Abstract
Pulmonary infiltrates in neutropenic hosts with invasive aspergillosis are caused by organism-mediated tissue injury, vascular invasion, and hemorrhagic infarction. Ultrafast computed tomography (UFCT) scanning reproducibly measures these lesions in experimental invasive pulmonary aspergillosis in persistently neutropenic rabbits. The pulmonary lesion score from UFCT scanning is a useful outcome variable for measuring differences in efficacy of antifungal compounds alone and in combination, as well as the virulence of different strains and species of Aspergillus. Several studies demonstrate that the course of pulmonary lesions treated with amphotericin B, lipid formulations of amphotericin B, triazoles, echinocandins, and combination therapy measured by serial UFCT scans correlate with those measured by survival, histopathological resolution of lesions, microbiological clearance of Aspergillus fumigatus, and resolution of galactomannan index. We further developed a multidimensional volumetric imaging (MDVI) method for analysis of the volume of pulmonary infiltrates over time in response to antifungal therapy. Volumetric data by MDVI correlate with UFCT pulmonary lesion scores and validated biological endpoints. A recent pilot clinical study demonstrated the applicability of MDVI to human pulmonary fungal infections. MDVI also improves objectivity of radiological assessment of therapeutic response to antifungal therapy and merits more extensive evaluation in patients with invasive aspergillosis, as well as other fungal and bacterial pneumonias.
Keywords: Invasive pulmonary aspergillosis, computed tomographic scanning, ultrafast CT scanning, multidimensional volumetric imagin, antifungal therapy
Introduction
Invasive aspergillosis is an important cause of morbidity and mortality in immunocompromised patients. Computed tomographic (CT) scan technology has greatly enhanced our capability for early diagnosis and for monitoring of therapeutic response. During the past 12 years we have applied CT scan technology to the monitoring of therapeutic response of experimental pulmonary aspergillosis.
The objectives of this review are to discuss the basic concepts of CT imaging (CTI), to illustrate applications of CTI to experimental invasive pulmonary aspergillosis, to discuss the development and applications of multi-dimensional volumetric imaging (MDVI), to review the applications of MDVI to the study of human invasive pulmonary aspergillosis, and to outline future directions for these and other CT scan technologies. In illustrating the applications of CTI, we will further discuss the role of CTI in understanding the basic pathophysiology of invasive pulmonary aspergillosis, review the role for monitoring of therapeutic response to antifungal therapy as it applies to both single agent treatments (monotherapy and combination therapy), and to outline this potential utility in understanding pathogenesis and virulence of Aspergillus species.
Definitions
CT scan: imaging technology where the x-ray beam moves in a circle around the body to obtain multiple views of the same organ or structure, resulting in greater data density than that obtained from conventional radiography. These data are processed, analyzed and displayed in two-dimensional images.
Ultrafast CT scan (UFCT): a new generation of CT scanning technology using electron-beam tomography that substantially reduces the scanning time per section.
Pixel: the smallest point in a two-dimensional image that contains information.
Voxel: the smallest informational element in a three dimensional image.
Multidimensional Volumetric Imaging: The assembly of CT data to provide the volume of one or more selected lesions in an organ.
History of Computed Tomography
Computed tomography, a scientific achievement that revolutionized medicine, was developed by Allan Cormack and Godfrey Hounsfield. In 1972, Hounsfield combined x-ray and computer technology to create a device that scanned the human brain in four and one-half minutes [1]. A British physicist with no formal university training, Hounsfield transformed his 1968 CT prototype into a machine capable of hospital use in only four years in his work at EMI Central Research Laboratory [1,2]. The extensive profits from the sale of Beatles’ album allowed EMI to fund his research. The original prototype developed by Hounsfield was installed at Atkinson Morley’s Hospital in London where clinical trials were performed on the brain. Hounsfield himself participated in the early scans where the safety and efficacy of the scanner was tested [1,3].
In 1973, the second CT scanner was installed in the United States at Mayo Clinic [4]. By 1976, Hounsfield modified the machine to perform scans of the entire body [1,5]. Subsequently, CT scanners became widely available, leading to wide usage of these diagnostic imaging systems. In recognition of the company’s contributions, the technology is often referred to as the ‘EMI scanner’ [2].
