Abstract
A meeting entitled ‘Current Perspective on the Use of Statins in the Treatment of Dyslipidemic Patients’ was held in Stresa, Italy, on 27–28th June 2019. The presentations covered the 2019 European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS) guidelines on dyslipidaemia, with discussion about the importance of controlling low-density lipoprotein cholesterol (LDL-C) and the pharmacological opportunities to reach the novel lipid goals. The roles of statins to manage dyslipidaemia in patients with different cardiovascular risks were also discussed. In particular, the efficacy and safety of pitavastatin for the treatment of dyslipidaemia were reviewed, highlighting its further advantages beyond LDL-C reduction. Therefore, the impact of statins on the glycaemic profile was discussed in view of the null/lower effect of pitavastatin as compared with other statins, as well as the interaction profile with other drugs commonly used. This meeting report summarizes the main messages of the discussion with a special focus on pitavastatin, whose main features in different settings are described.
Keywords: cardiovascular risk, diabetes, lipid, low-density lipoprotein, pitavastatin, statin
Introduction
The retention of low-density lipoprotein (LDL) and apolipoprotein B (apoB)-containing lipoproteins in the arterial wall is the key initiating event in atherogenesis. Recent European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) guidelines on dyslipidaemia revised LDL-C management goals as a function of cardiovascular (CV) risk. Statins play an important role in the management of hypercholesterolaemia by lowering the levels of LDL-C.
This review summarises the outcomes of an international meeting entitled ‘Current Perspective on the Use of Statins in the Treatment of Dyslipidemic Patients’, which was held in Stresa, Italy, on 27–28th June 2019. The meeting was endorsed by EAS and supported by an unrestricted grant from Recordati. It was chaired by Dr Alberico Catapano (Italy) and Dr Lale Tokgözoglu (Turkey).
The discussion involved many aspects of the use of statins in dyslipidaemia treatment based on the new 2019 ESC/EAS guidelines. The debate covered the importance of LDL-C control and the pharmacological opportunities currently available to reach the novel lipid goals proposed. The diagnosis and treatment of dyslipidaemia in the context of cardiometabolic and atherosclerotic cardiovascular disease (CVD) and the central role of statins in managing dyslipidaemia in patients with different CV risks were of particular interest to the specialists who attended the conference. Finally, specific attention was focused on pitavastatin and on its potential benefits beyond LDL-C reduction.
This report is based mainly on the presentations made at the meeting and has been updated with more recent literature data.
ESC/EAS guidelines for plasma lipid control and treatment goals: current status and future challenge
The causal role of LDL in the development of atherosclerosis is well established, and evidence suggests that global risk of CVD directly correlates with LDL-C levels, where LDL-C reduction was shown to result in disease remission.1
The choice of an intervention strategy should be based on the individual risk. The 2016 ESC/EAS guidelines recommended more ambitious goals and, therefore, more intensive lipid lowering in patients with higher risk of CVD. For example, patients with ≥120 mg/dL LDL-C and at low risk of CVD were advised to follow a healthy lifestyle, whereas patients with the same level of LDL-C and high risk of CVD were advised to add immediately a pharmacological treatment in addition to a healthy lifestyle.2
The new guidelines also highlighted the importance of assessing the overall CV risk and identified new specific LDL-C goals for each level of risk:3 an LDL-C reduction of ≥50% from baseline and an LDL-C goal of <55 mg/dL for patients at very high risk; an LDL-C reduction of ≥50% from baseline and an LDL-C goal of <70 mg/dL for patients at high risk; an LDL-C goal of <100 mg/dL for individuals at moderate risk or <116 m/dL for individuals at low risk (Table 1).
Table 1.
Novel cardiovascular risk categories and LDL-C target, as per 2019 ESC/EAS guidelines (adapted from ref 3).
