Abstract
Objective:
To evaluate YKL-40 and MMP-9 proteins as tumor biomarkers in serum samples from patients with primary central nervous system lymphoma (PCNSL).
Methods:
In this prospective longitudinal study, serum samples from consecutive patients with histologically confirmed PCNSL were collected concurrently with magnetic resonance imaging (MRI) scans at multiple time points and were analyzed for levels of YKL-40 and MMP-9 by enzyme-linked immunosorbent assay. Marker levels were correlated to disease status and survival.
Results:
Forty-five patients with PCNSL were accrued. Median follow-up for survivors was 25 months, and 21 (47%) died during the study. A total of 230 serum samples were collected, and 93% had corresponding MRI scans. PCNSL patients without evidence of radiographic disease (29 patients, 131 samples) had significantly lower levels of serum YKL-40 and MMP-9 than patients with active tumor (n = 34 patients, 84 samples; p = 0.03 and 0.01, respectively). There was a significant inverse correlation between survival and doubling of the YKL-40 level (hazard ratio, 1.7; p = 0.01).
Interpretation:
In patients with PCNSL, serum levels of YKL-40 and MMP-9 are associated with radiographic disease status. Longitudinal increase in serum levels of YKL-40, but not MMP-9, predicts survival in patients with PCNSL.
Primary central nervous system lymphoma (PCNSL) is a non-Hodgkin lymphoma usually of B-cell origin that is confined to the central nervous system at presentation and may involve the brain, leptomeninges, and eyes at diagnosis. It is rare, accounting for only 3 to 5% of newly diagnosed primary brain tumors.1 Although median survival ranges from 25 to 51 months, treatment for PCNSL may be potentially curative, but must be intensive and carries a significant risk of treatment-associated neurotoxicity.2 Age and performance status at diagnosis have been consistently identified as predictors of outcome for PCNSL.2 Despite this, treatment recommendations are uniformly applied to all patients, which may result in under- or overtreatment for some patients.
In addition to clinical prognostic factors, pathologic prognostic factors are beginning to emerge. Overexpression of BCL6 and reduced expression of STAT6 and/or activated STAT6, both involved in control of the apoptotic cascade, have been associated with improved survival.3–6 The presence of a reactive perivascular T-cell infiltrate was also correlated with a better outcome.7 Loss of heterozygosity on chromosome 6q was associated with worse survival in PCNSL patients compared to those with an intact 6q.8 These molecular analyses must be performed on tissue, which is a disadvantage, as most patients with PCNSL undergo only a biopsy and have limited tissue available for evaluation. Serum markers that correlate with disease status would obviate this difficulty. One example of a serum marker that may have utility in this regard is increased serum lactate dehydrogenase (LDH), which has been associated with poor outcome by some groups,9,10 but this finding could not be confirmed in larger cohorts.2
An essential component of the aggressiveness of PCNSL is its propensity for tissue infiltration, which requires degradation and remodeling of the extracellular matrix; proteins involved with or released into the circulation during tissue breakdown may be biomarkers for this disease.11 YKL-40 and MMP-9 (matrix metalloproteinase) are 2 important mediators of tissue remodeling.12 YKL-40, also known as chitinase 3—like 1 (CHI3L1), and human cartilage glycoprotein 39 (HCgp39), is a glycoprotein associated with the extracellular matrix, but its function remains unknown. It is overexpressed by cancer cells arising from brain, systemic organs, and lymph nodes.13,14 It has been found in tumor-associated macrophages, and is involved in inflammation-related carcinogenesis12,15 and vascular smooth muscle cell adhesion and migration.16 Elevated serum YKL-40 levels are an independent prognostic biomarker of short recurrence-free interval and survival in patients with acute myeloid leukemia and solid tumors.13 Proteomic analysis showed that YKL-40 was highly overexpressed in the cerebrospinal fluid (CSF) of central nervous system lymphoma patients.17 Recently, YKL-40 gene expression, as measured in microarray analysis from tumor tissue, and protein levels by immunohistochemical analysis were found to be significantly upregulated in PCNSL compared to systemic diffuse large B-cell lymphoma.11
MMP-9 belongs to the matrix metalloproteinases, a group of key enzymes involved in the degradation of the extracellular matrix during cellular invasion and permeability control of the blood-brain barrier.18–21 MMP-9 has been detected consistently by immunohistochemistry in PCNSL tissue.22 CSF MMP-9 levels determined by enzyme-linked immunosorbent assay (ELISA) correlate with active leptomeningeal disease in PCNSL and systemic lymphomas.23 Both YKL-40 and MMP-9 are secreted into the bloodstream and can be easily and reproducibly measured by ELISA.24,25
We hypothesized that serum levels of YKL-40 and MMP-9 will correlate with disease status and survival in PCNSL patients. To test these 2 proteins as biomarkers for PCNSL, we determined serum levels of YKL-40 and MMP-9 prospectively in consecutive patients with PCNSL at the time of their regularly scheduled neuroimaging follow-up.