Unknown to Hounsfield and EMI, Allan Cormack, a South African physicist, had previously developed a similar prototype in the 1950s. In a paper published in 1957, Cormack outlined the mechanism of tomography and described the reconstruction of the image using the Radon Transform [1]. In his job at the radiology department of a local hospital in Cape Town, Cormack was asked by his employer to measure how much x-ray energy was absorbed by various sections of the body in order to deliver more precise x-ray doses at specific parts of the body [6]. During his studies, Cormack realized the need for an x-ray map of the body that depicted the location of tissues and other materials. The map would be developed using tomography to construct an image of any specified cross-section of the body. After Cormack searched effortlessly through literature to find a mathematical method to produce tomography to no avail, he constructed a mathematical formula in 1957 to produce a high definition image from numerous x-ray readings. Using models and a calculator, he refined his hypothesis and as he described it, ‘left it to the engineers’ [6]. Although Cormack and Hounsfield did not collaborate during their research, both men shared the Nobel Prize in Medicine in 1979 for their contributions toward the development of CT [1,6,7].
Physics of CT scanning
CT scanning employs x-ray technology to create a three-dimensional image. X-rays and their medical application were first discovered by William Roentgen in 1895. In traditional x-ray images, x-rays pass through body and the shadow of the contents are projected to detectors that record the projection and produce a two-dimensional image in various shades of gray. Although bone is easily detectable in x-rays, soft tissue appears dark and indistinct [5]. CT addresses the limitations of conventional radiography as it offers a ‘cross-section’ view that displays a composite of three-dimensional data known in the form of voxels. In CT technology, the x-ray and detectors rotate around the patient and detectors record the x-ray absorption by the patient’s body in each direction (www.impactscan.org/slides/eanm2002/sld009. htm; http://docs.ksu.edu.sa/PDF/Articles27/Article270699.pdf).
From the intensity of the transmitted radiation, the linear attenuation coefficient (μ) is calculated. Attenuation coefficients measure the degree by which x-ray intensity is reduced by a given substance [8,9]. Each voxel in an image is assigned a CT number based on the average of the attenuation coefficients. The CT number is scaled to water, measured in Hounsfield units, and calculated using the equation: CT number [(μtissue – μwater)/μwater] * 1000 [5,9].
A mathematical algorithm is necessary to convert attenuation coefficients into a cross-sectional image. A coordinate system (x,y) locates measurement points and a density function: f(x,y) represents the attenuation coefficients. A primary goal of CT is to reproduce the density function as accurately as possible. The line integral of f(x,y) along (r,ϕ) is the projection ray P(r,ϕ) where r is the movement of the object in front of the detector and x-ray tube and ϕ is the angle of rotation of the (x,y) axes [5,9].
| (1) |
With the Beer-Lambert Law, another integral equation is derived:
| (2) |
where I0 and I are, respectively, the rates of the incident and the emerging photon beams; μ(x, y) is the linear attenuation coefficient at position (x, y); and ds is a length element along the ray r [5,9]. Using equations 1 and 2, a complete set of sum rays for a defined angle f, called projection is obtained. Utilizing computer analysis and data from a great number of sets of f, an image is produced [5,9].
Advancements in CT scanning
Since the 1970s, CT image quality has improved markedly by decreasing pixel size and increasing the number of pixels in an image. Pixel size has decreased from 3 × 3 mm in the 1970s to 0.5 × 0.5 mm in 2001, thus increasing the number of pixels in an image 36-fold [5].
The increased detail of CT is attributed to the development of slip ring technology in which the x-ray tube rotates continuously around patient. This advancement produced the multi-slice CT scanner in which data are gathered continuously instead of one slice at a time [5]. The durations of scans are decreased in the multi-slice CT scanner, eliminating errors from patient motion such as breathing and peristalsis. These advances in rapid scanning also contribute to the capacity of scanning animals with experimental invasive pulmonary aspergillosis. Scanning time also has markedly decreased as seen in 1972 at 4 min per rotation by early CT scanning to the approximate 7 sec per section of the thorax obtained by the initial UFCT (Imatron C-100) in the early 1990s to the 0.8 sec per section by the current generation of UFCT (GE Medical Systems CE 0459).
Relationship between CT imaging and pathophysiology of invasive pulmonary aspergillosis
Pulmonary infiltrates in neutropenic hosts with invasive aspergillosis are caused by vascular invasion, coagulative necrosis, and hemorrhagic infarction [10–12]. At the border of the infarctive process, there is a region of ischemia that is characterized by pulmonary edema. As the infarction progresses, the pulmonary edema is replaced by intra-alveolar hemorrhage, and finally by coagulative necrosis. This region of reversible ischemia correlates with the halo sign surrounding a dense nodule.
We hypothesized that CT imaging may provide a real-time objective parameter by which to measure organism-mediated tissue injury. This hypothesis was validated in a series of subsequent experiments, [13–18]. We therefore have monitored the course of pulmonary infiltrates by serial UFCT scanning in persistently neutropenic rabbits with experimental invasive pulmonary aspergillosis.
