Abstract
In this issue of Cell Stem Cell, Fumagalli et al. (2020) employ intravital microscopy of colorectal cancer organoid xenografts to investigate the cell of origin of metastases. While tumor-initiating cells are Lgr5+, most disseminated cancer cells are Lgr5− and seed liver metastases in which Lgr5+ cells then appear, showing that bidirectional plasticity of phenotypic states drives metastasis.
The cancer stem cell (CSC) hypothesis posits that tumor initiation and growth are driven by a small number of cells with indefinite self-renewal and differentiation capability (Reya et al., 2001). This model suggests an explanation for the regeneration of tumors following therapy from residual disease. It also evokes the attractive possibility of eliminating advanced, heterogeneous tumors by defining and targeting the critical requirements of a relatively homogeneous and fixed CSC population. However, whether CSCs truly represent static cell fates, or whether cancer cells may dynamically enter and exit CSC phenotypes, remains unclear. In this issue of Cell Stem Cell, Fumagalli et al. (2020) use intravital multiphoton microscopy to illuminate a dynamic plasticity between CSC and non-CSC differentiated states that is required to drive colorectal cancer metastasis.
In mouse models, cells expressing the Leucine-rich repeat-containing G protein-coupled receptor 5 (Lgr5) have been shown to act both as the homeo-static stem cells of the intestinal epithelium (Barker et al., 2007) and as the cell of origin for colorectal cancer (CRC) (Barker et al., 2009). The initiation of metastasis by disseminated single cells or small clusters, on the other hand, has parallels with tissue regeneration following epithelial injury, during which intestinal epithelial cells demonstrate tremendous plasticity (Ganesh et al., 2020). When epithelial integrity is disrupted by injury or Lgr5+ cell ablation, a wide range of differentiated Lgr5− cell types can drive wound repair by first transdifferentiating into a stem-like Lgr5+ state (de Sousa E Melo and de Sauvage, 2019; Murata et al., 2020). Clarifying the continued requirement for Lgr5+ cells in propagating metastasis in distant organs is of tremendous therapeutic significance since metastatic disease causes the vast majority of cancer deaths and is the target of most anti-cancer drugs.
The continued requirement for Lgr5+ CSCs in metastatic disease has only recently been explored. Using a diphtheria-toxin-mediated approach to genetically ablate Lgr5DTR cells, de Sousa e Melo and colleagues showed that selective depletion of Lgr5+ cells in primary tumors causes tumor stasis, but such tumors can be rapidly replenished by dedifferentiation of Lgr5− cells upon diphtheria toxin withdrawal (de Sousa e Melo et al., 2017). In contrast, the formation and maintenance of liver metastases required Lgr5+ cells, with little metastatic regrowth up to 2 weeks after diphtheria toxin withdrawal. These findings suggested a crucial requirement for Lgr5+ cells for metastasis. However, Lgr5+ cell depletion did not limit cancer cell invasion, leaving open the possibility that, as during wound healing, metastasis may involve passage through an Lgr5− state.
Fumagalli et al. now elucidate a functionally consequential dynamic plasticity between Lgr5− and Lgr5+ phenotypes that is required to drive CRC metastasis (Figure 1). They isolated murine CRC organoids harboring onco-genic mutations in APC, KRAS, and TP53 that also expressed RFP-Confetti and Lgr5DTR/eGFP fluorescent reporters. Orthotopic cecal transplantation of these organoids led to the formation of single primary tumors that spontaneously metastasized to the liver and lung. Although eGFP+Lgr5+ cells sorted from both organoids and primary cecal tumors re-initiated organoid growth with higher efficiency than eGFP−Lgr5− cells, intravital microscopy revealed that the vast majority of disseminating cancer cells migrating into the surrounding stroma, migrating into the portal venous circulation, or seeding liver metastasis were in fact Lgr5−. By performing in vivo microscopy of liver metastases, they found that while micrometastases initially contained only eGFP−Lgr5− cells, some Lgr5− cells underwent plasticity and gained Lgr5eGFP expression. Importantly, selective ablation of Lgr5DTR/eGFP cells in early metastases inhibited further growth and ultimately led to the regression of Lgr5− metastases, as also shown by de Sousa E Melo et al. To demonstrate that such cell state plasticity occurs in human CRC, the authors imaged xenograft tumors derived from human CRC organoids carrying a fluorescent Ascl2 reporter that has been previously shown to label Lgr5+ CSCs (Oost et al., 2018). Similar to the murine organoids, human Lgr5− cells were more efficient at disseminating from orthotopic cecal tumors and seeding metastases that also contained Lgr5+ cells.
Figure 1.
