:
We confirmed using spectroscopy that the chemical composition of the observed microwires is predominantly silver.
Conclusions:
These observations suggest mechanistic explanations for the structural reinforcement of carious dentin in addition to remineralization. We hypothesize that SDF may not only achieve its antimicrobial functions by biochemical interactions, but also through its inherent ability to integrate into the native tooth structure.
Keywords: Caries treatment, Remineralization, Synchrotron X-ray Micro-computed Tomography, Biological Materials, Silver Diamine Fluoride
Introduction
Dental caries (tooth decay) occurs when plaque microbiota metabolize dietary sugars into acids that demineralize the tooth, exposing the collagenous dentin matrix, which is then degraded by endogenous and bacterial proteases1. Meta-analysis has shown that application of 38% SDF arrests 81% of such decay2 and both AAPD and the American Dental Association have recommended its use3,4. A protocol for its use has been published5.
It is thought that SDF arrests lesions via silver ions lysing bacteria, and fluoride ions promoting remineralization to strengthen the lesion5. However, fluoride has antibacterial action at high concentrations6, and ex vivo studies show more resistance to demineralization following treatment with SDF than with matching fluoride solutions without silver7,8. Thus, both silver and fluoride may contribute to both bactericidal effects and lesion strengthening.
SDF-arrested caries lesions are roughly twice as hard as normal dentin9; this could not be caused by remineralization alone. Moreover, the outermost dentin is the most difficult to remineralize, due to matrix degradation. Reaction with the collagenous dentin matrix is supported by in vitro observations of silver particles on gelatin exposed to SDF10.
Previous work on silver-based antibacterial compounds focused on silver nitrate (SN). While SN has been observed greater than one mm deep in artificial lesions, dark SDF stains have been observed 200–300 µm into dentin11. Studies of SN found semi-spherical polyhedral particles by light microscopy to course throughout the dentinal tubules in treated artificially created lesions (drill holes) penetrating as far as 1.65 mm, or 90% of the distance to the pulp12. SDF was developed to combine the anti-caries effectiveness of silver and fluoride, and outperformed each separately in clinical and laboratory models13. Despite the positive anti-caries performance of SDF, a rigorous study of SDF penetration depth has been lacking. Approximately 45 years ago, exploratory microstructural work with scanning electron microscopy (SEM) of SDF-treated healthy dentin revealed sparse aggregates hundreds of micrometers into dentinal tubules14. However, this study was not conducted on clinically treated teeth, and the findings of irregularly distributed particles were inconsistent with a structure that would spread mechanical loads to reinforce lesions, as is observed clinically following SDF treatment. In this research we sought to characterize the deposition of solids in carious primary teeth following clinical SDF treatment.
Materials and Methods
Preparation of teeth
Five primary and two permanent teeth with natural cavitated carious lesions (exposing dentin: ICDAS 5–6) were collected following serial extraction for orthodontic treatment. The teeth were collected in accordance with de-identification procedures by the University of California, San Francisco Institutional Review Board (BUA BU032969–03M). The carious lesions had been treated eight to 36 months prior to extraction with 38% silver diamine fluoride (Advantage Arrest, Elevate Oral Care LLC, West Palm Beach, FL) and documented clinically to be arrested by visual and tactile examination. The teeth were stored in 0.1% thymol until used in this study. This manuscript describes analysis of one representative tooth; a comprehensive report on variations across more samples will follow.
Microscopy
Teeth were sectioned to two mm thickness mesio-distally with a slow speed metallurgical saw, polished with sandpaper (Buehler, USA), and allowed to dry before study.
Brightfield light microscopy was performed with an upright BX50 microscope (Olympus, Japan). Scanning electron micrographs (SEM) were obtained using a Gemini Ultra-55 Analytical Field Emission SEM (Zeiss, Germany). An operating voltage of ten keV was selected to differentiate the silver wires from dentin, whereas a lower voltage, usually between five-to-eight keV, distinguishes peritubular from intertubular dentin (Supplemental Figure 1). Elemental analyses were conducted with an AzTec Energy Dispersive X-ray Spectroscopy (EDS) system (Oxford Instruments, England) coupled to the SEM.