Conventional radiography in rabbits
The first series of experiments studied the normal anatomy of the rabbit thorax with conventional chest radiographs and UFCT. Following initial studies to optimize exposure of the normal rabbit lung by conventional chest radiography and by UFCT, a series of experiments was performed to compare the relative sensitivities of detection of pulmonary aspergillosis by conventional chest radiograph and by UFCT [13]. As UFCT was the state-of-the-art technology at the time for patients, earlier generations of CT scans were not studied. Conventional radiography in our studies was performed with rabbits placed recumbent in the left lateral and ventral-dorsal positions.
Our initial studies demonstrated that UFCTwas more sensitive than conventional chest radiography in the detection of the lesions of experimental invasive pulmonary aspergillosis. Conventional chest radiography detected lesions in 16 (29.6%) of 54 lobes, while UFCT revealed lesions in 29 (53.7%) of 54 lobes (P<0.05) of untreated controls. Similar results were obtained among treated animals, where chest radiography detected lesions in 70 (31.5%) of 222 lobes, while UFCT detected lesions in 99 (44.6%) of 222 lobes (P<0.01).
Methods of ultrafast CT scans in rabbits
A brief description of our UFCT methods is helpful to understand adaptation of this technology to rabbits. We originally studied experimental invasive pulmonary aspergillosis by UFCT with a C100 XL electron beam scanner (Imatron, Oyster Point, CA) [13]. The C100 was the first generation of UFCT scanners in clinical use and was the leading technology of its time being used in patients. We currently perform UFCT with the CE 0459, HiSpeed CT/i ultrafast electron beam CT scanner (GE Medical Systems, Milwaukee, WI). Before undergoing UFCT, rabbits are sedated and gently placed in a prone position head first on the scanning couch. Scans are made in the high resolution, table incremented, and volume acquisition mode. Sections measuring 1.25-mm in thickness are then performed every 4 sec. A 512 × 512 matrix is used, resulting in a pixel size of less than 1 mm. Scan parameters include a scan duration of 800 ms. Under these conditions, we found that 60 slices were sufficient to scan the entire rabbit thorax. Images are photographed using lung windows at a level −600 Hounsfield units and with a width of 1800 Hounsfield units.
Imaging characrteristics of CT scans in rabbits with experimental invasive pulmonary aspergillosis