| Very high | People with any of the following: Documented ASCVD, either clinical or unequivocal on imaging. Documented ASCVD includes previous ACS (MI or unstable angina), stable angina, coronary revascularisation (PCI, CABG, and other arterial revascularisation procedures), stroke and TIA, and peripheral arterial disease. Unequivocally documented ASCVD on imaging includes those findings that are known to be predictive of clinical events, such as significant plaque on coronary angiography or CT scan (multivessel coronary disease with two major epicardial arteries having >50% stenosis), or on carotid ultrasound. DM with target organ damage or at least three major risk factors or early onset of T1DM of long duration (>20 years). Severe CKD (eGFR _10% for 10-year risk of fatal CVD. FH with ASCVD or with another major risk factor. |
LDL-C reduction of ≥50% from baseline LDL-C goal of <55 mg/dL |
| High | People with: Markedly elevated single risk factors, in particular TC >8 mmol/L (>310 mg/dL), LDL-C >4.9 mmol/L (>190 mg/dL), or BP ≥180/110 mmHg. Patients with FH without other major risk factors. Patients with DM without target organ damage or with DM duration ≥10 years or another additional risk factor. Moderate CKD (eGFR 3059 mL/min/1.73 m2). A calculated SCORE ≥5% and <10% for 10-year risk of fatal CVD |
LDL-C reduction of ≥50% from baseline LDL-C goal of <70 mg/dL |
| Moderate | Young patients (T1DM <35 years; T2DM <50 years) with DM duration <10 years, without other risk factors. Calculated SCORE ≥1% and <5% for 10-year risk of fatal CVD. | LDL-C goal of <100 mg/dL for individuals |
| Low | Calculated SCORE <1% for 10-year risk of fatal CVD. | <116 m/dL for individuals |
ACS, acute coronary syndrome; ASCVD, atherosclerotic cardiovascular disease; BP, blood pressure; CABG, coronary artery bypass grafting; CKD, chronic kidney disease; CT, computed tomography; DM, diabetes mellitus; EAS, European Atherosclerosis Society; eGFR, estimated glomerular filtration rate; ESC, European Society of Cardiology; FH, familial hypercholesterolaemia; LDL-C, low-density lipoprotein cholesterol; MI, Myocardial infarction; PCI, Percutaneous coronary intervention; SCORE, Systematic Coronary Risk Evaluation; TC, total cholesterol; TIA, transient ischaemic attack.
Therefore, the best pharmacological approach will be based on the initial CV risk assessment. However, there is no standard method to assess the overall CV risk for each patient, considering that the risk estimate usually results from several factors combined. One of these factors is patient’s age. The Systematic Coronary Risk Evaluation (SCORE) system correlates CV risk with age: for example, given the presence of similar risk factors, the risk of death within 10 years is much higher for a 65-year-old than for a 40-year-old.4,5 Based on this model, the treatment should be proposed preferentially to older people. However, genetic data describe a linear relationship between per unit-change in LDL-C and the risk of CV disease, suggesting that an early treatment would be more effective than a late one.6 Therefore, the goal LDL-C level in patients with CV risk should be reached early in the treatment process and should be maintained calling for an effective and safe treatment.
Low levels of high-density lipoprotein-C (HDL-C) are associated with a higher CV risk;7 however, HDLs are not a therapeutic target in the 2019 ESC/EAS guidelines as no causality for the HDL-C in the atherosclerotic process has been shown. It should be noted that measuring the levels of non-HDL-C becomes relevant in the presence of high levels of triglycerides; furthermore, monitoring apoB levels is also recommended because it recapitulates the levels of all atherogenic lipoproteins (very-LDL [VLDL], remnants and LDL).3
It should also be considered that although it is evident that there is a clinical benefit of lowering LDL-C, this is proportional to the absolute LDL-C reduction in terms of both absolute and relative risk reduction (RRR) of CV events; as an example, lowering the LDL-C levels by 50% from 50 to 25 mg/dL does not deliver the same results as lowering the levels from 150 to 75 mg/dL (exactly the same 50% reduction). In patients at the same CV risk, treatment effectiveness is larger when baseline LDL-C is higher.7 In very high-risk patients, it is possible to consider a more aggressive approach and more stringent goals, keeping in mind that the absolute risk reduction (ARR) would be lower with lower LDL-C levels.
Statins as the foundation of the dyslipidaemia therapy
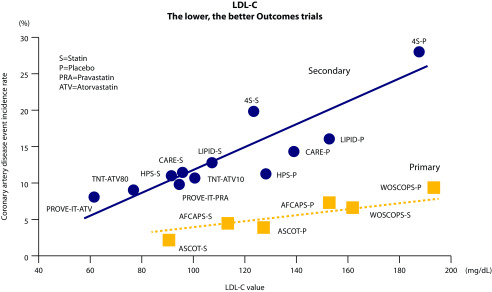
Statins were considered to be the pillars of anti-LDL-C treatments in the 2019 ESC/EAS guidelines.3 Statins have a class effect in reducing CV risk, but different statins have different degrees of LDL-C lowering, ranging from 30 to 50% reduction of LDL-C.8 The choice of which statin to use should be individualised on patients’ characteristics and depends on many factors, including baseline risk, goals to be reached and individual clinical variations.3 Numerous outcome trials have shown that the use of statins results in primary and secondary prevention of CV events (Figure 1).9 It has been shown that statins favour plaque stabilisation, as demonstrated by optical coherence tomography (OCT) and virtual histology (VH), and 1-year treatment with statin decreased lipid content and thickened fibrous cap, thus stabilising the plaque.10 The Plaque Regression With Cholesterol Absorption Inhibitor or Synthesis Inhibitor Evaluated by IntraVascular UltraSound (PRECISE-IVUS) and Progression of Atherosclerotic Plaque DetermIned by Computed Tomographic Angiography Imaging (PARADIGM) studies showed that statins monotherapy, and in combination with ezetimibe, can induce plaque regression, decrease the plaque volume and increase the calcification.10,11
Figure 1.
