Abstract
Severe acute respiratory syndrome–correlated new coronavirus (SARS-Cov-2) infection may result in neurological signs and symptoms through different mechanisms. Although direct infection of the central nervous system is uncertain or very rare and the para-infectious complications (e.g. inflammatory neuropathies) are rare, delirium and septic encephalopathy are common in severely ill patients. Smell dysfunction and headache are very common in mild cases, especially in younger people and females. Muscle pain is common in both mild and severe cases, and in the most compromised patients, it is accompanied by increased creatine kinase levels and by a likely true myopathic damage. Ischemic stroke has been reported as a possible complication of the hypercoagulability associated with severe SARS-Cov-2 infection, but further studies are needed. Most of the neurological manifestations may occur early in the illness. Therefore, during the pandemic period, neurologists need to be involved, alert, and prepared. Neurological practice will not be the same until a vaccine is available.
Keywords: anosmia, coronavirus, COVID-19, CPK, Guillain-Barré syndrome, myopathy, myositis, Sars-Cov-2, stroke
Introduction
The severe acute respiratory syndrome–correlated new coronavirus (SARS-Cov-2), first isolated from three Chinese patients in Wuhan, China, is spreading worldwide since the beginning of 2020, emerging as an unprecedented crisis. This disease has been called ‘coronavirus disease 2019 (COVID-19).’1
By March 11, 2020, the World Health Organization declared the pandemic status. Clinical features and risk factors are highly variable, making the clinical severity range from asymptomatic to fatal. Understanding of COVID-19 is ongoing. Respiratory droplet transmission is the main route, and it can also be transmitted through person-to-person contacts by asymptomatic carriers. The most common features at onset of COVID-19 are fever, cough, and fatigue, while other signs and symptoms include sputum production, headache, hemoptysis, diarrhea, dyspnea, and lymphopenia.2
Variable incubation period (5–14 days), highly transmissible nature, asymptomatic carriage, and wide spectrum of illness make this disease extremely challenging for healthcare systems.3
Several neurological symptoms were identified as part of the COVID-19 spectrum since the first detailed study from Wuhan. Symptoms included muscle pain (11%), confusion (9%), and headache (8%) in this first retrospective study performed on severely ill patients.4
The major clinical manifestations of the SARS-Cov-2 infection are due to pulmonary complications. Although most have mild symptoms, such as fever, headache, cough, dyspnea, myalgia, and anosmia, some develop acute respiratory distress syndrome (ARDS) that can result in death.5
A study on 113 Chinese patients who died from COVID-19 (compared with patients who ultimately survived) revealed that ARDS with respiratory failure, sepsis, acute cardiac injury with heart failure, and lastly hypoxic encephalopathy were the most common critical complications. The median age of the deceased patients was 68 years, and male sex was predominant (73%). Hypertension and other cardiovascular comorbidities were more frequent among the deceased patients. As expected, dyspnoea, chest tightness, and altered consciousness, as well as increased creatine kinase (CK) levels were more common in the deceased patients.1
A report of viral infiltration of the brainstem in a limited number of pathologic specimens (from patients infected with coronaviruses other than SARS-Cov-2) opened the debate about the neurological features of SARS-Cov-2 infections and the potential direct neuroinvasivity of this virus.6 However, to date, this hypothesis has not been confirmed and its relevance remains to be assessed.
A retrospective case series from Wuhan on 214 hospitalized patients with SARS-Cov-2 infection (severe in 41% of them) revealed that 78 patients (36%) had some neurological features. For instance, ‘dizziness’ (not further defined) was present in 17% of cases.7 Patients with severe disease had signs of neurological impairment such as ‘skeletal muscle injury’ (19%), impaired consciousness (15%), and acute cerebrovascular diseases (6%).7
This review aims to summarize early findings on the neurological features of COVID-19 and their treatment approaches. We searched PubMed until May 27, 2020, for all articles about “(covid* OR coronavirus* OR sars*) AND (neurolog* OR nervous OR brain OR *neuropath* OR nerve OR nerves OR neuron* OR muscle* OR muscular OR neuromuscular OR hyposmia OR anosmia OR myopath* OR stroke OR coagulati* OR seizure* OR delirium OR consciousness OR ataxi*),” where * is the Pubmed wildcard for every possible word beginning or ending. Then we reviewed the abstracts in order to identify all the relevant publications in English language.
Taste and smell
Olfactory and gustatory dysfunctions (common in mild cases)
A multicenter, specifically designed prospective European study performed on 417 mild and moderate COVID-19 patients showed that 86 and 88% of patients reported olfactory and gustatory dysfunctions, respectively.8
There was a significant positive association between olfactory and gustatory dysfunctions as expected, considering that the perceived gustatory impairment is usually secondary to the true olfactory dysfunction. Olfactory dysfunction appeared before the other symptoms in 12% of cases. Interestingly, females were significantly more affected by olfactory and gustatory impairments. In this study, olfactory and gustatory symptoms were more prevalent than general symptoms such as cough, myalgia (~58%), loss of appetite, diarrhea, fever, headache (~45%), and asthenia. The olfactory dysfunction persisted after the resolution of the other symptoms in 63% of cases. Among the patients who reported anosmia, the olfactory function recovered throughout the first 2 weeks following the resolution of the disease in >95% of cases. Many viruses may lead to olfactory dysfunction through an inflammatory reaction of the nasal mucosa and the development of rhinorrhea; the most familiar agents being rhinovirus, parainfluenza, Epstein–Barr virus, and some other coronaviruses. However, olfactory dysfunction linked to COVID-19 infection seems specific as it is not associated with rhinorrhea.8
Another large, multicenter, prospective European study performed on mild COVID-19 patients confirmed a high prevalence of loss of smell (70%) and gustatory dysfunction (54%). Young people more frequently reported loss of smell compared with older subjects; moreover, it was more prevalent in females compared with males.9
Interestingly, clinically evident new-onset smell and taste disorders were significantly more frequent among COVID-19 patients (39%) than influenza patients (13%). They usually had an acute onset and were commonly an initial manifestation. Concomitant nasal obstruction was rare in COVID-19 patients.10
Smell and taste impairments were also common in not-tested household contacts of mildly symptomatic home-isolated SARS-Cov-2-positive patients.11
The retrospective study from Wuhan reported a markedly lower prevalence of these manifestations (taste impairment 6% and smell impairment 5%). However, this study was performed on older, severely affected patients and was not specifically designed to asses these subtle symptoms, difficult to evaluate in severely ill subjects.7
Of note, transient bilateral olfactory bulbs edema was demonstrated by magnetic resonance imaging (MRI) in a patient with COVID-19-related anosmia.12
It has been suggested that the olfactory epithelium may represent a site of SARS-Cov-2 active replication and accumulation, considering that cells located there express the receptors required for efficient SARS-Cov-2 infection. A key, open question is whether SARS-Cov-2 may infect the brain through the uptake into ciliated dendrites/soma and subsequent anterograde axonal transport along the olfactory nerve.13 Further studies are strongly needed.