Patients and Methods
Patient Characteristics
Serum samples were collected prospectively from all patients with histologically confirmed PCNSL who enrolled in the study from August 2002 to July 2008. Patients were followed and samples collected until January 2009. Patients were allowed to enroll at any time during the course of their illness. Patients with suspected PCNSL identified by imaging, who had not yet undergone diagnostic biopsy, were eligible for the study. Patients enrolled before their initial surgical procedure were retained in the study only after histologic confirmation of PCNSL. All pathologic samples were reviewed centrally at our institution. Patients with concurrent systemic lymphoma revealed during their evaluation were excluded from the study. All patients were treated with a high-dose methotrexate-based regimen usually in combination with vincristine, procarbazine, and rituximab followed by radiotherapy in younger patients and consolidation with high-dose cytarabine in all. Patients with concurrent inflammatory illness, acute infection, human immunodeficiency virus, or other types of cancer were excluded from participating in the study. The Memorial Hospital Institutional Review Board approved the study, and all patients signed an informed consent.
Study Design
In this prospective longitudinal study, serum samples and imaging studies were obtained at baseline and every 2 to 3 months when patients were evaluated for regular clinical follow-up. The protocol required that blood samples were collected within 1 month of imaging, but most samples were obtained the same day as magnetic resonance imaging (MRI). Serum levels of YKL-40 and MMP-9 were determined by ELISA (Quidel Corp., San Diego, CA and Quantikine, R&D Systems, Minneapolis, MN, respectively) as previously described.26 In certain solid cancers, determination of MMP-9 was influenced by the method of blood collection.25 Therefore, in a subset of patients, both serum and plasma levels of MMP-9 were obtained to eliminate any spurious increase in the value resulting from the contribution of MMP-9 released by platelets during clotting.25 Plasma samples were collected in heparinized tubes and centrifuged at 4 °C for 10 minutes at 1,300 rpm, and the supernatant was stored at −80°C. MRIs of the brain were performed with 1.5T GE scanners (GE Medical Systems, Milwaukee, WI). All MRI studies included fluid attenuated inversion recovery (FLAIR), T2-weighted, and T1-weighted images before and after administration of gadolinium contrast material (gadopentetate dimeglumine). Tumor size was determined uni- and bidimensionally on gadolinium-enhanced T1-weighted and FLAIR images by at least 2 of the authors (A.F.H, F.M.I., and S.K.), who were blinded in regard to serum marker levels and clinical outcome.27 Response was assessed according to standardized response criteria for PCNSL.28
Statistics
Disease status was defined as no evidence of radiographic disease (complete response [CR]) versus radiographic disease (combined partial response [PR], stable disease [SD], and progression of disease [POD]). To examine the relationship between marker and presence of disease, all measurements were used in a logit model with generalized estimating equations to correct for within-patient correlations.29 As the distributions for YKL-40 and MMP-9 were skewed, the data were log transformed before all statistical testing. Correlations between the markers and tumor size and between MMP-9 in serum and in plasma were calculated across patients using the Pearson correlation coefficient. Survival was defined as time from registration date to date of death or last follow-up. The effect of YKL-40 and MMP-9 on survival was analyzed using each marker separately as a time-dependent covariate on the log scale in a Cox proportional hazards model.