Relationship between LDL-C and CVD (adapted from ref 8).
In secondary prevention, statins significantly decreased mortality and morbidity, regardless of gender, age and presence of risk factors or diabetes.12 The Cholesterol Treatment Trialist (CTT) analysis showed that a LDL-C reduction by 2 mmol/L would decrease the major CV events (MACE) risk by 45%.13
High LDL-C levels have a cumulative effect on CV risk; therefore, it is advisable to start antilipidaemic treatment as early as possible, in order to reduce the exposure to high LDL-C levels and to delay development of CV events.
Considering that high LDL-C levels (>190 mg/dL) lead to a 5-fold increase of 30-year risk of coronary artery disease (CAD), maintaining lower LDL-C levels from a young age helps reduce the CV risk.3 Therefore, the benefit-to-risk ratio for long-term use of statins is highly favourable as there are few potential side effects associated with this treatment. However, some categories of patients, such as the elderly, female and those with low body mass index, hypothyroidism, renal–hepatic impairment, polypharmacy that affects CYP 450 metabolism and vitamin D deficiency, may be at a higher risk of adverse events and should be monitored during therapy.3 Both in the USA and Europe, current guidelines recommend the use of statins in the elderly (>75 years old) for secondary prevention, as elderly patients are generally at a higher risk of CV events.3,14 In a meta-analysis, the use of statins was shown to result in reducing major CV events per mmol/L reduction of LDL-C by age at randomisation in the elderly population.15 This analysis also demonstrated that patients over the age of 75 benefited from statin treatment and, although RRR was lower than that in younger patients (RRR 13 versus 21%), the ARR was evident (ARR 0.5% per year/mmol/L reduction of LDL).15 However, the choice of therapy in elderly patients must be considered carefully, especially in light of potentially associated comorbidities.3 It is advisable to start at low doses and titrate up especially in frail patients.
Patients with chronic kidney disease (CKD) are also at high risk for CV events. Guidelines recommend the use of statins in patients with non-dialysis-dependent moderate-to-severe CKD; statins that are mainly eliminated by the hepatic route, such as atorvastatin, fluvastatin and pitavastatin, should be preferred, whilst those metabolised via CYP 3A4 should be avoided because they might lead to adverse events.3 Furthermore, the dose of statins should be decreased from standard levels in stage 5 renal disease (GFR <15 mL/min/1.73 m2).3
There is a concern about increased risk of diabetes with statins. Patients who suffer from hypertension, multiple risk factors, obesity and metabolic syndromes may have an increased risk of developing new onset diabetes, especially with high doses of statins.3 Studies have shown that pravastatin and pitavastatin have a neutral effect on glycaemic parameters, although the clinical relevance of the increased glycaemia by statins still needs to be fully understood.3
There is also some concern about a potential increased risk of intracerebral haemorrhage, especially in patients with a prior stroke raised by the Lipitor In The Prevention Of Stroke, For Patients Who Have Had A Previous Stroke (SPARCL) study.16 However, these data have not been substantiated in other studies, and a meta-analysis on statins in patients with previous stroke including more than 40 trials showed that these drugs clearly decreased mortality and recurrence in stroke survivors.17
In clinical practice, the main problem associated with prolonged use of statins is the loss of compliance over time. It has been reported that within 1 year of initiating statin therapy, about 50% of patients discontinue therapy.18 The EUROASPIRE IV Survey on Cardiovascular Disease Prevention and Diabetes study concluded that only 37% of patients with CAD remained on their original treatment dose after 6 or 12 months of follow-up.19 The main reason for discontinuation of statin therapy is the occurrence of side effects (mostly myalgia).18 Muscle symptoms associated with statins (SAMS) may be due to the so-called ‘nocebo’ effect and are very difficult to properly identify. The best way to manage SAMS is to interrupt statins for 4–6 weeks, check the creatine kinase (CK) levels and restart treatment with a lower dose or with another molecule. Therefore, it would be essential to follow-up the patients who are taking statins to ensure compliance to therapy.3 A structured, team-based, patient-centred intervention could be effective to improve compliance to therapy.