Micro-computer tomography (MicroCT) was conducted at the Lawrence Berkeley National Laboratory Advanced Light Source (ALS) synchrotron at Beamline 8.3.215. The ALS operates at a ring current of 500 mA. An X-ray energy of 30 keV was selected using a multilayer monochromator (chosen to be above the 25 keV absorption edge of silver), with a one mm aluminum filter to prevent leak through of lower energies. These data were collected by imaging a Ce:LuAG scintillator with an optical Mitutoyo 5X lens onto a 14-bit scientific sCMOS camera with 2560 × 2160 pixel area with six µm pixels (PCO, Germany), yielding 1.3 µm effective pixel size and an estimated 3.3 mm horizontal field of view. For each scan, a total of either 1025 or 2049 projections were acquired over a 180-degree scan range. Samples were scanned separately without and with phase contrast by placing the detector within ten cm or at two meters from the sample tooth to optimize accuracy and physical edge detection (e.g. between peritubular dentin and empty dentinal tubules), respectively. Reconstruction was carried out using a custom ImageJ plugin for image preprocessing and Octopus Imaging Software (Inside Matters, Belgium) for tomographic reconstruction. Reconstructed images were processed and compiled using Avizo 9.0 (FEI, USA) at the ALS Visualization Lab.
Results
Optical Light Microscopy
Light microscopy of sectioned the exemplar SDF-treated tooth showed black stain where SDF absorbed and oxidized (Figure 1A), coating the dentin lesion outer surface and extending 700 µm pulpally, as elongated striations matching the orientation of the dentinal tubules. The pulpal extent of the stain follows a pattern in the orientation of the dentinal tubules, although not all tubules are filled (Figure 1A inset).
Figure 1. Microwires form in dentinal tubules following SDF treatment of caries lesions.

A. Light imaging of primary tooth with natural caries lesion treated with SDF before extraction shows black and brown stains suggestive of silver deposition (5X, inset corresponds to white box: 30X). B. Synchrotron microCT imaging of the region of dentin outlined by the yellow box in panel A shows densities far greater than that of enamel, with a highly dense layer at the lesion surface, and structures of varying density extending pulpally. Volume rendering shown from occlusal, with visualization threshold set to show structures denser than dentin (100 µm scale bar). Colors in panel B denote increased density in order: yellow (density of enamel), orange, red, white. Arrows points to region of interest with silver microwires. All images were taken of same area of same tooth section.
Advanced Microscopy: Scanning electron microscopy and synchrotron X-ray micro-CT
Synchrotron microCT revealed silver densities far greater than that of enamel in every scanned tooth, matching the distribution and shape of stains observed by light microscopy. A highly dense, less than ten µm thick layer, coats the surface (Figure 1B and Figure 2), with some discontinuities. Structures of varying density extend up to 2,100 µm pulpally from the arrested lesion surface, with most ending in cylindrical forms of intense uniform density (Figure 1B). In the exemplar tooth, densities between that of enamel and dentin fills the first 175 µm (Figure 1B). Rendering to show only densities above that of enamel revealed irregular solids projecting generally in the orientation of dentinal tubules and bridging across some tubules presumably broken down by the caries process (Figure 1B and Figure 2).Uniform filamentous densities ranging from 50 to 2100 µm in length (maximum for each tooth: 2,100; 1,700; 1,100; 500; 200; 150; and 50 µm) and 2.5 to seven µm in diameter, present near each other in structures of bundled “microwires.” These novel structures have not been reported before in literature and are in contrast to the particles observed by other groups7,9. It is widely assumed that the silver component in SDF precipitates out as particles; however, we observe another phase of silver assuming the shape of the dentinal tubules.Less regular solids throughout the lesion range from five-500 µm long. By re-examining these areas with a phase contrast X-ray setup, whereby the edges of dentinal tubules are further contrasted and highlighted, it is confirmed that the densities increase and do indeed follow the course of the dentinal tubules towards the pulp (Figure 1).