Results
Patients Cohort
A total of 45 patients (18 women and 27 men, 40%:60%) with PCNSL were enrolled (Table 1). Twenty-six patients (58%) were enrolled within the first 3 months of diagnosis and 21 (47%) within 1 month. The median age at diagnosis of PCNSL was 59 years (range, 24–81 years). Twelve (27%) patients were 50 years of age or younger. At registration, 80% of patients had a Karnofsky performance status (KPS) ≥70. The majority of patients (78%) underwent a biopsy for diagnosis of PCNSL, whereas 17% of patients had a surgical resection. Surgical information was missing in 2 patients. In all patients, pathology revealed diffuse large B-cell lymphoma. A total of 230 measurements of YKL-40 and MMP-9 were available, with a median of 4 samples per patient. Median follow-up for survivors was 25 months (range, 6–73 months), and 21 (47%) patients died during the study.
TABLE 1:
Patient Cohort
| Characteristic | Value |
|---|---|
| No. of patients | 45 |
| Gender | 18 F, 27 M |
| Age at diagnosis, yr | |
| Median (range) | 59 (24–81) |
| ≤50 | 12 (27%) |
| Baseline KPS | |
| Median (IQR) | 80 (70–90) |
| <70 | 8 (20%) |
| ≥70 | 33 (80%) |
| Missing | 4 |
| Extent of tumor resection | |
| Biopsy | 35 (78%) |
| Resection (complete or incomplete) | 8 (17%) |
| Unknown | 2 (4%) |
| No. of patients followed from initial diagnosis | 21 (47%) |
| Median mo from diagnosis to enrollment (range) | 1.3 (0–92) |
| Total No. of samples | 230 |
| Median No. samples per patient (range) | 4 (1–15) |
| No. of samples correlated to MRI | 215 (93%) |
| Median follow-up for survivors, mo (range) | 25 (6–74) |
| No. of patients who died during study follow-up | 21 (47%) |
KPS = Karnofsky performance status; IQR = interquartile range; MRI = magnetic resonance imaging.
Serum Levels of MMP-9 Correlate with Plasma Levels
Serum and plasma samples were collected simultaneously in 121 measurements. There was a strong correlation between serum and plasma MMP-9 values (r = 0.63, p < 0.0001), showing that in PCNSL patients MMP-9 levels did not change significantly by either method of blood collection. Therefore, all MMP-9 analyses in this study used serum measurements.
Correlation of Serum Level with Radiographic Disease
Over the course of the study, 29 patients had a radiologic CR as assessed on gadolinium enhanced T1-weighted images, 12 had a PR, 6 had SD, and 29 had POD. A total of 215 of the 230 measurements (93%) had a matching MRI of the brain, which was performed on the same day as the measurement in 50% of measurements, within the same week in 26%, and within the same month in 24%. Serum YKL-40 levels were significantly lower in samples corresponding to MRIs without radio-graphic evidence of disease (mean, 117 ng/ml; range, 21–450 ng/ml) compared to samples with corresponding radiographic evidence of disease (mean, 185 ng/ml; range, 25–1,472 ng/ml) (p = 0.03) (Fig, A). Similarly, MMP-9 levels were significantly lower in samples corresponding to MRIs that showed no evidence of disease (mean, 326 ng/ml; range, 75–1,200 ng/ml) compared to samples corresponding with MRIs showing active disease (mean, 648 ng/ml; range, 36–2,952 ng/ml) (p = 0.01) (see Fig, B). Nonenhancing FLAIR hyperintensity was also measured in 24 patients followed within the first year from time of diagnosis. Serum MMP-9 levels were significantly lower in samples corresponding to FLAIR MRIs without radiographic evidence of disease (mean, 249 ng/ml; range, 28–472 ng/ml) compared to samples with corresponding radiographic evidence of FLAIR abnormalities (mean, 579 ng/ml; range, 23–3,341 ng/ml) (p = 0.005). However, YKL-40 levels were not significantly different in samples corresponding to MRIs with no evidence of disease (mean, 124 ng/ml; range, 29–235 ng/ml) compared to samples corresponding to MRIs showing FLAIR lesions (mean, 648 ng/ml; range, 30–1,472 ng/ml) (p = 0.89).