Achieving similar compliance levels between trials (around 80%) and real-world setting represents the next challenge, and significant effort must be made to optimise the adherence to treatment and drug intake.20
Focus on pitavastatin
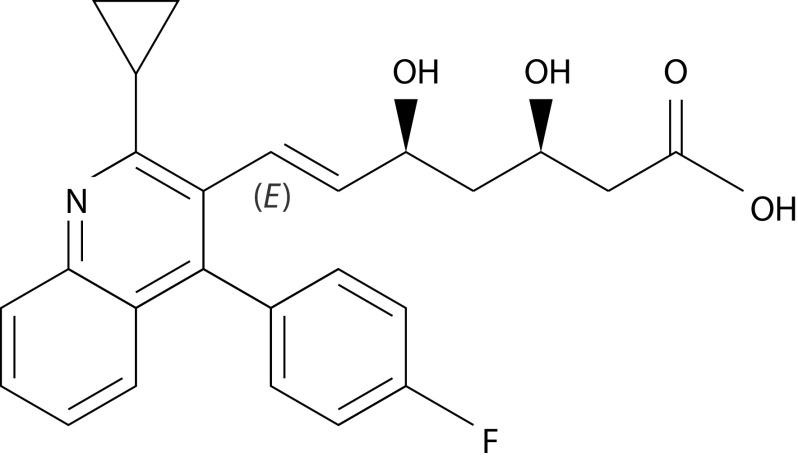
Pitavastatin is a competitive 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor that is available in the European Union (EU). It is used to reduce elevated total cholesterol and LDL-C levels in adults with primary hypercholesterolaemia and combined (mixed) dyslipidaemia (Table 2). The presence of the cyclopropyl group in its molecular structure (Figure 2) guarantees a high affinity binding to HMG-CoA reductase and reduces the extent of cholesterol synthesis, with a more potent effect than that reported for simvastatin and pravastatin.21
Table 2.
Summary of the effects of pitavastatin as monotherapy on lipid profile.
| Lipid | Effect (up to) |
|---|---|
| LDL-C | −47% |
| HDL-C | +29% |
| TC | −22% |
| TG | −32% |
| Non-HDL-C | −41% |
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol
Figure 2.
Molecular structure of pitavastatin.
Six large (n>325), randomised, double-blind, double-dummy, active comparator-controlled, multinational or multicentre, phase III or IV studies evaluated the short-term efficacy of pitavastatin in adult patients with uncontrolled primary hypercholesterolaemia or combined (mixed) dyslipidaemia regardless of dietary measures.22–26 Across the studies, patients were treated with pitavastatin (1, 2 or 4 mg) or an active comparator (atorvastatin or simvastatin at an equipotent dosage, or pravastatin at a dosage corresponding to that currently indicated in the USA and Europe) once daily for 12 weeks. Pitavastatin was generally equally effective compared to equipotent dosages of atorvastatin and simvastatin22–24 and was superior to pravastatin25,26 concerning the mean change from baseline of LDL, HDL, total cholesterol, triglycerides and rate of patients who achieved target LDL-C level. Assessment of the long-term effectiveness of pitavastatin showed that the drug provided sustained reduction of LDL-C levels compared with baseline over a 44–60 weeks treatment period, where the National Cholesterol Education Program and EAS LDL-C target levels were generally maintained in four extension studies.27
Based on the results from clinical trials and on the concept that the clinical benefit derives from LDL-C lowering, the 2016 ESC/EAS guidelines included pitavastatin amongst the three most potent monotherapy statins (along with atorvastatin and rosuvastatin) in reducing LDL-C by 50%.2 When monotherapy is not effective in reducing LDL-C, the 2019 ESC/EAS guidelines recommend combination therapy, between high-potent statins with ezetimibe, fibrates and Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors.3
As pitavastatin is characterised by a low potential for drug–drug interactions, its use as part of combined therapy is possible even in presence of polypharmacy. Indeed, the cyclopropyl group on pitavastatin diverts the drug away from metabolism by CYP 3A4 and allows only a small percentage of the drug to be transformed by CYP 2C9, whereas other statins are predominantly metabolised by cytochromes (lovastatin, simvastatin and atorvastatin by CYP 3A4, although fluvastatin and rosuvastatin are metabolised by CYP 2C9). In human hepatic microsomes, both pitavastatin acid and lactone were poorly metabolized, whereas lactone metabolites from other statins were shortly removed by CYP isoenzymes.27 This may explain why the concomitant administration of pitavastatin with drugs that inhibit CYP isoenzymes did not modify the incidence of muscle-related adverse drug reactions, which were described in the post-hoc analysis of the Livazo Effectiveness and Safety (LIVES) study.28 To date, pitavastatin is contraindicated only in patients treated with cyclosporin or lopinavir/ritonavir combination therapy, and it should be used with caution in people treated with fibrates or niacin.