The microbiological, immunological, and histological characteristics of the persistently neutropenic rabbit model of invasive pulmonary aspergillosis have been described in detail elsewhere [19]. Fig. 1 depicts an image of an ultrafast high resolution CT scan of a persistently neutropenic rabbit with invasive pulmonary aspergillosis. This section depicts features of nodular pulmonary infiltrates, bronchopneumonia, and normal lung parenchyma. As rabbits are placed supine in the CT scan for better positional stability, the scans depict the right side of the thorax on the right side of the image. This is the opposite of CT images in humans, wherein the patient lies supine during the procedure.
Fig. 1.
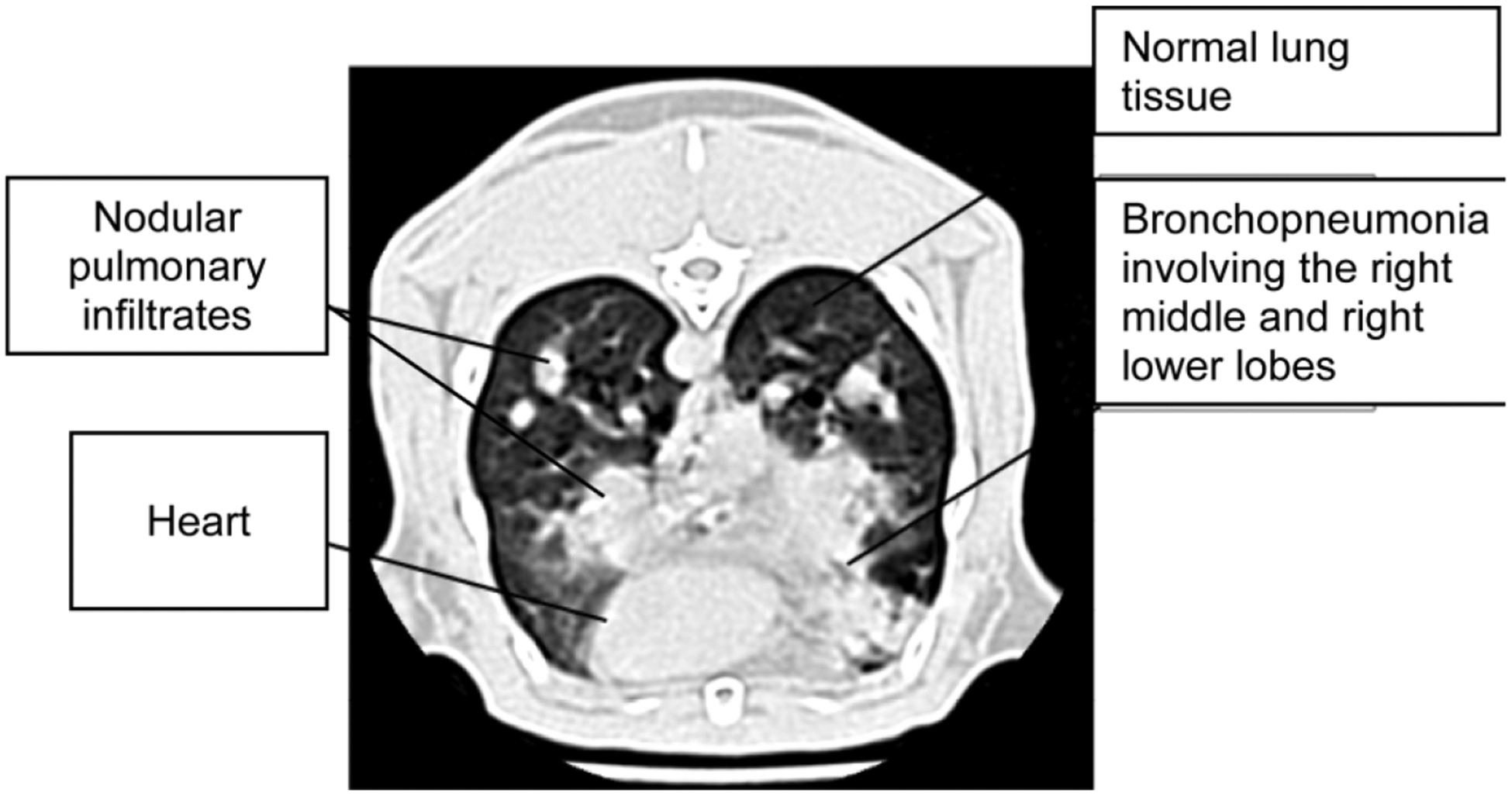
Radiological features of experimental invasive pulmonary aspergillosis.
UFCT imaging of normal rabbits delineates the anatomy of the thoracic cavity, including heart, lungs, tracheobronchial tree, and great vessels more clearly than does conventional chest radiograph [13]. UFCT in rabbits with invasive pulmonary aspergillosis also demonstrates lesions of bronchopneumonia, pulmonary nodules, halo signs, consolidation, and pleural effusions. Images also reveal bronchopneumonia, segmental pulmonary infiltrates, and pulmonary nodules with halo signs that progressed into dense multi-lobar consolidation. Although UFCT is a qualitative technique, the time course of lesions may be analyzed quantitatively. When rabbits are treated for invasive aspergillosis with polyenes, triazoles or echinocandins, these lesions diminish in size and number over time. This resolution of lesions allows quantitation of therapeutic response by the CT pulmonary lesion score (PLS).
In order to characterize the degree of organism-mediated pulmonary injury, an algorithm for determining pulmonary lesions was established by evaluating the infiltrate in each lobe. The pulmonary lesion score in each lobe was initially 0. Thereafter, each lobe was evaluated and scored independently over time by blinded observers. If the pulmonary infiltrate within the lobe demonstrated worsening, stabilization, or improvement, a score of +0.5, +1, 0, −0.5, or −1 respectively, was added to the previous score. Hence, the mean pulmonary lesion score at a particular time point represents the mean of these scores in a particular experimental group. A CT pulmonary lesion score can be calculated over time for individual rabbits as well as groups of rabbits within treatment cohorts. The scores are then analyzed statistically for differences in patterns and trends that reflect differences over time in organism-mediated pulmonary injury.