Limited evidence-based treatments exist for anosmia.14 However, smell and taste dysfunctions are self-limiting in the great majority of COVID-19 patients and do not require specific treatments.
Pain
Headache (common in mild cases)
A meta-analysis inclusive of 61 studies (59,254 patients) reported that headache was present in 12%, representing the fifth clinical feature (after fever, cough, muscle pain and/or fatigue, dyspnea).15 Headache was also reported in the retrospective case series from Wuhan with nearly exactly the same prevalence (13%).7
A large, multicenter, prospective European study performed on mild COVID-19 patients reported a higher prevalence of headache (70%).9 Young people more frequently had headache compared with older subjects; moreover, it was more prevalent in females compared with males. The prospective nature of this latter study and the fact that it was performed on milder and younger (mean age 39 years)9 patients are the likely explanations of this markedly different percentage.
Several types of headaches can appear during COVID-19. It has been suggested that the headache occurring from the seventh day after the clinical onset could be related to the cytokine storm typical of this infection,16 but further studies are needed.
As no specific treatment options for COVID-19-related headache were reported, a careful pain management is recommended.
Central nervous system
Impaired consciousness and delirium (common in severe cases)
Chen and coworkers reported that altered consciousness at the time of hospital admission was more common in the patients who subsequently passed away (22%) than in the subjects who eventually recovered from the disease (1%), as expected. Unfortunately, the exact meaning of ‘altered consciousness’ in this setting was not provided.1
Another study reported the neurological features of 58 patients with ARDS due to COVID-19.17 ‘Agitation’ (likely corresponding to hyperkinetic delirium) was present in 40 patients (69%) when neuromuscular blockade was discontinued. Subsequently, 33% of the patients who have been discharged were reported to have a ‘dysexecutive syndrome’ consisting of inattention, disorientation, and poorly organized movements in response to command. Cerebrospinal fluid (CSF) samples were negative for SARS-Cov-2 in seven patients who underwent lumbar puncture. Electroencephalography (EEG) and brain MRI showed nonspecific findings (only two patients had a small acute ischemic stroke). Further studies are needed to determine which of these ‘encephalopathic’ features were due to critical illness–related encephalopathy, cytokines, and/or medications and which features were specific of SARS-Cov-2 infection, if any.17
The retrospective study from Wuhan also showed that nervous system manifestations were more frequently found in severe SARS-Cov-2 infections compared with the less severe ones. For instance, ‘impaired consciousness’ was reported in ~15% of the most severely ill subjects compared with ~2% of the other patients.7
Impaired consciousness and delirium are likely to be associated with pyramidal signs (i.e., enhanced tendon reflexes, ankle clonus, bilateral extensor plantar reflexes), which were reported in 67% of ARDS patients.17
COVID-19-related impairment of consciousness and delirium are probably due to infectious toxic (or ‘septic’) encephalopathy, a type of reversible brain dysfunction syndrome caused by factors such as systemic inflammatory response syndrome–related toxemia and hypoxia during the process of acute pulmonary infection.18 Detailed studies are greatly required.
It has been suggested that the implementation of excellent delirium prevention and management at the bedside should be a priority during the COVID-19 pandemic.19
A study performed in a palliative care hospital revealed that agitation could respond well to benzodiazepines.20 However, benzodiazepines may be dangerous for patients with respiratory failure who are not ventilated, and a special attention is needed. For everything else, even if it has been suggested that hyperactive delirium could require a more aggressive management in these patients,21 COVID-19-related delirium should probably not be treated differently from delirium due to other causes.22
Ischemic stroke (rare)
In the retrospective study from Wuhan,7 ischemic stroke was reported in five of the severely ill subjects (~5%) compared with only one (~1%) in the other group (moderately affected patients). Interestingly, patients with severe infection had higher D-dimer level, suggestive of consumptive coagulation system. Unfortunately, the relationship between stroke and D-dimer levels was not provided.7
A retrospective Dutch research studied 184 patients with proven COVID-19 pneumonia admitted to the intensive care unit (ICU) and observed the following clinically evident thrombotic complications: ~14% pulmonary thromboembolism, ~2% different venous thromboembolic events, and ~2% ischemic strokes.23
An Italian study from Milan reported the same rate of ischemic stroke (2.5%). Three of these patients with stroke (n=9) were in the ICU and six on the general ward. In six patients, stroke was the primary reason for hospitalization.24
A study from the United States reported ischemic stroke in 1.1% of hospitalized COVID-19 patients.25
Furthermore, a 36-year-old Spanish woman was reported with an infarct in the territory of the left middle cerebral artery associated with a free-floating thrombus in the ascending aorta, during SARS-Cov-2 infection.26
A case series from London, UK, reported six consecutive patients with acute ischemic stroke and COVID-19. All six patients had large vessel occlusion with markedly elevated D-dimer levels (≥1000 μg/L). Five of these patients had a positive lupus anticoagulant – but its pathogenic relevance is uncertain. Three patients had multiterritory infarcts, two had concurrent venous thrombosis, and, in two, ischemic strokes occurred despite therapeutic anticoagulation.27
A recent paper from New York, United States, reported five cases of large-vessel stroke in patients who had SARS-Cov-2 infection and were younger than 50. On admission, the mean National Institutes of Health Stroke Scale score was 17, consistent with severe stroke. One patient had a previous history of stroke.28
However, New York and London are very large cities importantly affected by COVID-19 pandemic, and further investigation is needed to confirm that the association between large-vessel stroke and COVID-19 was not simply due to chance.