FIGURE.
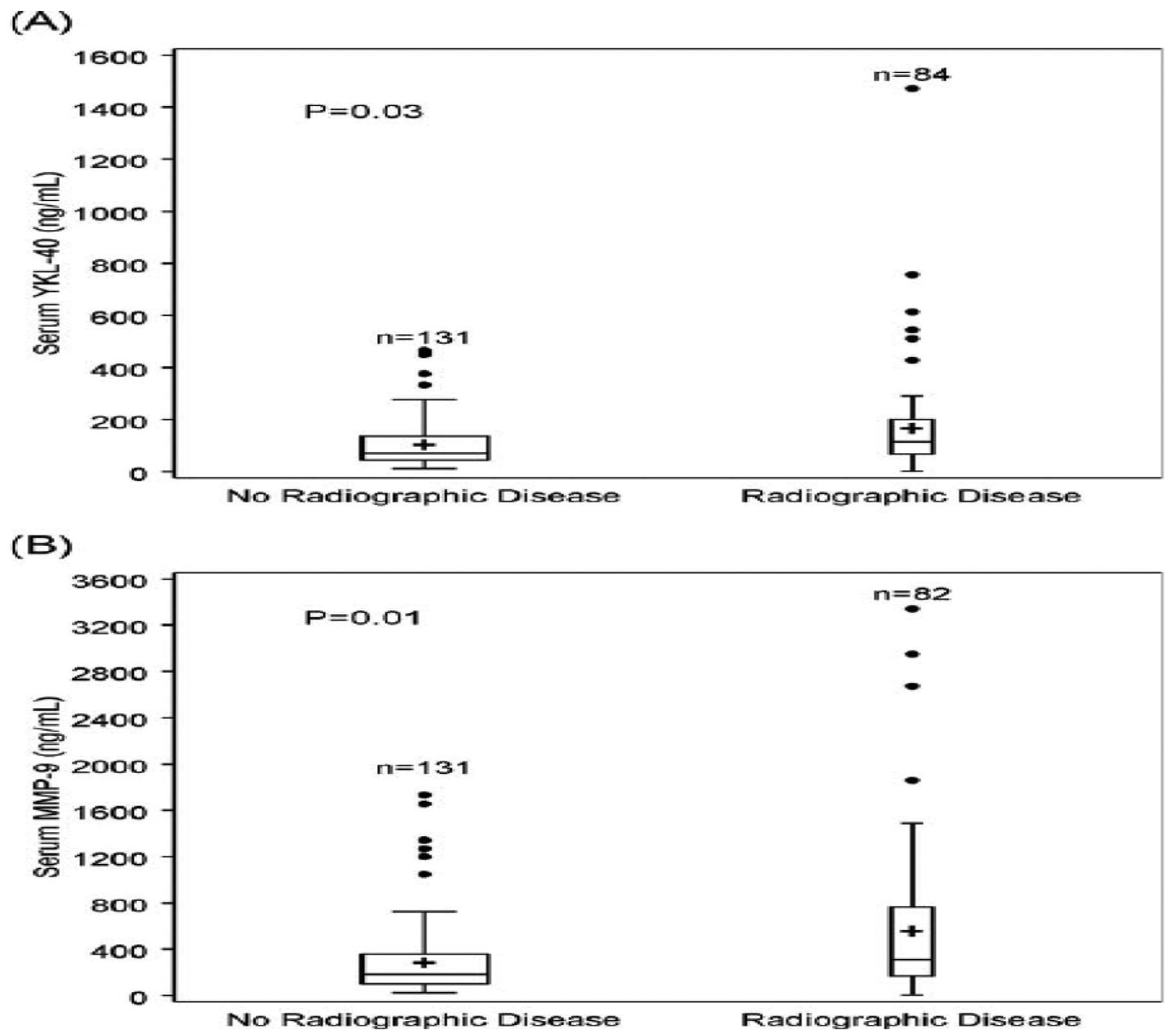
Box plots representing serum (A) YKL-140 and (B) MMP-9 values in complete response (no radiographic evidence of disease) versus presence of disease (partial response, stable disease, progression of disease). Serum YKL-40 and MMP-9 were significantly higher in patients with radiographic disease (p = 0.03 and p = 0.01, respectively). Upper line of boxes = 75th percentile; lower line of boxes = 25th percentile; + = mean; middle horizontal line = median. Two measurements were not performed for MMP-9.