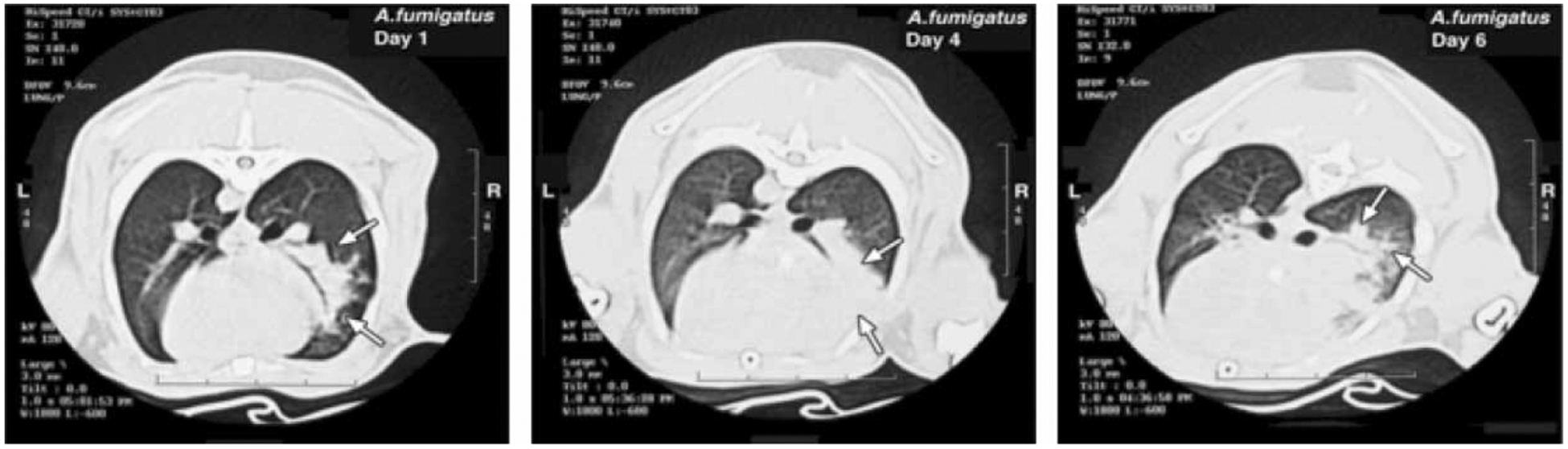
The evolution of pulmonary infiltrates in experimental invasive pulmonary aspergillosis is depicted in Fig. 2. On day 1 postinoculation, a dense segmental focal pulmonary infiltrate is present in the right middle lobe. By day 4, the infiltrate has involved the entire right middle lobe. A delicate alveolar infiltrate is present at the advancing border of the pneumonic process. By day 6, the pulmonary infiltrate has extended into the right lower lobe with an expansion of alveolar infiltrate at the advancing border.
Fig. 2.
Data acquisition of CT scanner (http://docs.ksu.edu.sa/PDF/Articles27/Article270699.pdf).
UFCT has since been applied to the study of invasive pulmonary aspergillosis for measurement of therapeutic response to amphotericin B [13], antifungal triazoles [14,17], echinocandin [15], and combinations of antifungal agents in treatment of experimental therapy [16]. The UFCT-defined PLS is one of six outcome variables used to study experimental invasive pulmonary aspergillosis. These outcome variables consist of (1) residual fungal burden in lung tissue (measured by log colony forming unit (CFU)/g), (2) survival, (3) serum galactomannan antigenemia, (4) total lung weight, (5) pulmonary infarct score (measured as number of lobes with hemorrhagic infarcts), and (6) pulmonary lesion score measure by UFCT. The latter three markers are indicators of organism-mediated tissue injury.
The initial UFCT scans provided a new system by which to evaluate the response of experimental invasive pulmonary aspergillosis to antifungal therapy. In comparison to pulmonary lesion scores (PLSs) of untreated controls, those of animals treated with lipid formulation of amphotericin B (LFAB) (5 and 10 mg kg/day) were significantly reduced (P ≤50.005 and P ≤50.05, respectively) on days 7 10 of treatment. The PLS of animals treated with LFAB (1 mg/kg/day) and deoxycholate amphotericin B (DAmB) (1 mg/kg/day) also were significantly reduced compared with those of untreated controls on day 9 (P ≤50.05 and P ≤50.005, respectively).
The initial studies of UFCT also revealed a CT PLS that increased within the first three days of treatment with DAmB and LFAB [13]. The PLS declined, thereafter, in response to therapy. This pattern of an initial rise and fall of lesions in experimental pulmonary aspergillosis is similar to that reported in patients by Caillot et al. [20]. The initial rise in PLS may reflect a delay in response to therapy before response ensues. Alternatively, the initial increase in PLS may occur as the result of a release of cytokines in response to polyene-induced hyphal injury.
The efficacy of antifungal triazoles was studied using UFCT as one of the therapeutic indices. During the comparative study of posaconazole and itraconazole in persistently neutropenic rabbits with invasive pulmonary aspergillosis, the mean PLS increased during the first 6 days in untreated control and treated animals [14]. Due to mortality in untreated controls the PLS curve ended on day 6. Whereas, the PLS curve declined following day 6 in both the posaconazole and itraconazole treatment groups. The PLS curve for itraconazole ended on day 8 due to mortality while that for posaconazole continued to improve through day 10. These UFCT findings were consistent with the five other therapeutic outcome variables in a pattern consistent with the more potent activity of posaconazole at similar plasma concentrations. A similar pattern was observed in a separate study of the efficacy, safety, and plasma pharmacokinetics of escalating dosages of ravuconazole in experimental invasive pulmonary aspergillosis. During the first 4 days of ravuconazole treatment, there was an increase in the PLS in rabbits treated with 2.5, 5.0, and 10 mg/kg/day. However, the magnitude of the pulmonary infiltrate scores in rabbits treated with 5 mg/kg/day and 10 mg/kg/day within the first 4 days was lower than that in 2.5 mg/kg-treated rabbits or untreated controls. Consistent with the dose-dependent reduction of organism-mediated pulmonary injury, UFCT scans demonstrated a similar pattern of resolution of pulmonary infiltrates in rabbits treated with ravuconazole at 5 and 10 mg/kg/day with a significant dose-related effect in the reduction of PLS in treated animals in comparison to untreated controls (P = 0.05 by analysis of variance (ANOVA)).