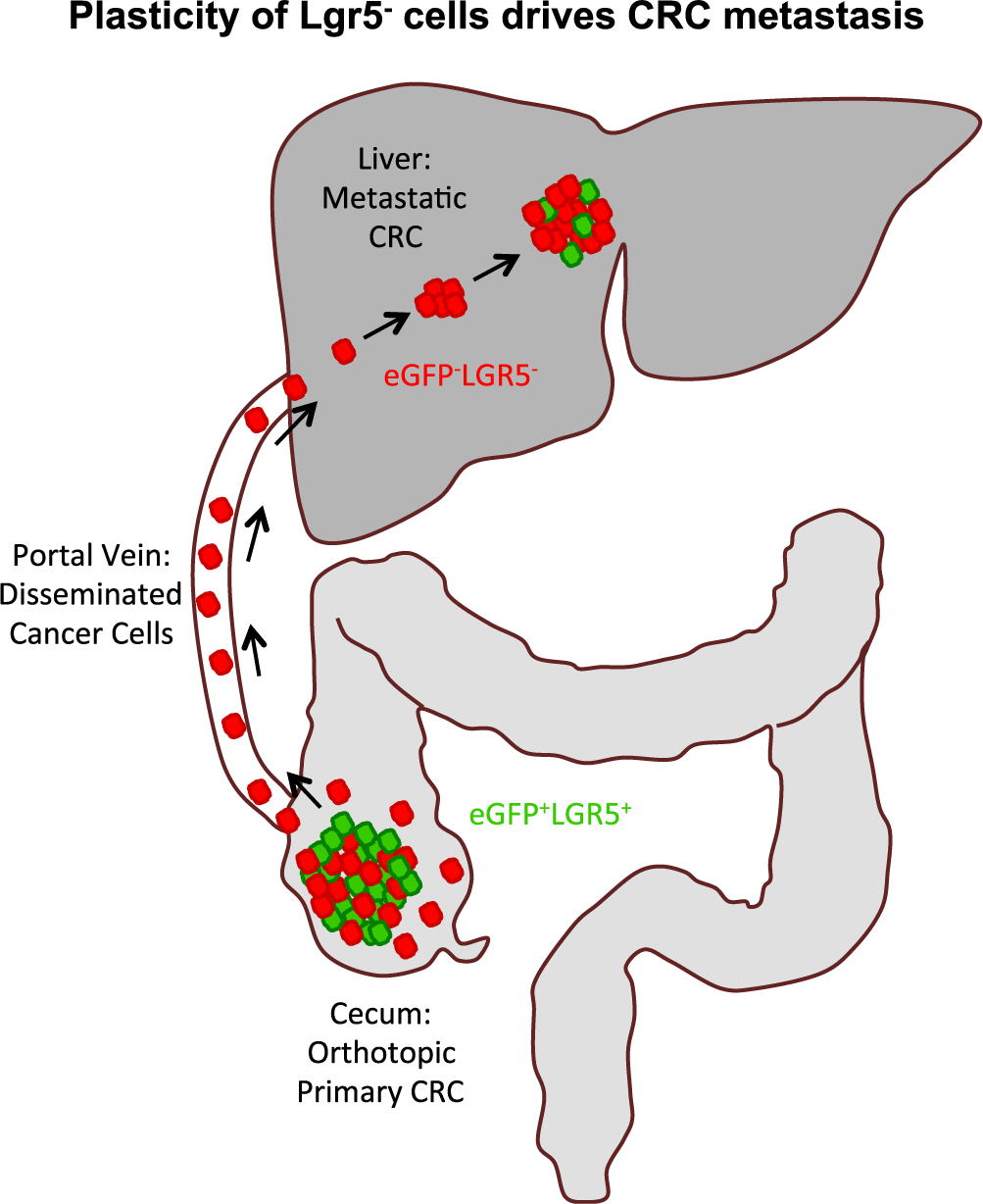
Plasticity of Lgr5– Cells Initiates Colorectal Cancer Metastasis Organoid-derived orthotopic tumor xenografts are initiated by eGFP+Lgr5+ cells (green), which generate eGFP−Lgr5− (red) differentiated progeny. The majority of cells invading the stroma, disseminating via the portal vein, or seeding liver metastasis are eGFP−Lgr5− (red). Liver metastasis is initiated by eGFP−Lgr5− (red) cells, a subset of which undergo plasticity to generate eGFP+Lgr5+ cells (green). Diphtheria-toxin-driven ablation of Lgr5DTR/eGFP cells causes collapse of orthotopic cecal tumors and regression of liver metastases. Bidirectional plasticity of Lgr5 cells is required to initiate and propagate CRC metastases.
These findings in the mouse are consistent with recent observations in clinical samples that patient CRC metastases and organoids are initiated by Lgr5lowL1CAMhigh cells that are dispensable in primary tumors, but emerge dynamically from Lgr5high primary tumors upon the loss of epithelial integrity during self-disruptive tumor invasion and are required for the survival of disseminated cancer cells and re-initiation of growth in distant organs (Ganesh et al., 2020). Human tumors typically become progressively “poorly differentiated” over time, bearing less and less morphological resemblance to the normal tissue from which they are derived. Whether advanced human cancers remain dependent on re-entry into the Lgr5+ state for tumor propagation, or whether they have evolved alternate Lgr5-independent pathways to sustain regrowth, remains to be determined.