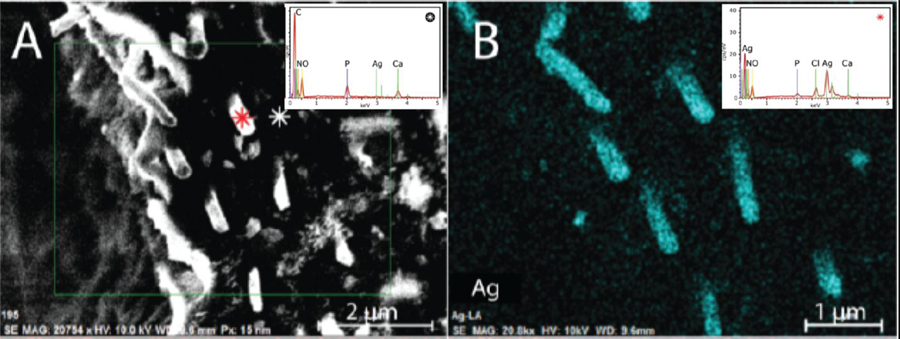
Figure 2. SDF-induced microwires are made of silver and chlorine.
SEM-EDS imaging of a cross-section orthogonal to the dentinal tubules, showing A. the surface (electron density; 2 µm scale bar), B. silver (spectral composition, 1 µm scale bar). Insets show EDS spectral traces for the positions corresponding to the red, and white asterisks in panel A. The microwires are comprised by approximately four parts silver to one part chlorine. keV: kilo-electron volts, obs: observations, cps counts per second.
Discussion
These results demonstrate the penetration of SDF to be three times further into lesions than was previously thought11. This is approximately the median of samples we have analyzed thus far, which range from 0.3 to 2.1 µm. These silver structures were not observed despite previous attempts to image treated dentin using microCT, including studies demonstrating prevention of demineralization7,16 or promotion of remineralization, and evaluation of clinically treated successfully arrested teeth17. The previous limitation of microCT appears to be the resolution; near-micrometer pixel sizes (1.3 µm) were necessary to image these silver microwires. Indeed, teeth not treated with SDF imaged with the same microCT do not show these filamentous silver microwires (e.g. Supplemental Figure S1), nor does dentin distant from the treatment area in SDF-treated teeth (Supplemental Figure S2 panel A). The shape and size of these microwires may enable distribution of forces across the dentin, reinforcing the lesion like rebar in cement. An analogous observation has been made for peritubular dentin, increasing hardness and stiffness of dentin with its more dense form lining the dentinal tubules18,19. However, the physical relationship of the wires to dentin substructures is not visible at this resolution (Supplemental Figures S2 panel B, S3 panel A).
SEM confirmed this novel observation of microwires in the exemplar tooth, protruding from tubules near a fracture in the dentin in a deep cross-section (Figure 2A), 250 nm in diameter - significantly smaller than observed using microCT. The differential electron density of silver enables application of high-energy X-ray and electron imaging techniques to probe the effect of SDF on treated tooth structures with sub-micrometer spatial resolution. Yet the observation of three-to-seven µm diameter microwires by microCT, amidst detection of 250 nm microwires by SEM suggests that many thin microwires are below the detection limit of microCT with the settings used here, perhaps more of these microwires exist in the tooth structure.