An important cohort study from Northern Italy (Brescia) compared neurological patients with and without COVID-19 admitted during the same period.29 Patients with COVID-19 were older and had a different distribution regarding admission diagnoses, including cerebrovascular disorders (77 versus 58%). In-hospital mortality rates (38 versus 4%) and incident delirium (27 versus 8%) were significantly higher in the COVID-19 group. Stroke had similar baseline characteristics in both groups, but patients with COVID-19 had a worst outcome at discharge. Therefore, COVID-19 patients admitted with neurological disease, including stroke, had a significantly higher in-hospital mortality, incident delirium, and higher disability than patients without COVID-19.29
The procoagulant pattern of COVID-19 patients may justify the clinical reports of thromboembolic complications, including stroke, during the course of the disease. COVID-19 patients with ARDS showed a procoagulant profile characterized by an increased clot strength due to both platelet and fibrinogen contribution, elevated D-dimer levels, and hyperfibrinogenemia (possibly linked to increased interleukin-6, a powerful pro-inflammatory cytokine).30 At least in the most severe cases, an aggressive antithrombotic therapy may be warranted (i.e. low molecular weight heparin 6000 IU, two times a day).30 Further studies are also needed to assess the best prophylaxis and treatment of this condition. A randomized controlled trial is being planned to study whether prophylactic-dose enoxaparin (versus no treatment) may reduce early, all-cause mortality and unplanned hospitalizations in adult symptomatic ambulatory COVID-19 patients with no other indications to receive anticoagulation.24
Another study confirmed that coagulation dysfunction is common in patients with COVID-19, especially fibrinogen and D-dimer elevation, and the degree of elevation is related to the severity of the disease. As the patient recovers, fibrinogen and activated partial thromboplastin time also return to normal.31
However, the issue of stroke in SARS-Cov-2 infections is still debated. For instance, there has not been any evident increase in stroke incidence during COVID-19 pandemic in some of the most affected areas, such as Piacenza and its province in Northern Itay.32 This is also true for our area. Our province (Lucca) is one of the most hit in Tuscany (Central Italy) with 351 confirmed COVID-19 cases per 100,000 inhabitants up to May 27, 2020. Our Neurological Unit is the Stroke Unit for an area comprising ~228,000 persons. However, we are aware of only one stroke case in a SARS-Cov-2 positive subject in our area. Furthermore, this patient was recovering from COVID-19 and had several other ‘conventional’ risk factors for stroke (Figure 1). Therefore, we don’t believe that SARS-Cov-2 infection had a major role in causing this minor stroke.
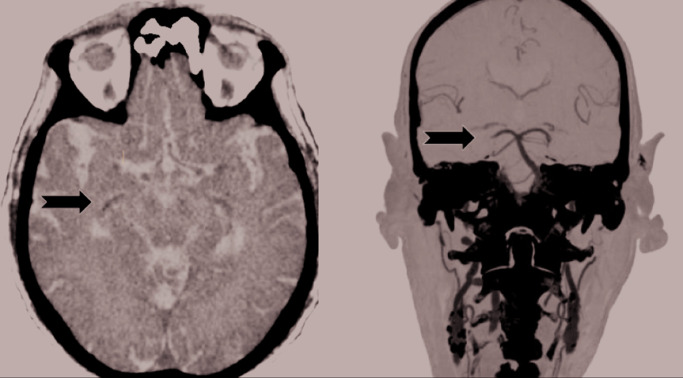
Figure 1.
Minor stroke in a COVID-19-positive subject.
Our patient presented at 70 years with transitory sensory and motor disturbances on the left side of the body (~6 hours). His past medical history was remarkable for hypertension, type 2 diabetes, chronic renal disease, dyslipidemia, and ischemic heart disease with a myocardial infarction. He had an implantable cardioverter-defibrillator and pacemaker (not MRI compatible). He was a smoker. The day before he was discharged from the pneumological unit of our hospital, where he had been hospitalized for 27 days because of COVID-19-related bilateral pneumonia. SARS-Cov-2 RNA was still detectable in his nasopharyngeal specimens by reverse-transcription polymerase chain reaction. Brain CT (left) and angioCT (right) revealed a thrombus in the right cerebral posterior artery (arrows). When he was evaluated, the disturbances were resolved and the neurological examination was normal. Therefore, there were not any criteria for systemic thrombolysis or mechanical thrombectomy, and he was treated with standard medical therapy.
During the last weeks, further case reports of ischemic stroke in subjects with SARS-Cov-2 have appeared.33–40 In at least some of these cases, a causal link is possible, and specifically designed longitudinal studies are strongly needed.
Importantly, even if some adaptations in the real-life management of stroke may be needed,41,42 COVID-19 pandemic should not alter the inclusion and exclusion criteria for acute stroke treatments, such as systemic fibrinolysis and mechanical thrombectomy.43 This also applies to stroke patients with suspected or confirmed SARS-Cov-2 infection.44,45
Rarer central neurological features
There are reports of rare patients with various neurological features during the course of COVID-19, including intracerebral hemorrhage,7,46–50 cerebral venous thrombosis,51–53 slight neck stiffness (with no SARS-Cov-2 genomes in the CSF),54 generalized myoclonus,55 seizures,7,56,57 status epilepticus58,59 and acute epileptic encephalopathy,60 hemorrhagic posterior reversible encephalopathy syndrome,61 acute necrotizing encephalopathy,62 white matter and globus pallidum inflammatory lesions,63 diffuse leukoencephalopathy with microhemorrhages,64,65 ‘steroid-responsive encephalitis’,66 neuroleptic malignant syndrome,67 and post-infectious acute transverse myelitis.68 Furthermore, a 6-week-old term male infant was reported with episodes characterized by sustained upward gaze, dystonic bilateral leg extension, and altered responsiveness in the setting of COVID-19 and rhinovirus coinfection.69
A recent neuropathological case report revealed a range of neuropathological lesions, with features resembling both vascular and demyelinating etiologies.70 Hemorrhagic white matter lesions were present throughout the cerebral hemispheres with surrounding axonal injury and macrophages. The subcortical white matter had scattered clusters of macrophages, a range of associated axonal injury, and a perivascular acute disseminated encephalomyelitis (ADEM)-like appearance.70 Surprisingly, SARS-Cov-2 genomes (and/or viral particles) were not searched in these lesions.
There is only one report of meningoencephalitis with finding of SARS-Cov-2 genomes in the CSF.71 In another case of meningoencephalitis started with seizures in a young COVID-19 positive woman in the United States, SARS-Cov-2 genomes were not researched in the CSF72 (note: in a subsequent paper, which appeared when this paper was under review, other researchers claim that “CSF was subsequently found to be positive for SARS-Cov-2” in this patient).73 Therefore, the issue of direct central nervous system (CNS) infection by SARS-Cov-2 remains unconfirmed to date. Even if confirmed, meningoencephalitis is likely a very rare complication of COVID-19.
More cases with epidemiological data are necessary to support a direct causal relationship between SARS-Cov-2 and most of the above-reported rarer neurological features. For instance, seizures are a common symptom in elderly patients with fever due to any cause and may not be directly related to this specific viral infection.
It has been suggested, on the basis of purely theoretical considerations, that when visiting critical COVID-19 patients who have a change in mental status, one should make sure that nonconvulsive status epilepticus is not ongoing, by means of continuous EEG monitoring.74 Unfortunately, there are not yet any clinical data supporting (or not supporting) this approach.