Given its clinical effectiveness and its favourable pharmacokinetic profile, the use of pitavastatin was investigated in various clinical settings, such as acute coronary syndrome (ACS), CAD, diabetes and metabolic syndrome, human immunodeficiency virus (HIV), and kidney disease. Similar to other statins (atorvastatin), pitavastatin increases HDL-C levels and is neutral on the incidence of new onset diabetes or alteration in the glycaemic metabolism.29
In patients with HIV, pitavastatin shows a lower incidence of liver or muscle toxicity and other toxicities that may result from statin therapy. HIV-infected patients face an increased risk for CVD, estimated at 1.5 to 2-fold as compared with an HIV-uninfected person. Statins are the preferred agents for reducing the risk for CVD amongst HIV-infected populations based on guidance extrapolated from general population (HIV-uninfected) cholesterol-treatment guidelines. Pitavastatin represents the statin of choice for this class of patients, as it has similar cholesterol-lowering effects compared to rosuvastatin and atorvastatin, with very little adverse drug interactions with antiretroviral therapy or effects on glucose metabolism.30 The ‘Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE)’ showed that in comparison with placebo, the use of pitavastatin calcium (4 mg/day) resulted in the primary prevention of major adverse CV events (MACE) in 7500 people with HIV with low-to-moderate traditional risk and stable antiretroviral therapy.31
Furthermore, as pitavastatin is mainly eliminated via the liver, a dose reduction is not required in patients with kidney disease.32
HDL-C: an insight into lipidomics
The levels of HDL-C associated with the lowest risk of CV-related death, coronary heart disease (CHD) and myocardial infarction (MI) have been defined based on data from the Copenhagen cohorts: 40–80 mg/dL (1–2 mmol/L) in men and 100 mg/dL (2.5 mmol/L) in women.33
Statin therapy is frequently associated with changes in HDL-C levels that are inversely proportional to the progression of coronary atherosclerosis, even in patients with low levels of LDL-C.34 HDL increases regulated by statins seem to enhance the ‘reverse cholesterol transport’ pathway, in which excess cholesterol is eliminated from peripheral cells and moved into the liver via HDL to be excreted in the bile.35 Statins are already known to favour HDL-C production, and pitavastatin produces significantly greater and more stable HDL elevations over time than other statins. However, it is unclear whether this effect provides a further CV benefit.
Patients with type 2 diabetes mellitus (T2DM), metabolic syndrome, HIV infection and kidney disease are usually diagnosed with atherogenic dyslipidaemia and show dysfunctions in HDL-C.36 For instance, data from the Framingham study showed an association between low HDL-C levels and high CV risk in patients with insulin resistance in the presence of T2DM or metabolic syndrome.37 Similarly, patients with high levels of triglycerides and insulin resistance have a remarkably high CV risk, because of the pathogenic role of triglyceride-rich lipoproteins in atherosclerosis development.37 Furthermore, pitavastatin greatly reduced the plaque volume per 1% increase in HDL-C compared with other statins (atorvastatin, pravastatin, rosuvastatin, simvastatin).38 All these findings suggest that pitavastatin may play a role in improving HDL levels and functionality.
The analysis of HDL lipidome in patients with T2DM with dysfunctional HDL in the Copenhagen cohort indicated that abnormal HDL particles were smaller than those retrieved from healthy participants, with an unbalanced plasmalogen/apo-A I ratio.39 A larger cohort study performed in Australia found three types of abnormal lipids (ceramides and two plasmalogen) in patients with T2DM.40
The CAPITAIN study (An Open Label Study of the Chronic and Acute Effects of Pitavastatin on Monocyte Phenotype, Endothelial Dysfunction, and HDL Atheroprotective Function in Subjects with Metabolic Syndrome) showed that pitavastatin progressively normalised the triglycerides-to-cholesterol ratio within 6 months of treatment, without modifying glucose metabolism or significantly changing HDL levels.41 Furthermore, the study suggested that pitavastatin enhances plasmalogen production, and, although the biochemical mechanism is not completely understood, this might be clinically relevant in reducing oxidative stress and inflammation.41 Moreover, an effectiveness analysis showed that pitavastatin treatment resulted in a significant decrease of high-sensitivity C-reactive protein (CRP) levels in patients with metabolic syndrome, whereas high-molecular-weight adiponectin levels did not change.42 This observation suggests that pitavastatin can delay the progression of coronary atheroma by affecting HDL-related inflammation and oxidation, which are common in people with metabolic syndrome and T2DM.
Therefore, pitavastatin seems to regulate the metabolism of HDL-C, an effect that may reduce atherosclerotic disease by decreasing both oxidation and inflammation.
New onset diabetes
Observational studies and meta-analyses of randomised clinical trial data have reported a 2–12% increased risk of new-onset diabetes associated with statin therapy depending on the population treated and the statin used.43 The risk-to-benefit ratio decreases using statins at moderate or low doses and choosing pravastatin, lovastatin and pitavastatin over other statins.44 Potential actions of statins on beta cell functions have been identified: reducing glucose transporters (GLUT2) expression, thereby limiting glucose uptake; upregulating LDL receptors, thus increasing the cholesterol uptake; reducing coenzyme Q10 levels, thus impairing mitochondrial function and adenosine triphosphate (ATP) production and inhibiting L-type calcium channels, thus decreasing the amount of calcium in the cytosol, with negative influence on insulin secretion.43 All these effects might perturb glucose regulation and induce diabetes.