While silver is the only element that can account for the densities observed in the microwires, the exact material composition remained unclear. Examination of the microwires with EDS measurements coupled with SEM (SEM-EDS) revealed primarily silver composition (Figure 2B and inset), and not calcium, carbon, fluoride, oxygen, nitrogen, nor phosphorus (Supplemental Figure S4). Overlay of silver, calcium, and carbon shows the wires descend into the tubules surrounded by highly calcified peritubular dentin (Supplemental Figure S4 panel H). Spectral characterization showed the microwires are comprised by silver and chlorine (Figure 2B and inset), determined by ratiometric analysis to be approximately four parts to one, respectively. While features of the dentin can be distinguished with SEM measurements coupled with EDS (Supplemental Figure S3 panel B), more sensitive methods are required to determine the physical relationship of the wires to dentin substructures.
In summary, silver microwires 50–2,100 µm long and 0.25–7.0 µm in diameter are cast in situ in dentinal tubules by treatment of dental caries lesions with SDF. Diverse morphologies of the microwires are observed down to 2,100 µm from the surface (700 µm in the tooth shown), seeming to expand throughout dentinal tubules that were broken down by the caries process. The microwires may provide a reservoir of silver for antimicrobial action, prevent fluid flow through tubules, and increase hardness of the lesion as reported clinically. Hardness can arise from a densification of the soft matrix and the microwires distributing forces from the lesion throughout the intact dentin. Fluid flow in tubules causes pain, and SDF clinically decreases sensitivity20.
Conclusion
SDF clinically acts across several length-scales to treat dental caries lesions.
At the level of the lesion, SDF reinforces the lesion, perhaps by silver complexes forming in the voids left by the carious process and the densification of the remaining organic matrix, instead of fluoride-driven remineralization as reported previously8. This hypothesis is contrary to previous assumptions that fluoride strengthens the weakened tooth structure and silver merely acts as an antimicrobial agent.
At the level of the tooth microstructure, we observe that SDF intercalates into adjacent interfaces, even in non-caries affected areas, as seen in the case of neighboring dentinal tubules and carious areas closer to the pulp (Supplemental Figure S5). Comparison of these dentinal tubules in SDF and non-SDF affected states indicates that the silver does not alter the microstructure, but rather fills in the spaces where intra-dentinal tubule fluid previously existed. This possible mechanism indicates that SDF pervades these structural spaces like a highly hydrophilic compound in the treated tooth.
Subsequently, SDF undergoes a phase transition within the treated tooth from liquid to solid silver under the spatial confines of the tooth microstructure.
Further work is needed to assess the correspondence of silver microwire physico-chemical features to clinical outcomes. Structural characteristics such as microwire dimensions or distribution across tubules may be predictive of treatment success and can be observed in other teeth (Supplemental Figure S5). It is pertinent to further characterize the nano-biomechanical features of these silver structures to assess their individual contribution to the “hardening” effect. Such measurements will be critical, given that there are presently no parameters to guide clinical protocols for maximizing effectiveness of treatment.
Supplementary Material
Figure S1. Silver microwires are not observed in teeth not treated with silver diamine fluoride. A portion of a tooth scanned and rendered using the same settings and instruments shows an absence of high density structures.
Figure S2. No high-density structures are observed in unaffected dentin underlying sound enamel. A. Zoom out from Figure 1 panel C (yellow square in Figure 1 panel A) with dentin rendered in gray to demonstrate complete absence of high density in areas of dentin not reached by SDF (yellow arrow). B. Cross-sectional slice of microCT showing context of wires; the resolution is not sufficient to determine the relationship with or state of the peritubular dentin.
Figure S3. Features of dentin with microCT and SEM images. Images of the same tooth show features of the dentin in which the microwires are observed. A. A cross-sectional view of the microCT image shows that features such as peritubular dentin may be beyond the resolution used here. B. Using a lower accelerating voltage (six keV) than that used to show the silver microwires (ten keV) shows differential charging of peritubular dentin (yellow arrow), versus the surrounding intertubular dentin (yellow triangle). The green arrow denotes the area in which the silver microwires are observed with higher accelerating voltage.