A recent specifically designed retrospective study from China confirmed that seizures are rare during COVID-19. A total of 304 people were studied, of whom 108 had a severe condition. Only two people had seizure-like symptoms during the hospitalization. Therefore, there was no evidence suggesting an important additional risk of seizures in people with COVID-19.75
When treating epilepsy in a given patient with SARS-Cov-2 infection, it is important to check the pharmacological interactions between antiepileptic drugs and the drugs used to treat COVID-19 in that subject. A special attention is needed for carbamazepine, phenytoin, phenobarbital, and primidone. An updated list provided by the University of Liverpool is available here: https://www.covid19-druginteractions.org.
Muscle and nerve
Muscular involvement (common)
The prospective European study performed on mild COVID-19 patients reported a high prevalence of muscle pain in this setting (63%).9 In contrast, a large, prospective study from New York performed on critically ill patients reported that muscle pain was present in 26%.76
The previous Chinese retrospective study performed on severe cases reported muscle pain as an onset symptom in 22% of the patients. Median CK levels (normal values < 190) were higher in the patients who subsequently passed away (189 U/L) than in the other patients (84 U/L).1
The retrospective study from Wuhan reported a similar finding: ‘skeletal muscle injury’ (defined in the following way: “when a patient had skeletal muscle pain and elevated serum CK,” greater than 200 U/L) was significantly more common in severe infections compared with less severe infections (19 versus 5%). Median CK levels were higher in the severe group: 83 U/L (range 9–12,216) compared with 59 U/L (19–1260). Of note, patients with muscle injury had multiorgan damage, including more serious liver and kidney abnormalities.7
Severe rhabdomyolysis may be a rare, late complication associated with COVID-19.77
Even if electromyography, muscle imaging, or muscle histopathology are not available to date, on the basis of the available data, coronavirus infections may likely cause a viral myositis.78 Furthermore, very sick patients may develop weakness due to muscle fiber atrophy from disuse and/or critical illness myopathy (and/or polyneuropathy), but specifically designed studies are still pending.78
Inflammatory neuropathies (rare)
There are few reports of patients who experienced typical acute inflammatory demyelinating polyneuropathy during79–82 or after83–86 the course of COVID-19.87 A case of acute polyradiculoneuritis with locked-in syndrome in a patient with COVID-19 was also reported.88 Another patient was reported with acute motor-sensory axonal neuropathy during the course of the infection, but its CSF was not sampled.89 Most of those patients have been treated with intravenous immunoglobulins (IVIg) with variable outcome.
Furthermore, a recent paper reported two Spanish cases highlighting the rare occurrence of Miller Fisher syndrome and polyneuritis cranialis during the COVID-2 pandemic. The first subject was treated with IVIg. Two weeks later, both patients made a complete neurological recovery.90 Two patients who were diagnosed with COVID-19 after presenting with diplopia and ophthalmoparesis were also reported.91
A detailed case series from Northern Italy described five patients who had Guillain–Barré syndrome after the onset of COVID-19.92 Four of these patients had a positive nasopharyngeal swab for SARS-Cov-2 at the onset of the neurologic syndrome, whereas the fifth subject had a positive serologic test. The first symptoms of Guillain–Barré syndrome were lower-limb weakness and paresthesia in four patients, and facial diplegia followed by ataxia and sensory disturbances in one patient. Generalized, flaccid tetraparesis or tetraplegia evolved over a period of 36 hours to 4 days in four patients; three received mechanical ventilation. The interval between the onset of symptoms of COVID-19 and the first symptoms of Guillain–Barré syndrome ranged from 5 to 10 days. On analysis of the CSF, two patients had a normal protein level and all the patients had a normal white-cell count. Antiganglioside antibodies were absent. In all the patients, SARS-Cov-2 genomes were not found in the CSF. Electrophysiological findings were consistent with an axonal variant of Guillain–Barré syndrome in three patients and with a demyelinating process in two patients. MRI showed enhancement of the caudal nerve roots in two patients and of the facial nerve in another patient. All the patients were treated with IVIg; two received a second course of IVIg and one started plasma exchange. At 4 weeks after treatment, only one patient had been discharged and was able to walk independently.92
The effect of reduced vital capacity due to neuromuscular failure from Guillain–Barré syndrome should be considered if findings on chest imaging are not commensurate with the severity of respiratory insufficiency in COVID-19 patients.92
Future research is needed to characterize the clinical/electrophysiological pattern of the new cases of acute para- or post-infectious autoimmune polineuropathies (Guillain–Barré syndrome and its variants) observed in the context of COVID-19 pandemic and to definitively confirm the causal relationship.
The treatment of SARS-Cov-2 infection-related Guillain–Barré syndrome does not differ from the treatment of any other inflammatory acute neuropathy.93 However, plasma exchange may pose more organizational issues than IVIg infusions, especially in those patients who are still infectious.