Correlation of Serum Levels with Tumor Size
We evaluated whether serum levels of YKL40 and MMP-9 correlated with the size of contrast-enhancing disease and FLAIR on MRI (defined as either length or area of the tumor). There was no correlation between tumor size of contrast-enhancing disease and levels of YKL-40 (r = 0.13 for length, r = 0.09 for area). Tumor size correlated very weakly with MMP-9 levels for both unidimensional (r = 0.23) and bidimensional measurements (r = 0.20). Similarly, YKL-40 and MMP-9 correlated weakly with unidimensional (r = 0.12 and r = 0.24, respectively) and bidimensional measurements (r = 0.14 and r = 026, respectively) of FLAIR hyperintensity in 71 MRI scans of 24 patients who were included in the study from time of diagnosis to 1-year follow-up.
Correlation of Serum Levels with Survival
We evaluated whether serum levels of YKL-40 or MMP-9 correlated with survival in PCNSL patients. The median follow-up for survivors was 25 months (range, 6–74 months). The estimated 2-year survival from registration was 67% (95% confidence interval [CI], 51–79%). We looked at longitudinal changes in YKL-40 and MMP-9 serum markers (Table 2). A doubling in the value of YKL-40 was associated with a significant risk of death (hazard ratio [HR], 1.7; 95% CI, 1.2–2.4; p = 0.001). This association was not observed for MMP-9 (HR, 0.8; 95% CI, 0.6–1.1; p = 0.13).
TABLE 2:
Univariate Analysis of the Effect of Longitudinal Increases in Serum YKL-40 and MMP-9 on Survival
| Hazard Ratio (95% CI) | P | |
|---|---|---|
| YKL-40 per doubling in actual value | 1.7 (1.2–2.4) | 0.001 |
| MMP-9 per doubling in actual value | 0.8 (0.6–1.1) | 0.13 |
Number of patients = 45; deaths = 21. CI = confidence interval.
Discussion
We report on the clinical relevance of YKL-40 and MMP-9 as serum markers for patients with PCNSL, by measuring their values in 230 serial serum samples from 45 PCNSL patients with a median follow-up of 25 months. High serum levels of YKL-40 predicted worse survival in patients with PCNSL. A genome-wide gene expression array associated increased expression and activity of YKL-40 with active proliferation of malignant B cells.11 An immunohistological study showed positive nuclear staining of lymphoma cells for YKL-40 in 73% of PCNSL patients,11 suggesting heterogeneous subsets of the disease that may predict more aggressive forms carrying a worse prognosis. Our data demonstrate that elevated YKL-40 is associated with active disease on MRI and poor outcome. It is unknown if YKL-40 is simply a marker of tumor resistance or plays an intricate role in conferring that resistance. For instance, high expression of YKL-40 in tumor tissue of newly diagnosed glioblastoma correlated with tumor radioresistance and worse prognosis.30
Serum levels of MMP-9 were not associated with survival in our study. This is in contrast to patients with systemic non-Hodgkin lymphoma, in whom elevated serum levels of MMP-9 correlated with more aggressive disease and decreased survival.31–33 PCNSL is a subset of non-Hodgkin lymphoma, but whether it is a unique entity with a specific gene expression profile remains to be defined.34 Our data suggest that regulation of MMP-9 in PCNSL differs from what is found in systemic non-Hodgkin lymphoma, highlighting 1 biologic difference. However, these results may simply reflect variation of expression of MMP-9 by different cell types, such as tumor versus stromal cells in PCNSL and systemic lymphoma.22,35
We showed that increased serum levels of YKL-40 and MMP-9 were associated with the presence of active disease, and that low levels were associated with absence of disease as determined by MRI. The optimal cutpoint level of YKL-40 and MMP-9 to distinguish normal from elevated values has not been determined for PCNSL or any other cancer.36 As serum levels were measured repetitively over time in our patients, our analyses are based on longitudinal changes of serial YKL-40 and MMP-9 levels rather than a predetermined cutpoint. The association between serum levels of MMP-9 or YKL-40 and disease status has been reported in other cancers. In systemic non-Hodgkin lymphoma, successful therapy was associated with decreased levels of serum MMP-9.32 Serum YKL-40 levels allowed monitoring of response to therapy in gliomas and colorectal cancer.26,37