Fumagalli et al. provide direct evidence for the existence and importance of cell state plasticity in driving CRC metastasis. The results identify two discrete stages required for successful metastasis: first, plasticity of the Lgr5+ tumor-initiating state into an Lgr5− state required for migration, survival during dissemination, and metastatic seeding; and second, reversion of Lgr5− cells into an Lgr5+ state to sustain expansion of nascent metastatic seeds into macrometastatic colonies. These findings further under-score the close relationship between wound healing and metastasis—both conditions in which detached cells must first activate emergency responses to survive the loss of an intact epithelial niche and next regenerate functional epithelial structures. Whether Lgr5−/Lgr5+ plasticity also occurs during cancer treatment, when epithelial structures within tumors are again disrupted, is not clear. The recognition of dynamic Lgr5−/Lgr5+ plasticity in multiple contexts suggests that at least some tissue stem cells exist not in a hierarchy of line-age-defined static cell fates, but in a dynamic hierarchy of plastic cell states. The extent to which Lgr5−/Lgr5+ plasticity is driven by shared signaling pathways and epigenetic programs during wound healing and metastasis remains to be un-covered. Intriguingly, the two-stage Lgr5−/Lgr5+ transition is reminiscent of the EMT/MET transition also associated with metastatic progression (Shibue and Weinberg, 2017). Future studies may elucidate the relationships between these two forms of metastatic plasticity.
Devising therapeutic strategies to contain highly plastic metastatic tumors is daunting, but one promising approach may be to identify and target critical, shared bottlenecks in the metastatic cascade, such as surviving the stress of epithelial detachment. At present it is unclear whether Lgr5+ and Lgr5− states themselves represent an aggregate of multiple sub-states, each of which might be different in human tumors with different genotypes or clinical characteristics. Large-scale human tumor phenotyping efforts are required for the identification of conserved states in metastasis that can then be interrogated to define their therapeutic vulnerabilities. Notably, only a minority of Lgr5− cells reverted to an Lgr5+ state in growing metastases, but how plasticity is constrained to a select sub-population of cells is un-known. Investigating the molecular mechanisms and microenvironmental signals that can license some cancer cells to undergo metastatic plasticity may yield exciting new strategies for treating metastatic cancer.
REFERENCES
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, and Clevers H (2007). Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007. [DOI] [PubMed] [Google Scholar]
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, and Clevers H (2009). Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457, 608–611. [DOI] [PubMed] [Google Scholar]
- de Sousa E Melo F, and de Sauvage FJ (2019). Cellular Plasticity in Intestinal Homeostasis and Disease. Cell Stem Cell 24, 54–64. [DOI] [PubMed] [Google Scholar]
- de Sousa e Melo F, Kurtova AV, Harnoss JM, Kljavin N, Hoeck JD, Hung J, Anderson JE, Storm EE, Modrusan Z, Koeppen H, et al. (2017). A distinct role for Lgr5+ stem cells in primary and metastatic colon cancer. Nature 543, 676–680. [DOI] [PubMed] [Google Scholar]
- Fumagalli AO, Oost KC, Kester L, Morgner J, Bornes L, Bruens L, Spaargaren L, Azkanaz M, Schelfhorst T, Beerling E, et al. (2020). Plasticity of Lgr5-negative cancer cells drives metastasis in colorectal cancer. Cell Stem Cell 26, this issue, 569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh K, Basnet H, Kaygusuz Y, Laughney AM, He L, Sharma R, O’Rourke KP, Reuter VP, Huang Y-H, Turkekul M, et al. (2020). L1CAM defines the regenerative origin of metastasis-initiating cells in colorectal cancer. Nat. Cancer 1, 28–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata K, Jadhav U, Madha S, van Es J, Dean J, Cavazza A, Wucherpfennig K, Michor F, Clevers H, and Shivdasani RA (2020). Ascl2-Dependent Cell Dedifferentiation Drives Regeneration of Ablated Intestinal Stem Cells. Cell Stem Cell 26, 377–390 e376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oost KC, van Voorthuijsen L, Fumagalli A, Lindeboom RGH, Sprangers J, Omerzu M, Rodriguez-Colman MJ, Heinz MC, Verlaan-Klink I, Maurice MM, et al. (2018). Specific Labeling of Stem Cell Activity in Human Colorectal Organoids Using an ASCL2-Responsive Minigene. Cell Rep. 22, 1600–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, and Weissman IL (2001). Stem cells, cancer, and cancer stem cells. Nature 414, 105–111. [DOI] [PubMed] [Google Scholar]
- Shibue T, and Weinberg RA (2017). EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat. Rev. Clin. Oncol 14, 611–629. [DOI] [PMC free article] [PubMed] [Google Scholar]