Each statin has a peculiar, potential diabetogenic effect, with pravastatin and fluvastatin that seem to exhibit neutral effects on glycaemic parameters.45 A post-hoc analysis from the Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22 (PROVE-IT TIMI 22) trial comparing glycaemic control between patients with T2DM, who received atorvastatin 10 mg, pravastatin 10 mg or pitavastatin 2 mg per day (n=279), showed blood glucose levels and glycated haemoglobin (HbA1c) only increased amongst atorvastatin-treated patients.46 These results are consistent with those observed in a subanalysis of the Collaborative Study on Hypercholesterolemia Drug Intervention and Their Benefits for Atherosclerosis Prevention (CHIBA) study in which 45 Japanese patients with T2DM and hypercholesterolaemia received pitavastatin 2 mg or atorvastatin 10 mg for 12 weeks.47 The use of atorvastatin significantly increased serum glycoalbumin and slightly HbA1c, whereas pitavastatin did not show an obvious effect on the levels of these proteins.47 The CAPITAIN study showed that a 6-month treatment with pitavastatin 4 mg did not change the mean fasting plasma glucose, homeostasis model assessment index, insulin levels, insulin/glucose ratio or HbA1c levels from baseline in people with metabolic syndrome.41 In the Livalo Effectiveness and Safety (LIVES) study, HbA1c levels were significantly reduced (0.28%; p<0.001) in amongst 308 patients with T2DM after 104 weeks of pitavastatin treatment.48 Results from larger trials are awaited to fully confirm these observations.
The position of current guidelines on the use of statins in patients with T2DM is multifaceted, but all refer to the results of the Collaborative Atorvastatin Diabetes Study (CARDS) study (2004 patients). The CARDS study compared atorvastatin 10 mg to placebo: the time to first occurrence of acute CHD events, coronary revascularisation or stroke were significantly prolonged in patients with diabetes without prior CV events, treated with atorvastatin.49 The net benefit presented in the CARDS study supports the recommendation of statins for primary and secondary prevention of diabetes in the current American College of Cardiology (ACC), American Diabetes Association and ESC/EAS guidelines. Only young people with type 1 diabetes should be excluded from statin therapy.
Adherence to therapy
In a study conducted in 1996 patients with recent MI (median age 54 years), the probability of acquiring definite familial hypercholesterolaemia (FH) was present in 9% of patients, where 42.8% of them were not on statins prior to their MI. Amongst FH patients, 63.3% were discharged on high-intensity statins, but only 17.8% had LDL-C<70 mg/dL at their 1-year follow-up.50 In another observational study in about 1000 elderly patients with hypercholesterolaemia (93% hypertension, 31% previous MI or stable angina), statins were recommended to 77% of patients: 39.8% adhered to the treatment constantly, 41.2% intermittently and 18% refused it. Furthermore, most of the patients stopped the therapy less than 3 months following prescription, reporting as main reasons for discontinuation fear of adverse reactions (46%), lack of motivation (29.4%), polypharmacy (27.6%), memory problems (26.5%), side effects (11.7%) and cost (13.5%).51 In a large dataset from a Veteran Affairs Study (n=347,104), during a mean follow-up of 2.9 years, 85,930 patients (24.8%) died.52 A low adherence to high-intensity statins was associated with a greater risk of death, compared with moderate- or low-intensity statins. Even in those patients, who had an adherence of 70–89% to statin therapy, the risk of mortality was higher (+7%) in the higher-intensity versus the moderate- or low-intensity groups.53
CV risk reduction
Intensive versus non-intensive lipid-lowering treatment has been evaluated in secondary prevention in many different trials. High-intensity regimen further decreased primary endpoint (a composite of CV death, non-fatal MI, non-fatal ischaemic stroke or unstable angina requiring emergency hospitalisation).53 In the Randomized Evaluation of Aggressive or Moderate Lipid Lowering Therapy With Pitavastatin in Coronary Artery Disease (REAL-CAD) study, two pitavastatin dosages (4 versus 1 mg) were compared; 4 mg reduced the incidence of CV death, non-fatal MI, non-fatal ischemic stroke or unstable angina by 19%.54
Many clinical trials demonstrated that early and intensive statin treatment in CAD is effective in reducing CV events. Statins are recommended in all patients with MI, according to the recent 2019 ESC and ACC guidelines.3,14 As stated in the 2019 American Heart Association (AHA)/ACC guidelines, high-intensity statin treatment should be started as early as possible especially in patients at very high risk of arteriosclerotic CVD.14 The 2018 guidelines of the Japanese Circulation Society recommend to administer the maximum tolerable dose of a strong statin in patients with ACS.55 Since 1994, prospective trials with statins have been conducted in Western countries and Japan: in the USA and Europe, the LDL-C target to achieve a clinical benefit was established below 70 mg/dL, whilst in Japan, it was higher. Only recently, with the increase of patients at high CV risk in the general population, the Japanese