Although MMP-9 levels correlated with presence of tumor, there was only a weak correlation with tumor size on MRI as determined by gadolinium contrast enhancement. We found no correlation between tumor size on MRI scans and serum levels of YKL-40. This poor correlation may be due to a number of factors: First, MMP-9 and YKL-40 may be involved in pathogenic processes that are not directly linked to the mechanisms that lead to blood-brain barrier disruption and accumulation of gadolinium visualized as contrast enhancement on MRI. Second, it is possible that the relationship between tumor size and serum levels of our markers was confounded by the fact that not all PCNSLs express YKL-40 and MMP-9.11 Third, YKL-40 and MMP-9 expression by nonlymphoma cells may contribute to poor correlation between serum level and tumor size.11,35 Fourth, YKL-40 and MMP-9 are both enzymes that break down glycosamino-glycans and gelatin, respectively, in a catalytic but nonlinear fashion.13,38 The tumor may only need to secrete small amounts of these enzymes into the extracellular matrix to facilitate tumor progression. Therefore, tumor size and these biomarkers may be better correlated in a catalytic assay, like zymography for MMP-9. A substrate-based assay for YKL-40 is yet to be developed. Alternatively, the mechanisms by which these enzymes exert their tumoral effect may be independent of its direct catalytic activity.13,39
The correlative analysis of the biomarkers and infiltrative component of the tumor, as measured by nonenhancing FLAIR hyperintense areas, did not offer further insight into the degree of response. It is well established that FLAIR abnormalities can result from a number of confounding factors, such as ischemic injury, seizures, vasogenic edema, or treatment-related leukoencephalopathy.
The comparison of YKL-40 to serum LDH could have helped elucidate the usefulness of serum LDH as a prognostic factor, and resolve conflicting reports including 1 of ours.2,9,10 Later in our study, we tried to assay LDH on the patients’ stored serum samples that were used to determine YKL-40 and MMP-9. Unfortunately, those samples were old; we did not see variation of the values among the prospective samples, and concluded that they were no longer reliable to determine the enzymatic activity of the LDH. We also looked for prospective determination of LDH, but only a few patients had such a determination while on the study. Therefore, we do not feel confident in using a small number of samples for a comparative analysis. Certainly, LDH should be included in future studies together with the other 2 putative markers.
Our study includes a large number of patients with a rare disease accrued by a single institution and spanning an extended period of time, but the small number of events observed in our cohort precludes multivariate analysis to control for other known prognostic factors, such as age and KPS.2 Patients were captured throughout the course of their disease. This allowed for the evaluation of these markers in actual patient care conditions. However, as a result of this design, the patient population was not controlled in terms of the timing of their enrollment. All patients were treated with a methotrexate-based regimen, but patients were captured before or after chemotherapy, during or after radiotherapy or consolidation treatment, or even at recurrence, when another type of treatment may have been utilized. Therefore, evaluation of the markers in disease status or resistance to a specific form of treatment cannot be assessed specifically. Only a prospective study of these markers in a clinical trial will determine definitively whether these biomarkers are useful tools.
Acknowledgment
Supported by the Society for Memorial Sloan-Kettering Cancer Center (A.H.).
Footnotes
Potential Conflicts of Interest
Nothing to report.