Neurological practice during COVID-19 pandemic
Neurological practice is affected by the COVID-19 pandemic in several key ways.94 Patients with neuromuscular disorders (e.g. myasthenia gravis)95 and neurodegenerative conditions (e.g. dementia, Parkinsons disease) may be particularly susceptible to SARS-Cov-2 infection and its complications, and this pandemic has forced a rapid reorganization of clinical care delivery, including telemedicine and telephonic contacts.78 Telemedicine can be fundamental for the follow-up of chronic neurological disorders such as amyotrophic lateral sclerosis96 and multiple sclerosis.97
The COVID-19 pandemic necessitated cancellation of elective or nonurgent contact with the healthcare system, including nonurgent electrodiagnostic studies and other neurological diagnostic tests.98
A very recent meta-analysis showed that the pooled percentage for having a pre-existing neurological disease in patients with severe COVID-19 was 8%. The presence of pre-existing neurological disease was frequently not specified in detail.3 Futhermore, there is a 2.5-fold increase in odds of severe COVID-19 illness with a previous history of cerebrovascular disease.99
Moreover, many patients with neurological autoimmune disease such as multiple sclerosis, neuromyelitis optica, myasthenia gravis, inflammatory myopathies, and neuropathies are on a wide variety of immunosuppressive therapies. It might be prudent for such patients to take extra precautions to prevent exposure to the virus and in selected instances to reevaluate the dosages of the medications.5 Holding or suspending oral corticosteroids or subcutaneous immunoglobulins is not routinely recommended, whereas requirement for intravenous infusions needs to be taken into risk/benefit discussions as reliance on health workers may increase the overall risks.78
When treating a neurological patient with SARS-Cov-2 infection, it is important to check the pharmacological interactions (see https://www.covid19-druginteractions.org ). Furthermore, the possibility of drug toxicity should be considered before using (hydroxy)-chloroquine (with or without azithromycin), particularly in individuals who may be more susceptible to these effects, including subjects with epilepsy, porphyria, and myasthenia gravis.2
Conclusion
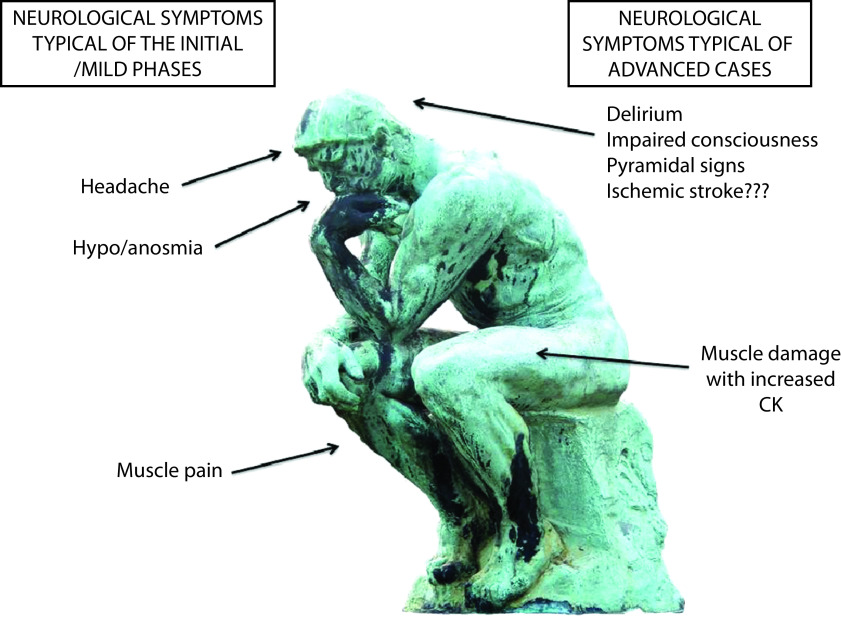
SARS-Cov-2 infection may result in neurological signs and symptoms, through different mechanisms. Although direct infection of the CNS is uncertain or very rare and para-infectious complications (e.g. inflammatory neuropathies and possibly ADEM) are rare, delirium and septic encephalopathy are common in severely ill patients.100 A schematic representation of the most typical neurological features associated with COVID-19 is presented in Figure 2. Smell dysfunction is a fairly specific,101 ‘key’ symptom of COVID-19 infection (more frequent in mild cases and especially in younger people and females).9 Headache follows a similar pattern. Muscle pain is common in both mild and severe cases, and in the most compromised patients, it is accompanied by increased CK levels and by a likely true myopathic damage. Ischemic stroke has been reported as a possible complication of the hypercoagulability associated with severe SARS-Cov-2 infection, but further studies are needed.
Figure 2.
The most typical neurological features associated with mild (left) and severe (right) coronavirus disease 2019.
During the epidemic period of COVID-19, when seeing patients with these neurological manifestations, clinicians should suspect a possible SARS-Cov-2 infection. Most neurological manifestations may occur early during the course of the illness.7
Interestingly, angiotensin converting enzyme 2, which was identified as the functional receptor for SARS-Cov-2, is present in multiple human tissues, including nervous system and skeletal muscle.7 However to date only very limited anedoctal data suggest a systematic neuroinvasive potential of SARS-Cov-2. It has been hypothesized that SARS-Cov-2 can enter the human CNS, based upon the observation that several patients need mechanical ventilation to treat respiratory failure.6 However, respiratory failure caused by pneumonia is clinically distinct from that caused by brainstem dysfunction. The known respiratory clinical features of COVID-19 do not suggest that involvement of the CNS is a common cause of respiratory failure in these patients, and the possibility of CNS entry by SARS-Cov-2 remains plausible, but unproven.102 SARS-Cov-2 genomes were not found in two COVID-19 patients with neurological symptoms,103 and direct SARS-Cov-2 meningoencephalitis is likely very rare.
Given that COVID-19 patients can present with neurological symptoms and signs, neurologists need to be involved, alert, and prepared.104 Furthermore, the longitudinal follow-up of people who have been infected by SARS-Cov-2 should include a careful assessment of the nervous system,105 in order to evaluate the possible development of late complications.
Neurological practice will not be the same until a vaccine is available.
Acknowledgements
The authors are grateful to Dr Alessandro Taliani (Unit of Radiology, San Luca Hospital, Lucca, Italy) for the technical support.
Footnotes
Contributions: All authors contributed to the preparation of this review. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: The authors declare that they have no conflicts of interest. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2020/05/dic.2020-5-1-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2020 Orsucci D, Caldarazzo Ienco E, Nocita G, Napolitano A, Vista M. https://doi.org/10.7573/dic.2020-5-1. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Article URL: https://www.drugsincontext.com/neurological-features-of-covid-19-and-their-treatment:-a-review
Provenance: invited; externally peer reviewed.