Atherosclerotic Society decided to decrease the LDL-C target to ≤70 mg/dL.56 Furthermore, in Japan in the ESTABLISH study, ACS patients who successfully underwent percutaneous coronary intervention were randomised to receive atorvastatin 20 mg/day or to control their diet. In the control group, atherosclerotic plaques increased after 6 months, whereas in the atorvastatin group, the number of plaques significantly decreased.56 Furthermore, statins significantly reduced LDL-C levels by 41%.57 In the JAPAN–ACS trial, atorvastatin and pitavastatin were compared in a larger cohort of ACS patients. Both drugs significantly decreased LDL-C and non-HDL-C levels and reduced the plaque volume in 10 months,58 thus confirming the results achieved in the ESTABLISH trial. In the ex-EXTABLISH study, the 5-year CVD event rate was significantly reduced by 46% in patients with plaque regression compared with that achieved in patients with plaque progression, suggesting that plaque regression was associated with long-term positive clinical outcomes.59
In another retrospective, observational study, patients with stable CAD achieved plaque stabilisation with pitavastatin (4 mg/day), but not with a 9-month controlled diet, and plaque stabilisation was dependent on the degree of LDL-C reduction.60
High-intensity pitavastatin is beneficial compared with low-dose statins in patients with stable CAD. REAL-CAD was a prospective, multi-centre, randomised, open-label study that enrolled more than 13,000 patients who were randomised to receive high (4 mg/day) or low (1 mg/day) doses of pitavastatin and were followed for 36–60 months. The primary endpoints of the study were CV death, non-fatal MI and cerebral infarction and unstable angina pectoris requiring emergency hospitalisation. High-dose pitavastatin achieved a higher reduction in LDL-C levels, with a stable difference of 14.7 mg/dL over time.54 High-sensitivity CRP levels were also significantly reduced in the high dose group and were unchanged in the low dose group compared with baseline values.54 After a 5-year follow-up, the primary endpoints were significantly reduced (RRR: 19%) in the high-dose group.55 Concerning the safety outcomes, there were no differences between the two groups, in term of rhabdomyolysis, muscle complaints, new onset of diabetes mellitus and laboratory test abnormalities.54 The REAL-CAD study demonstrated that the administration of maximum doses of statins would be the preferred strategy in patients with established CAD, irrespective of the baseline LDL-C levels.
Conclusion
Recent ESC/EAS guidelines have proposed lower LDL-C goals to reduce the CV risk. There are many challenges to achieve these novel goals, such as defining the most convenient therapeutic strategy to reduce and maintain low LDL-C levels over time, reducing potential side effects of long-term therapy and optimising the adherence to therapy. The early use of high-efficacy statins (atorvastatin, rosuvastatin, pitavastatin) should be advised in order to lower LDL-C levels, especially in patients who are at moderate or high CV risk. In this respect, the choice of pitavastatin versus other strong statins shows many potential advantages, given the same anti-lipidaemic properties. The null effect of pitavastatin on glycaemic profile reduces the risk of new-onset diabetes occurrence in patients with metabolic syndrome and in the presence of non-metabolic conditions such as HIV, all of which are suggested to alter glucose regulation. Low drug–drug interactions and the prevalent hepatic metabolism allow the use of pitavastatin in patients treated with polypharmacy, with concomitant disease, and in particular renal disease. Thanks to these distinct features, pitavastatin represents a valuable option to accomplish the goals proposed in the 2019 ESC/EAS guidelines.
Acknowledgements
The authors thank Content Ed Net (Rome, Italy) for their editorial assistance, with the helpful contribution of Elisa Sala, PhD, medical writer, in drafting the text and Mr Bilal Bham and Hussein Hijazi in the final reading of the manuscript for English language improvement.
Footnotes
Contributions: Both authors contributed equally to the preparation of this report. Both named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: JZ received speaker fees from Pfizer, Recordati, Amgen and Sanofi. LT is consultant for Abbott, Amgen, Bayer, MSD, Mylan and Sanofi; she has served as a speaker for Abbott, Actelion, Amgen, Astra, Bayer, MSD, Mylan, Novartis, Novo Nordisk, Sanofi, Servier, Pfizer and Recordati. She also declares her participation in an Amgen trial; she received honoraria from Daiichi Sankyo. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2020/05/dic.2020-4-4-COI.pdf
Funding declaration: The meeting ‘Current Perspective on the Use of Statins in the Treatment of Dyslipidemic Patients’ was held in Stresa, Italy, on 27–28th June 2019. It was endorsed by EAS and supported by an unrestricted grant from Recordati (Milan, Italy). The authors thank Recordati (Milan, Italy) for an unrestricted grant for editorial assistance.
Correct attribution: Copyright © 2020 Tokgözoglu L, Zamorano JL. https://doi.org/10.7573/dic.2020-4-4. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: submitted; externally peer reviewed.