References
- 1.Panageas KS, Elkin EB, DeAngelis LM, et al. Trends in survival from primary central nervous system lymphoma, 1975–1999: a population-based analysis. Cancer 2005;104:2466–2472. [DOI] [PubMed] [Google Scholar]
- 2.Abrey LE, Ben-Porat L, Panageas KS, et al. Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol 2006;24:5711–5715. [DOI] [PubMed] [Google Scholar]
- 3.Braaten KM, Betensky RA, de Leval L, et al. BCL-6 expression predicts improved survival in patients with primary central nervous system lymphoma. Clin Cancer Res 2003;9:1063–1069. [PubMed] [Google Scholar]
- 4.Levy O, Deangelis LM, Filippa DA, et al. Bcl-6 predicts improved prognosis in primary central nervous system lymphoma. Cancer 2008;112:151–156. [DOI] [PubMed] [Google Scholar]
- 5.Rubenstein JL, Shen A, Batchelor TT, et al. Differential gene expression in central nervous system lymphoma. Blood 2009;113: 266–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubenstein JL, Fridlyand J, Shen A, et al. Gene expression and angiotropism in primary CNS lymphoma. Blood 2006;107: 3716–3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ponzoni M, Berger F, Chassagne-Clement C, et al. Reactive perivascular T-cell infiltrate predicts survival in primary central nervous system B-cell lymphomas. Br J Haematol 2007;138:316–323. [DOI] [PubMed] [Google Scholar]
- 8.Weber T, Weber RG, Kaulich K, et al. Characteristic chromosomal imbalances in primary central nervous system lymphomas of the diffuse large B-cell type. Brain Pathol 2000;10:73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreri AJ, Blay JY, Reni M, et al. Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol 2003;21:266–272. [DOI] [PubMed] [Google Scholar]
- 10.Hayabuchi N, Shibamoto Y, Onizuka Y. Primary central nervous system lymphoma in Japan: a nationwide survey. Int J Radiat Oncol Biol Phys 1999;44:265–272. [DOI] [PubMed] [Google Scholar]
- 11.Tun HW, Personett D, Baskerville KA, et al. Pathway analysis of primary central nervous system lymphoma. Blood 2008;111: 3200–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hakala BE, White C, Recklies AD. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J Biol Chem 1993;268:25803–25810. [PubMed] [Google Scholar]
- 13.Johansen JS, Schultz NA, Jensen BV. Plasma YKL-40: a potential new cancer biomarker? Future Oncol 2009;5:1065–1082. [DOI] [PubMed] [Google Scholar]
- 14.Tanwar MK, Gilbert MR, Holland EC. Gene expression microarray analysis reveals YKL-40 to be a potential serum marker for malignant character in human glioma. Cancer Res 2002;62:4364–4368. [PubMed] [Google Scholar]
- 15.Rehli M, Krause SW, Andreesen R. Molecular characterization of the gene for human cartilage gp-39 (CHI3L1), a member of the chitinase protein family and marker for late stages of macrophage differentiation. Genomics 1997;43:221–225. [DOI] [PubMed] [Google Scholar]
- 16.Nishikawa KC, Millis AJ. gp38k (CHI3L1) is a novel adhesion and migration factor for vascular cells. Exp Cell Res 2003;287: 79–87. [DOI] [PubMed] [Google Scholar]
- 17.Roy S, Josephson SA, Fridlyand J, et al. Protein biomarker identification in the CSF of patients with CNS lymphoma. J Clin Oncol 2008;26:96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raithatha SA, Muzik H, Muzik H, et al. Localization of gelatinase-A and gelatinase-B mRNA and protein in human gliomas. Neuro Oncol 2000;2:145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao JS. Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer 2003;3:489–501. [DOI] [PubMed] [Google Scholar]
- 20.Wild-Bode C, Weller M, Wick W. Molecular determinants of glioma cell migration and invasion. J Neurosurg 2001. ;94: 978–984. [DOI] [PubMed] [Google Scholar]