Caspofungin was studied as proof of concept of the efficacy, safety, and plasma pharmacokinetics of an echinocandin in the treatment and prevention of experimental invasive pulmonary aspergillosis. This study demonstrated that the PLS curves of serial CT scans from treated animals and untreated control animals increased during the first 7 days. However, animals treated with the echinocandin displayed significant resolution of pulmonary infiltrates in comparison to untreated controls (P <0.0001 by ANOVA).
UFCT also has been used to characterize the refractoriness of pulmonary aspergillosis in polyene-resistant Aspergillus terreus versus polyene-susceptible Aspergillus fumigatus to DAmB and LFAB [21]. In parallel with other outcome variables, the UFCT consistently reflected resistance of A. terreus-induced infection and response of A. fumigatus infection to polyene therapy. Consistent with the slower rate of growth in vitro of A. terreus, UFCT also demonstrated that pulmonary infiltrates developed more slowly in animals infected with A. terreus.
Multidimensional volumetric imaging
Monitoring the dynamics of pulmonary infiltrates of invasive aspergillosis by UFCT is an important tool for assessing clinical response to antifungal therapy. A more quantitative assessment of the development of pulmonary infiltrates caused by Aspergillus spp. may facilitate therapeutic monitoring for patient care, clinical trials, and laboratory investigation. We therefore introduced and validated a MDVI method for analysis of the response of the volume of pulmonary infiltrates over time to antifungal therapy in experimental invasive pulmonary aspergillosis in persistently neutropenic rabbits [20]. We further developed a semi-automatic method to measure the volume of lung lesions, which was implemented as an extension of the MEDx visualization and analysis software.
Therapeutic monitoring of pulmonary infiltrates using MDVI demonstrated a significant decrease in the infiltrate volume in DAmB-treated rabbits in comparison to untreated controls (P ≤0.001) (Fig. 3). Volumetric data by MDVI correlated with conventional CT pulmonary score (r = 0.83, P ≤0.001). Data from the MDVI correlated with the following validated biological endpoints: pulmonary infarct scores (r = 0.85, P ≤0.001), lung weights (r=0.76, P ≤50.01), residual fungal burden (r=0.65, P ≤0.05), and GMI (r=0.78, P ≤0.01). Thus, MDVI correlated with key biological markers and improved the objectivity of radiological assessment of therapeutic response to antifungal therapy.
Fig. 3.
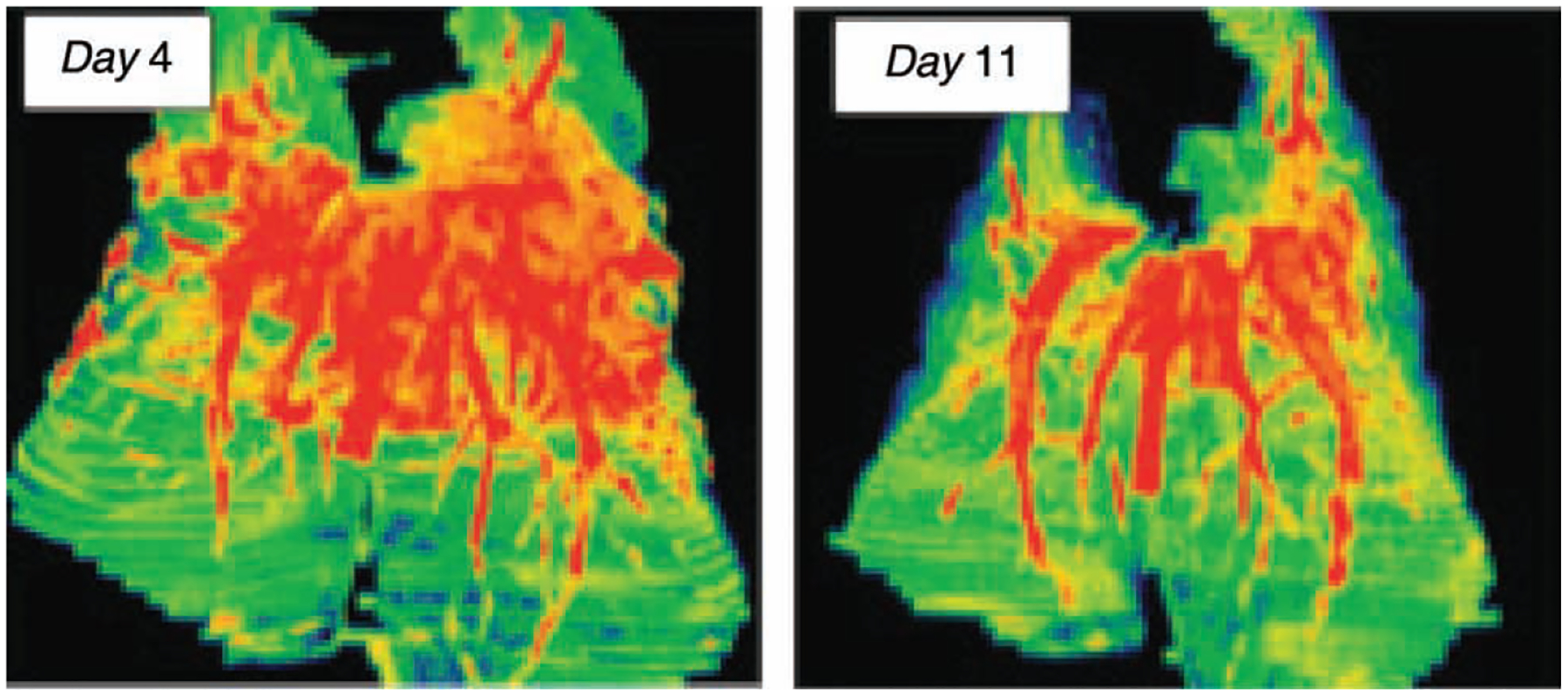
Multidimensional volumetric imaging of experimental invasive pulmonary aspergillosis in persistently neutropenic rabbits. The deep orange-red colouration depicts vessels and hemorrhagic lesions. Following antifungal therapy with amphotericin B, the pulmonary lesions have significantly diminished.