Peer review comments to author: 23 May 2020
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office dic.editorial@bioexcelpublishing.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Gennaro F, Pizzol D, Marotta C, et al. Coronavirus diseases (COVID-19) current status and future perspectives: a narrative review. Int J Environ Res Public Health. 2020;17(8) doi: 10.3390/ijerph17082690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herman C, Mayer K, Sarwal A. Scoping review of prevalence of neurologic comorbidities in patients hospitalized for COVID-19. Neurology. 2020 doi: 10.1212/WNL.0000000000009673. [DOI] [PubMed] [Google Scholar]
- 4.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nath A. Neurologic complications of coronavirus infections. Neurology. 2020 doi: 10.1212/WNL.0000000000009455. [DOI] [PubMed] [Google Scholar]
- 6.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of sars-cov2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020 doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020 doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lechien JR, Chiesa-Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1,420 european patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020 doi: 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beltran-Corbellini A, Chico-Garcia JL, Martinez-Poles J, et al. Acute-onset smell and taste disorders in the context of COVID-19: a pilot multicenter PCR-based case-control study. Eur J Neurol. 2020 doi: 10.1111/ene.14273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boscolo-Rizzo P, Borsetto D, Spinato G, et al. New onset of loss of smell or taste in household contacts of home-isolated SARS-CoV-2-positive subjects. Eur Arch Otorhinolaryngol. 2020 doi: 10.1007/s00405-020-06066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laurendon T, Radulesco T, Mugnier J, et al. Bilateral transient olfactory bulbs edema during COVID-19-related anosmia. Neurology. 2020 doi: 10.1212/WNL.0000000000009850. [DOI] [PubMed] [Google Scholar]
- 13.Butowt R, Bilinska K. Sars-cov-2: olfaction, brain infection, and the urgent need for clinical samples allowing earlier virus detection. ACS Chem Neurosci. 2020 doi: 10.1021/acschemneuro.0c00172. [DOI] [PubMed] [Google Scholar]
- 14.Boesveldt S, Postma EM, Boak D, et al. Anosmia-a clinical review. Chem Senses. 2017;42(7):513–523. doi: 10.1093/chemse/bjx025. http://doi.org/3844730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borges do Nascimento IJ, Cacic N, Abdulazeem HM, et al. Novel coronavirus infection (COVID-19) in humans: a scoping review and meta-analysis. J Clin Med. 2020;9(4) doi: 10.3390/jcm9040941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belvis R. Headaches during COVID-19: my clinical case and review of the literature. Headache. 2020 doi: 10.1111/head.13841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helms J, Kremer S, Merdji H, et al. Neurologic features in severe sars-cov-2 infection. N Engl J Med. 2020 doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020 doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotfis K, Williams Roberson S, Wilson JE, Dabrowski W, Pun BT, Ely EW. COVID-19: ICU delirium management during sars-cov-2 pandemic. Crit Care. 2020;24(1):176. doi: 10.1186/s13054-020-02882-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lovell N, Maddocks M, Etkind SN, et al. Characteristics, symptom management and outcomes of 101 patients with COVID-19 referred for hospital palliative care. J Pain Symptom Manage. 2020 doi: 10.1016/j.jpainsymman.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanders BJ, Bakar M, Mehta S, et al. Hyperactive delirium requires more aggressive management in patients with COVID-19: temporarily rethinking “low and slow”. J Pain Symptom Manage. 2020;(20):S0885–3924. 30389–4. doi: 10.1016/j.jpainsymman.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slooter AJ, Van De Leur RR, Zaal IJ. Delirium in critically ill patients. Handb Clin Neurol. 2017;141:449–466. doi: 10.1016/B978-0-444-63599-0.00025-9. [DOI] [PubMed] [Google Scholar]
- 23.Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020 doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain R, Young M, Dogra S, et al. COVID-19 related neuroimaging findings: a signal of thromboembolic complications and a strong prognostic marker of poor patient outcome. J Neurol Sci. 2020;414:116923. doi: 10.1016/j.jns.2020.116923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez-Pinto T, Luna-Rodriguez A, Moreno-Estebanez A, Agirre-Beitia G, Rodriguez-Antiguedad A, Ruiz-Lopez M. Emergency room neurology in times of COVID-19: malignant ischemic stroke and sars-cov2 infection. Eur J Neurol. 2020 doi: 10.1111/ene.14286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beyrouti R, Adams ME, Benjamin L, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020 doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oxley TJ, Mocco J, Majidi S, et al. Large-vessel stroke as a presenting feature of COVID-19 in the young. N Engl J Med. 2020 doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benussi A, Pilotto A, Premi E, et al. Clinical characteristics and outcomes of inpatients with neurologic disease and COVID-19 in Brescia, Lombardy, Italy. Neurology. 2020. [DOI] [PubMed]
- 30.Ranucci M, Ballotta A, Di Dedda U, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020 doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zou Y, Guo H, Zhang Y, et al. Analysis of coagulation parameters in patients with COVID-19 in Shanghai, China. Biosci Trends. 2020 doi: 10.5582/bst.2020.03086. [DOI] [PubMed] [Google Scholar]
- 32.Morelli N, Rota E, Terracciano C, et al. The baffling case of ischemic stroke disappearance from the casualty department in the COVID-19 era. Eur Neurol. 2020;2020:1–3. doi: 10.1159/000507666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moshayedi P, Ryan TE, Mejia LLP, Nour M, Liebeskind DS. Triage of acute ischemic stroke in confirmed COVID-19: large vessel occlusion associated with coronavirus infection. Front Neurol. 2020;11:353. doi: 10.3389/fneur.2020.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zayet S, Klopfenstein T, Kovẚcs R, Stancescu S, Hagenkötter B. Acute cerebral stroke with multiple infarctions and COVID-19, France, 2020. Emerg Infect Dis. 2020;26 doi: 10.3201/eid2609.201791. doi: 10.3201/eid2609.201791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrios-López JM, Rego-García I, Muñoz Martínez C, et al. Ischaemic stroke and SARS-CoV-2 infection: a causal or incidental association? Neurologia. 2020;(20):S0213–4853. 30095–5. doi: 10.1016/j.nrl.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gunasekaran K, Amoah K, Rajasurya V, Buscher MG. Stroke in a young COVID -19 patient. QJM. 2020. hcaa177. [DOI] [PMC free article] [PubMed]