Figure S4. Elaboration of elemental characterization of SDF-induced microwires. SEM-EDS imaging as in Figure 2, showing A. the surface (electron density; two µm scale bar), B. silver, C. calcium, D. phosphorus, E. carbon, F. oxygen, G. nitrogen, and H. overlay of B-G (one µm scale bars). Peritubular dentin is observed in the tubules as high levels of calcium (red, panel C) and phosphorus (green, panel D).
Figure S5. The observation of SDF microwires in other pediatric cases with synchrotron X-ray tomography. A-C Diverse 2D tomograms demonstrating the overview of SDF microwires with respect to the native tooth structure in different pediatric teeth [scale-bar = one mm].
Acknowledgements
The Authors thank Cate Quas and Steve Duffin for clinical samples and acknowledge Howard Barnard (Advanced Light Source, Berkeley, CA) for technical assistance and Alireza Sadr (University of Washington) for help with translation of references. This work was supported, in part, by NIH NIDCR grant T32-DE007306 and the User Programs at the Advanced Light Source and the Molecular Foundry at the Lawrence Berkeley National Laboratory. This research utilized resources of the Advanced Light Source and the Molecular Foundry, which are DOE Office of Science User Facilities under contract no. DE-AC02–05CH11231.
Footnotes
The Authors declare no conflicts of interest related to the presented work.
References
- 1.Featherstone J. The continuum of dental caries--evidence for a dynamic disease process. J Dent Res 2004;83(Spec No C):C39–C42. [DOI] [PubMed] [Google Scholar]
- 2.Gao S, Zhang S, Mei M, Lo E, Chu C. Caries remineralisation and arresting effect in children by professionally applied fluoride treatment - a systematic review. BMC Oral Health. 2016;16(12):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crystal Y, Marhalani A, Ureles S, et al. Use of Silver Diamine Fluoride for Dental Caries Management in Children and Adolescents, Including Those with Special Health Care Needs. Pediatr Dent 2017;39(5):135–145. [PubMed] [Google Scholar]
- 4.Slayton R, Urquhart O, Araujo M, et al. Evidence-based clinical practice guideline on nonrestorative treatments for carious lesions: A report from the American Dental Association. J Am Dent Assoc 2018;149(10):837–849. [DOI] [PubMed] [Google Scholar]
- 5.Horst J, Ellenikiotis H, Milgrom P. UCSF Protocol for Caries Arrest Using Silver Diamine Fluoride: Rationale, Indications and Consent. J Calif Dent Assoc 2016;44(1):16–28. [PMC free article] [PubMed] [Google Scholar]
- 6.Van Loveren C. Antimicrobial activity of fluoride and its in vivo importance: identification of research questions. Caries Res 2001;35(Suppl 1):65–70. [DOI] [PubMed] [Google Scholar]
- 7.Mei M, Ito L, Cao Y, Li Q, Lo E, Chu C. Inhibitory effect of silver diamine fluoride on dentine demineralisation and collagen degradation. J Dent 2013;41(9):809–817. [DOI] [PubMed] [Google Scholar]
- 8.Zhi Q, Lo E, Kwok A. An in vitro study of silver and fluoride ions on remineralization of demineralized enamel and dentine. Aust Dent J 2013;58(1):50–56. [DOI] [PubMed] [Google Scholar]
- 9.Chu C, Lo E. Microhardness of dentine in primary teeth after topical fluoride applications. J Dent 2008;36(6):387–391. [DOI] [PubMed] [Google Scholar]
- 10.Lou Y, Botelho M, Darvell B. Reaction of silver diamine fluoride with hydroxyapatite and protein. J Dent 2011;39(9):612–618. [DOI] [PubMed] [Google Scholar]
- 11.Shah S, Bhaskar V, Venkatraghaven K, Choudhary P, Ganesh M, Trivedi K. Silver Diamine Fluoride: A Review and Current Applications. J Adv Oral Res 2014;5(1):25–35. [Google Scholar]