Application of MDVI to human infection
These volumetric methods have potential applicability to a wide range of focal pneumonias, including those caused by bacteria and fungi. In order to further understand the application of MDVI to human infection, we conducted a retrospective pilot study of fungal pneumonias in hospitalized patients of the Washington Hospital Center who had CT scans performed as part of their care [22]. This study monitored six patients with pulmonary infections caused by Aspergillus spp. in three cases, Cryptococcus neoformans in two cases, and a Zygomycete in one case. Compared with standard computed tomography images, multidimensional CT scan imaging provided increased detail of the volume and geometry of the pulmonary infiltrates. The MDVI method measured volumes over a >50-fold range. Resolution of pulmonary infiltrates correlated with response to antifungal therapy. Serial measurements of pulmonary infiltrate volumes may provide an adjunct for monitoring disease progression and therapeutic response.
Conclusions
UFCT scanning is a reproducible system for measuring pulmonary infiltrates and organism-mediated pulmonary injury in experimental invasive pulmonary aspergillosis. When applied to the persistently neutropenic rabbit model, CT scanning can be used as a valuable end point for measuring differences in the efficacy of antifungal compounds alone and in combination, the virulence of different strains and species of Aspergillus, and the effects of immunomodulatory therapy. The current UFCT technology used in experimental aspergillosis and widely used in clinical imaging facilities is a powerful tool for detection and monitoring of therapeutic response in immunocompromised patients. Advances in volumetric imaging processing can provide clinicians with objective criteria for assessing response to therapy for invasive aspergillosis. Advances achieved in CT imaging technology in the study of invasive pulmonary aspergillosis also will have important applications in the study and management of fungal and bacterial pneumonias.
Footnotes
Declaration of interest: The author reports no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Telegraph. 2004. Sir Godfrey Hounsfield. http://www.telegraph.co.uk/news/main.jhtml?xml/news/2004/08/17/db1701.xml
- 2.The Whittington Hospital, National Hospital Service. The Beatles Greatest Gift… is to Science. http://www.whittington.nhs.uk/default.asp?=2804.
- 3.Beckmann EC. CT scanning in the early days. Br J Radiol 2006; 79: 5–8. [DOI] [PubMed] [Google Scholar]
- 4.Medical News Today. Mayo Clinic first in USA to scan with new Computed Tomography (CT) system, 2004. http://www.medicalnewstoday.com/articles/13731.php.
- 5.American Physics Society. CT Scans http://www.physicscentral.com/action/2002/scans.html.