- 37.Morassi M, Bagatto D, Cobelli M, et al. Stroke in patients with SARS-CoV-2 infection: case series. J Neurol. 2020;2020:1–8. doi: 10.1007/s00415-020-09885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tunç A, Ünlübaş Y, Alemdar M, Akyüz E. Coexistence of COVID-19 and acute ischemic stroke report of four cases. J Clin Neurosci. 2020 doi: 10.1016/j.jocn.2020.05.018. S0967-5868(20)31081-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldberg MF, Goldberg MF, Cerejo R, Tayal AH. Cerebrovascular disease in COVID-19. AJNR Am J Neuroradiol. 2020 doi: 10.3174/ajnr.A6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Viguier A, Delamarre L, Duplantier J, Olivot JM, Bonneville F. Acute ischemic stroke complicating common carotid artery thrombosis during a severe COVID-19 infection. J Neuroradiol. 2020 doi: 10.1016/j.neurad.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khosravani H, Rajendram P, Notario L, Chapman MG, Menon BK. Protected code stroke: hyperacute stroke management during the coronavirus disease 2019 (COVID-19) pandemic. Stroke. 2020 doi: 10.1161/STROKEAHA.120.029838. STROKEAHA120029838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baracchini C, Pieroni A, Viaro F, et al. Acute stroke management pathway during coronavirus-19 pandemic. Neurol Sci. 2020 doi: 10.1007/s10072-020-04375-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fraser JF, Arthur AS, Chen M, et al. Society of neurointerventional surgery recommendations for the care of emergent neurointerventional patients in the setting of COVID-19. J Neurointerv Surg. 2020 doi: 10.1136/neurintsurg-2020-016098. [DOI] [PubMed] [Google Scholar]
- 44.Wang A, Mandigo GK, Yim PD, Meyers PM, Lavine SD. Stroke and mechanical thrombectomy in patients with COVID-19: technical observations and patient characteristics. J Neurointerv Surg. 2020 doi: 10.1136/neurintsurg-2020-016220. [DOI] [PubMed] [Google Scholar]
- 45.Qureshi AI, Abd-Allah F, Al-Senani F, et al. Management of acute ischemic stroke in patients with COVID-19 infection: insights from an international panel. Am J Emerg Med. 2020;(20):S0735–6757. 30356–9. doi: 10.1016/j.ajem.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharifi-Razavi A, Karimi N, Rouhani N. COVID-19 and intracerebral haemorrhage: causative or coincidental? New Microbes New Infect. 2020;35:100669. doi: 10.1016/j.nmni.2020.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carroll E, Lewis A. Catastrophic intracranial hemorrhage in two critically ill patients with COVID-19. Neurocrit Care. 2020 doi: 10.1007/s12028-020-00993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al-Olama M, Rashid A, Garozzo D. COVID-19-associated meningoencephalitis complicated with intracranial hemorrhage: a case report. Acta Neurochir (Wien) 2020:1–5. doi: 10.1007/s00701-020-04402-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heman-Ackah SM, Su YS, Spadola M, et al. Neurologically devastating intraparenchymal hemorrhage in COVID-19 patients on extracorporeal membrane oxygenation: a case series. Neurosurgery. 2020. nyaa198. [DOI] [PMC free article] [PubMed]
- 50.Muhammad S, Petridis A, Cornelius JF, Hänggi D. Letter to editor: severe brain haemorrhage and concomitant COVID-19 Infection: a neurovascular complication of COVID-19. Brain Behav Immun. 2020;(20):S0889–1591. 30802–3. doi: 10.1016/j.bbi.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hughes C, Nichols T, Pike M, Subbe C, Elghenzai S. Cerebral venous sinus thrombosis as a presentation of COVID-19. Eur J Case Rep Intern Med. 2020;7:001691. doi: 10.12890/2020_001691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hemasian H, Ansari B. First case of COVID-19 presented with cerebral venous thrombosis: a rare and dreaded case. Rev Neurol (Paris) 2020 doi: 10.1016/j.neurol.2020.04.013. S0035-3787(20)30557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poillon G, Obadia M, Perrin M, Savatovsky J, Lecler A. Cerebral venous thrombosis associated with COVID-19 infection: causality or coincidence? J Neuroradiol. 2020 doi: 10.1016/j.neurad.2020.05.003. S0150-9861(20)30167-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yin R, Feng W, Wang T, et al. Concomitant neurological symptoms observed in a patient diagnosed with coronavirus disease 2019. J Med Virol. 2020 doi: 10.1002/jmv.25888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rábano-Suárez P, Bermejo-Guerrero L, Méndez-Guerrero A, et al. Generalized myoclonus in COVID-19. Neurology. 2020. [DOI] [PMC free article] [PubMed]
- 56.Vollono C, Rollo E, Romozzi M, et al. Focal status epilepticus as unique clinical feature of COVID-19: a case report. Seizure. 2020;78:109–112. doi: 10.1016/j.seizure.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fasano A, Cavallieri F, Canali E, Valzania F. First motor seizure as presenting symptom of SARS-CoV-2 infection. Neurol Sci. 2020:1–3. doi: 10.1007/s10072-020-04460-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Balloy G, Mahé PJ, Leclair-Visonneau L, et al. Non-lesional status epilepticus in a patient with coronavirus disease 2019. Clin Neurophysiol. 2020 doi: 10.1016/j.clinph.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Somani S, Pati S, Gaston T, Chitlangia A, Agnihotri S. De Novo status epilepticus in patients with COVID-19. Ann Clin Transl Neurol. 2020 doi: 10.1002/acn3.51071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mahammedi A, Saba L, Vagal A, et al. Imaging in neurological disease of hospitalized COVID-19 patients: an Italian multicenter retrospective observational study. Radiology. 2020 doi: 10.1148/radiol.2020201933. 201933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Franceschi AM, Ahmed O, Giliberto L, Castillo M. Hemorrhagic Posterior Reversible Encephalopathy Syndrome as a manifestation of COVID-19 infection. AJNR Am J Neuroradiol. 2020 doi: 10.3174/ajnr.A6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dixon L, Varley J, Gontsarova A, et al. COVID-19-related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e789. doi: 10.1212/NXI.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brun G, Hak J, Coze S, et al. COVID-19 – White matter and globus pallidum lesions demyelination or small-vessel vasculitis? Neurol Neuroimmunol Neuroinflamm. 2020;7(4):e777. doi: 10.1212/NXI.0000000000000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Radmanesh A, Derman A, Lui YW, et al. COVID-19 -associated diffuse leukoencephalopathy and microhemorrhages. Radiology. 2020:202040. doi: 10.1148/radiol.2020202040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sachs JR, Gibbs KW, Swor DE, et al. COVID-19-Associated leukoencephalopathy. Radiology. 2020:201753. doi: 10.1148/radiol.2020201753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pilotto A, Odolini S, Stefano Masciocchi S, et al. Steroid-responsive encephalitis in COVID-19 disease. Ann Neurol. 2020 doi: 10.1002/ana.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kajani R, Apramian A, Vega A, Ubhayakar N, Xu P, Liu A. Neuroleptic Malignant Syndrome in a COVID-19 patient. Brain Behav Immun. 2020 doi: 10.1016/j.bbi.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Munz M, Wessendorf S, Koretsis G, et al. Acute transverse myelitis after COVID-19 pneumonia. J Neurol. 2020 doi: 10.1007/s00415-020-09934-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dugue R, Cay-Martinez KC, Thakur K, et al. Neurologic manifestations in an infant with COVID-19. Neurology. 2020 doi: 10.1212/WNL.0000000000009653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reichard RR, Kashani KB, Boire NA, Constantopoulos E, Guo Y, Lucchinetti CF. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020 doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with sars-coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duong L, Xu P, Liu A. Meningoencephalitis without respiratory failure in a young female patient with COVID-19 infection in downtown Los Angeles, early April 2020. Brain Behav Immun. 2020 doi: 10.1016/j.bbi.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang YH, Jiang D, Huang JT. SARS-CoV-2 detected in cerebrospinal fluid by PCR in a case of COVID-19 encephalitis. Brain Behav Immun. 2020;(20):S0889–1591. 30770–4. doi: 10.1016/j.bbi.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Asadi-Pooya AA, Simani L. Central nervous system manifestations of COVID-19: a systematic review. J Neurol Sci. 2020;413:116832. doi: 10.1016/j.jns.2020.116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu L, Xiong W, Liu D, et al. New-onset acute symptomatic seizure and risk factors in corona virus disease 2019: a retrospective multicenter study. Epilepsia. 2020 doi: 10.1111/epi.16524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;(20):S0140–6736. 31189–2. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jin M, Tong Q. Rhabdomyolysis as potential late complication associated with COVID-19. Emerg Infect Dis. 2020;26(7) doi: 10.3201/eid2607.200445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guidon AC, Amato AA. COVID-19 and neuromuscular disorders. Neurology. 2020 doi: 10.1212/WNL.0000000000009566. [DOI] [PubMed] [Google Scholar]
- 79.Alberti P, Beretta S, Piatti M, et al. Guillain-Barre syndrome related to COVID-19 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7(4) doi: 10.1212/NXI.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barre syndrome associated with sars-cov-2 infection: causality or coincidence? Lancet Neurol. 2020;19(5):383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ottaviani D, Boso F, Tranquillini E, et al. Early Guillain-Barré syndrome in coronavirus disease 2019 (COVID-19): a case report from an Italian COVID-hospital. Neurol Sci. 2020:1–4. doi: 10.1007/s10072-020-04449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Su XW, Palka SV, Rao RR, Chen FS, Brackney CR, Cambi F. SARS-CoV-2 associated Guillain-Barre Syndrome with dysautonomia. Muscle Nerve. 2020 doi: 10.1002/mus.26988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Padroni M, Mastrangelo V, Asioli GM, et al. Guillain-Barre syndrome following COVID-19: new infection, old complication? J Neurol. 2020 doi: 10.1007/s00415-020-09849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Camdessanche JP, Morel J, Pozzetto B, Paul S, Tholance Y, Botelho-Nevers E. COVID-19 may induce Guillain-Barre syndrome. Rev Neurol (Paris) 2020 doi: 10.1016/j.neurol.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arnaud S, Budowski C, Ng Wing Tin S, Degos B. Post SARS-CoV-2 Guillain-Barré syndrome. Clin Neurophysiol. 2020;131(7):1652–1654. doi: 10.1016/j.clinph.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Scheidl E, Canseco DD, Hadji-Naumov A, Bereznai B. Guillain-Barré syndrome during SARS-CoV-2 pandemic: a case report and review of recent literature. J Peripher Nerv Syst. 2020 doi: 10.1111/jns.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gigli GL, Bax F, Marini A, et al. Guillain-Barré syndrome in the COVID-19 era: just an occasional cluster? J Neurol. 2020:1–3. doi: 10.1007/s00415-020-09911-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pfefferkorn T, Dabitz R, von Wernitz-Keibel T, et al. Acute polyradiculoneuritis with locked-in syndrome in a patient with COVID-19. J Neurol. 2020:1–2. doi: 10.1007/s00415-020-09897-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sedaghat Z, Karimi N. Guillain-Barre syndrome associated with COVID-19 infection: a case report. J Clin Neurosci. 2020 doi: 10.1016/j.jocn.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gutierrez-Ortiz C, Mendez A, Rodrigo-Rey S, et al. Miller fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020 doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- 91.Dinkin M, Gao V, Kahan J, et al. COVID-19 presenting with ophthalmoparesis from cranial nerve palsy. Neurology. 2020 doi: 10.1212/WNL.0000000000009700. [DOI] [PubMed] [Google Scholar]
- 92.Toscano G, Palmerini F, Ravaglia S, et al. Guillain-Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020 doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wijdicks EF, Klein CJ. Guillain-Barre syndrome. Mayo Clin Proc. 2017;92(3):467–479. doi: 10.1016/j.mayocp.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 94.Majersik JJ, Reddy VK. Acute neurology during the COVID-19 pandemic: supporting the front line. Neurology. 2020 doi: 10.1212/WNL.0000000000009564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Delly F, Syed MJ, Lisak RP, Zutshi D. Myasthenic crisis in COVID-19. J Neurol Sci. 2020;414:116888. doi: 10.1016/j.jns.2020.116888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Andrews JA, Berry JD, Baloh RH, et al. Amyotrophic lateral sclerosis care and research in the USA during the COVID-19 pandemic: challenges and opportunities. Muscle Nerve. 2020 doi: 10.1002/mus.26989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.D’Haeseleer M, Eelen P, Sadeghi N, D’Hooghe MB, Van Schependom J, Nagels G. Feasibility of real-time internet-based teleconsultation in patients with multiple sclerosis: interventional pilot study. J Med Internet Res. 2020 doi: 10.2196/18178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kassardjian CD, Desai U, Narayanaswami P. Practical guidance for managing EMG requests and testing during the COVID-19 pandemic. Muscle Nerve. 2020 doi: 10.1002/mus.26891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aggarwal G, Lippi G, Michael Henry B. Cerebrovascular disease is associated with an increased disease severity in patients with coronavirus disease 2019 (COVID-19): a pooled analysis of published literature. Int J Stroke. 2020;15(4):385–389. doi: 10.1177/1747493020921664. [DOI] [PubMed] [Google Scholar]
- 100.Needham EJ, Chou SH, Coles AJ, Menon DK. Neurological implications of COVID-19 infections. Neurocrit Care. 2020 doi: 10.1007/s12028-020-00978-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pleasure SJ, Green AJ, Josephson SA. The spectrum of neurologic disease in the severe acute respiratory syndrome coronavirus 2 pandemic infection: neurologists move to the frontlines. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1065. [DOI] [PubMed] [Google Scholar]
- 102.Turtle L. Respiratory failure alone does not suggest central nervous system invasion by sars-cov-2. J Med Virol. 2020 doi: 10.1002/jmv.25828. [DOI] [PubMed] [Google Scholar]
- 103.Al Saiegh F, Ghosh R, Leibold A, et al. Status of sars-cov-2 in cerebrospinal fluid of patients with COVID-19 and stroke. J Neurol Neurosurg Psychiatry. 2020 doi: 10.1136/jnnp-2020-323522. [DOI] [PubMed] [Google Scholar]
- 104.Liu K, Pan M, Xiao Z, Xu X. Neurological manifestations of the coronavirus (sars-cov-2) pandemic 2019–2020. J Neurol Neurosurg Psychiatry. 2020 doi: 10.1136/jnnp-2020-323177. [DOI] [PubMed] [Google Scholar]
- 105.Matias-Guiu J, Gomez-Pinedo U, Montero-Escribano P, Gomez-Iglesias P, Porta-Etessam J, Matias-Guiu JA. Should we expect neurological symptoms in the sars-cov-2 epidemic? Neurologia. 2020;35(3):170–175. doi: 10.1016/j.nrl.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]