- 12.Zander H, Burrill D. The Penetration of Silver Nitrate Solution into Dentin. J Dent Res 1943;22(2):85–89. [Google Scholar]
- 13.Yamaga R, Nishino M, Yoshida S, Yokomizo I. Diamine silver fluoride and its clinical application. J Osaka Univ Dent Sch 1972;12:1–20. [PubMed] [Google Scholar]
- 14.Shimooka S. On the penetration of silver nitrate and ammoniacal silver fluoride into microstructure of the sound dentin. Shigaku. 1972;59(6):534–566. [PubMed] [Google Scholar]
- 15.MacDowell A, Parkinson D, Haboub A, et al. X-ray micro-tomography at the Advanced Light Source. SPIE Proceedings. 2012;8506(18):1–14. [Google Scholar]
- 16.Liu B, Lo E, Li C. Effect of silver and fluoride ions on enamel demineralization: a quantitative study using micro-computed tomography. Aust Dent J 2012;57(1):65–70. [DOI] [PubMed] [Google Scholar]
- 17.Mei M, Ito L, Cao Y, Lo E, Li Q, Chu C. An ex vivo study of arrested primary teeth caries with silver diamine fluoride therapy. J Dent 2014;42(4):395–402. [DOI] [PubMed] [Google Scholar]
- 18.Weiner S, Veis A, Beniash E, et al. Peritubular dentin formation: crystal organization and the macromolecular constituents in human teeth. J Struct Biol 1999;126(1):27–41. [DOI] [PubMed] [Google Scholar]
- 19.Zaslansky P, Friesem A, Weiner S. Structure and mechanical properties of the soft zone separating bulk dentin and enamel in crowns of human teeth: insight into tooth function. j Struct Biol 2006;153(2):188–199. [DOI] [PubMed] [Google Scholar]
- 20.Castillo J, Rivera S, Aparicio T, et al. The short-term effects of diammine silver fluoride on tooth sensitivty: a randomized controlled trial. J Dent Res 2011;90(2):203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Silver microwires are not observed in teeth not treated with silver diamine fluoride. A portion of a tooth scanned and rendered using the same settings and instruments shows an absence of high density structures.
Figure S2. No high-density structures are observed in unaffected dentin underlying sound enamel. A. Zoom out from Figure 1 panel C (yellow square in Figure 1 panel A) with dentin rendered in gray to demonstrate complete absence of high density in areas of dentin not reached by SDF (yellow arrow). B. Cross-sectional slice of microCT showing context of wires; the resolution is not sufficient to determine the relationship with or state of the peritubular dentin.
Figure S3. Features of dentin with microCT and SEM images. Images of the same tooth show features of the dentin in which the microwires are observed. A. A cross-sectional view of the microCT image shows that features such as peritubular dentin may be beyond the resolution used here. B. Using a lower accelerating voltage (six keV) than that used to show the silver microwires (ten keV) shows differential charging of peritubular dentin (yellow arrow), versus the surrounding intertubular dentin (yellow triangle). The green arrow denotes the area in which the silver microwires are observed with higher accelerating voltage.
Figure S4. Elaboration of elemental characterization of SDF-induced microwires. SEM-EDS imaging as in Figure 2, showing A. the surface (electron density; two µm scale bar), B. silver, C. calcium, D. phosphorus, E. carbon, F. oxygen, G. nitrogen, and H. overlay of B-G (one µm scale bars). Peritubular dentin is observed in the tubules as high levels of calcium (red, panel C) and phosphorus (green, panel D).
Figure S5. The observation of SDF microwires in other pediatric cases with synchrotron X-ray tomography. A-C Diverse 2D tomograms demonstrating the overview of SDF microwires with respect to the native tooth structure in different pediatric teeth [scale-bar = one mm].