- 6.Thomas RMCG Jr. Allan Cormack, 74, Nobelist who helped invent CAT scan. New York Times; 9 May 1999. http://query.nytimes.com/gst/fullpage.html?res=9F01E1D61031F93AA35756C0A96E958260. [Google Scholar]
- 7.Nobel Prize.org The Nobel Prize in Physiology or Medicine in 1979. http://nobelprize.org/nobel_prizes/medicine/laureates/1979/
- 8.Platten D. Multi-slice Helical CT Physics and Technology. ImPACT, UK: Department of Health; http://www.impactscan.org/slides/eanm2002/sld001.htm. [Google Scholar]
- 9.Kansas State University. Introduction to CT Physics. http://docs.ksu.edu.sa/PDF/Articles27/Article270699.pdf.
- 10.Berenguer J, Allende MC, Lee JW, et al. Pathogenesis of invasive pulmonary aspergillosis during persistent granulocytopenia versus cyclosporine and methylprednisolone-induced immunosuppression. Am J Resp Crit Care Med 1995; 152: 1079–1086. [DOI] [PubMed] [Google Scholar]
- 11.Shaukat A, Bakri F, Young P, et al. Invasive filamentous fungal infections in hematopoietic stem cell transplant recipients after recovery from neutropenia: clinical, radiologic, and pathologic characteristics. Mycopathologia 2005; 159: 181–188. [DOI] [PubMed] [Google Scholar]
- 12.Stergiopoulou T, Meletiadis J, Roilides E, et al. Host-dependent patterns of tissue injury in invasive pulmonary aspergillosis. Am J Clin Pathol 2006; 127: 1–7. [DOI] [PubMed] [Google Scholar]
- 13.Walsh TJ, Garrett K, Feuerstein E, et al. Therapeutic monitoring of experimental invasive pulmonary aspergillosis by ultrafast computerized tomography: a novel non-invasive method for measuring responses of organism-mediated tissue injury. Antimicrob Agents Chemother 1995; 39: 1065–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petraitiene R, Petraitis V, Groll AH, et al. Antifungal activity and plasma pharmacokinetics of posaconazole (SCH 56592) in treatment and prevention of experimental invasive pulmonary aspergillosis: correlation with galactomannan antigenemia. Antimicrob Agents Chemother 2001; 45: 857–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petraitiene R, Petraitis V, Groll A, et al. Antifungal efficacy of caspofungin (MK-0991) in experimental pulmonary aspergillosis in persistently neutropenic rabbits: pharmacokinetics, drug disposition and relationship to galactomannan antigenemia. Antimicrob Agents Chemother 2002; 46: 12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petraitis V, Petraitiene R, Sarafandi AA, et al. Combination therapy in treatment of experimental pulmonary aspergillosis: synergistic interaction between an antifungal triazole and echinocandin. J Infect Dis 2003; 187: 1834–1843. [DOI] [PubMed] [Google Scholar]
- 17.Petraitiene R, Petraitis V, Lyman CA, et al. Efficacy, safety, and plasma pharmacokinetics of escalating dosages of intravenously administered ravuconazole di-lysine phosphoester in experimental pulmonary aspergillosis in persistently neutropenic rabbits. Antimicrob Agents Chemother 2004; 48: 1188–11196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petraitis V, Petraitiene R, Solomon J, et al. Multi-dimensional volumetric imaging of pulmonary infiltrates in measuring therapeutic response to antifungal therapy in experimental invasive pulmonary aspergillosis. Antimicrob Agents Chemother 2006; 50: 1510–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francis P, Lee JW, Hoffman A, et al. Efficacy of unilamellar liposomal amphotericin B in treatment of pulmonary aspergillosis in persistently granulocytopenic rabbits: the potential role of bronchoalveolar lavage D-mannitol and galactomannan as markers of infection. J Infect Dis 1994; 169: 356–368. [DOI] [PubMed] [Google Scholar]
- 20.Caillot D, Couaillier JF, Bernard A, et al. Increasing volume and changing characteristics of invasive pulmonary aspergillosis on sequential thoracic computed tomography scans in patients with neutropenia. J Clin Oncol 2001; 19: 253–259. [DOI] [PubMed] [Google Scholar]
- 21.Walsh TJ, Petraitis V, Petraitiene R, et al. Experimental pulmonary aspergillosis due to Aspergillus terreus: pathogenesis and treatment of an emerging fungal pathogen resistant to amphotericin B. J Infect Dis 2003; 188: 305–319. [DOI] [PubMed] [Google Scholar]
- 22.Pyrgos VJ, Petraitis V, Petraitiene R, et al. Multi-dimensional volumetric imaging method (MDVIM) in assessment of pulmon ary infiltrates in patients with invasive fungal pulmonary infections. Abstracts of the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy, 2007; p. 445, Abstr. M-1180. [Google Scholar]