- 21.Pagenstecher A, Wussler EM, Opdenakker G, et al. Distinct expression patterns and levels of enzymatic activity of matrix metalloproteinases and their inhibitors in primary brain tumors. J Neuropathol Exp Neurol 2001;60:598–612. [DOI] [PubMed] [Google Scholar]
- 22.Kinoshita M, Izumoto S, Hashimoto N, et al. Immunohistochemical analysis of adhesion molecules and matrix metalloproteinases in malignant CNS lymphomas: a study comparing primary CNS malignant and CNS intravascular lymphomas. Brain Tumor Pathol 2008;25:73–78. [DOI] [PubMed] [Google Scholar]
- 23.Wong ET, Lee D, Tam A, et al. Matrix metalloprotease-9 in cerebrospinal fluid correlates with disease activity in lymphomatous meningitis. Clin Lymphoma Myeloma 2007;7:305–308. [DOI] [PubMed] [Google Scholar]
- 24.Johansen JS, Lottenburger T, Nielsen HJ, et al. Diurnal, weekly, and long-time variation in serum concentrations of YKL-40 in healthy subjects. Cancer Epidemiol Biomarkers Prev 2008;17: 2603–2608. [DOI] [PubMed] [Google Scholar]
- 25.Jung K, Lein M, Laube C, Lichtinghagen R. Blood specimen collection methods influence the concentration and the diagnostic validity of matrix metalloproteinase 9 in blood. Clin Chim Acta 2001;314:241–244. [DOI] [PubMed] [Google Scholar]
- 26.Hormigo A, Gu B, Karimi S, et al. YKL-40 and matrix metalloproteinase-9 as potential serum biomarkers for patients with high-grade gliomas. Clin Cancer Res 2006;12:5698–5704. [DOI] [PubMed] [Google Scholar]
- 27.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–216. [DOI] [PubMed] [Google Scholar]
- 28.Abrey LE, Batchelor TT, Ferreri AJ, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol 2005;23: 5034–5043. [DOI] [PubMed] [Google Scholar]
- 29.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986;42:121–130. [PubMed] [Google Scholar]
- 30.Pelloski CE, Mahajan A, Maor M, et al. YKL-40 expression is associated with poorer response to radiation and shorter overall survival in glioblastoma. Clin Cancer Res 2005;11: 3326–3334. [DOI] [PubMed] [Google Scholar]
- 31.Hazar B, Polat G, Seyrek E, et al. Prognostic value of matrix metalloproteinases (MMP-2 and MMP-9) in Hodgkin’s and non-Hodgkin’s lymphoma. Int J Clin Pract 2004;58:139–143. [DOI] [PubMed] [Google Scholar]
- 32.Negaard HF, Svennevig K, Kolset SO, et al. Alterations in regulators of the extracellular matrix in non-Hodgkin lymphomas. Leuk Lymphoma 2009;50:998–1004. [DOI] [PubMed] [Google Scholar]
- 33.Sakata K, Satoh M, Someya M, et al. Expression of matrix metallo-proteinase 9 is a prognostic factor in patients with non-Hodgkin lymphoma. Cancer 2004;100:356–365. [DOI] [PubMed] [Google Scholar]
- 34.Mrugala MM, Rubenstein JL, Ponzoni M, Batchelor TT. Insights into the biology of primary central nervous system lymphoma. Curr Oncol Rep 2009;11:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouchard F, Belanger SD, Biron-Pain K, St-Pierre Y. EGR-1 activation by EGF inhibits MMP-9 expression and lymphoma growth. Blood 2010;116:759–766. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt H, Johansen JS, Sjoegren P, et al. Serum YKL-40 predicts relapse-free and overall survival in patients with American Joint Committee on Cancer stage I and II melanoma. J Clin Oncol 2006;24:798–804. [DOI] [PubMed] [Google Scholar]
- 37.Cintin C, Johansen JS, Christensen IJ, et al. High serum YKL-40 level after surgery for colorectal carcinoma is related to short survival. Cancer 2002;95:267–274. [DOI] [PubMed] [Google Scholar]
- 38.Xu D, McKee CM, Cao Y, et al. Matrix metalloproteinase-9 regulates tumor cell invasion through cleavage of protease nexin-1. Cancer Res 2010;70:6988–6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel bio-markers and potential therapeutic targets in human cancer. J Clin Oncol 2009;27:5287–5297. [DOI] [PMC free article] [PubMed] [Google Scholar]