Peer review comments to author: 29 April 2020
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editor-in-Chief gordon.mallarkey@bioexcelpublishing.com
For all permissions, rights, and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;16:1289–1297. doi: 10.1001/jama.2016.13985. [DOI] [PubMed] [Google Scholar]
- 2.Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS Guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37(39):2999–3058. doi: 10.1093/eurheartj/ehw272. [DOI] [PubMed] [Google Scholar]
- 3.Mach F, Baigent C, Catapano A, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 4.Cooney MT, Selmer R, Lindman A, et al. Cardiovascular risk estimation in older persons: SCORE O.P. Eur J Prev Cardiol. 2016;23(10):1093–1103. doi: 10.1177/2047487315588390. [DOI] [PubMed] [Google Scholar]
- 5.Berry JD, Dyer A, Cai X, et al. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366(4):321–329. doi: 10.1056/NEJMoa1012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ference BA, Yoo W, Alesh I, et al. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: a Mendelian randomization analysis. J Am Coll Cardiol. 2012;60(25):2631–2639. doi: 10.1016/j.jacc.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Laufs U, Descamps OS, Catapano AL, et al. Understanding IMPROVE-IT and the cardinal role of LDL-C lowering in CVD prevention. Eur Heart J. 2014;35(30):1996–2000. doi: 10.1093/eurheartj/ehu228. [DOI] [PubMed] [Google Scholar]
- 8.Rosenson RS. Statins: can the new generation make an impression? Expert Opin Emerg Drugs. 2004;9(2):269–279. doi: 10.1517/14728214.9.2.269. [DOI] [PubMed] [Google Scholar]
- 9.Ylä-Herttuala S, Bentzon JF, Daemen M, et al. Stabilization of atherosclerotic plaques: an update. Eur Heart J. 2013;34(42):3251–3258. doi: 10.1093/eurheartj/eht301. [DOI] [PubMed] [Google Scholar]
- 10.Tsujita K, Sugiyama S, Sumida H, et al. Impact of dual lipid-lowering strategy with ezetimibe and atorvastatin on coronary plaque regression in patients with percutaneous coronary intervention: the multicenter randomized controlled PRECISE-IVUS trial. J Am Coll Cardiol. 2015;66(5):495–507. doi: 10.1016/j.jacc.2015.05.065. [DOI] [PubMed] [Google Scholar]
- 11.Lee SE, Chang HJ, Sung JM, et al. Effects of statins on coronary atherosclerotic plaques: the PARADIGM study. JACC Cardiovasc Imaging. 2018;11(10):1475–1484. doi: 10.1016/j.jcmg.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344(8934):1383–1389. doi: 10.1016/S0140-6736(94)90566-5. [DOI] [PubMed] [Google Scholar]
- 13.Cholesterol Treatment Trialists Collaboration. Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a metaanalysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393(10170):407–415. doi: 10.1016/S0140-6736(18)31942-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amarenco P, Bogousslavsky J, Callahan A, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355(6):549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 17.Hackam DG, Woodward M, Newby LK, et al. Statins and intracerebral hemorrhage: collaborative systematic review and meta-analysis. Circulation. 2011;124(20):2233–2242. doi: 10.1161/CIRCULATIONAHA.111.055269. [DOI] [PubMed] [Google Scholar]
- 18.Wei MY, Ito MK, Cohen JD, et al. Predictors of statin adherence, switching, and discontinuation in the USAGE survey: understanding the use of statins in America and gaps in patient education. J Clin Lipidol. 2013;7(5):472–483. doi: 10.1016/j.jacl.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 19.De Smedt D, Annemans L, De Backer G, et al. Cost-effectiveness of optimized adherence to prevention guidelines in European patients with coronary heart disease: results from the EUROASPIRE IV survey. Int J Cardiol. 2018;272:20–25. doi: 10.1016/j.ijcard.2018.06.104. [DOI] [PubMed] [Google Scholar]
- 20.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297(2):177–186. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 21.Barrios V, Escobar C, Zamorano JL. Searching the place of pitavastatin in the current treatment of patients with dyslipidemia. Expert Rev Cardiovasc Ther. 2013;11(12):1597–1612. doi: 10.1586/14779072.2013.844546. [DOI] [PubMed] [Google Scholar]
- 22.Gumprecht J, Gosho M, Budinski D, et al. Comparative long-term efficacy and tolerability of pitavastatin 4 mg and atorvastatin 20–40 mg in patients with type 2 diabetes mellitus and combined (mixed) dyslipidaemia. Diabetes Obes Metab. 2011;13(11):1047–1055. doi: 10.1111/j.1463-1326.2011.01477.x. [DOI] [PubMed] [Google Scholar]
- 23.Eriksson M, Budinski D, Hounslow N. Comparative efficacy of pitavastatin and simvastatin in high-risk patients: a randomized controlled trial. Adv Ther. 2011;28(9):811–823. doi: 10.1007/s12325-011-0056-7. [DOI] [PubMed] [Google Scholar]
- 24.Ose L, Budinski D, Hounslow N, et al. Comparison of pitavastatin with simvastatin in primary hypercholesterolaemia or combined dyslipidaemia. Curr Med Res Opin. 2009;25(11):2755–2764. doi: 10.1185/03007990903290886. [DOI] [PubMed] [Google Scholar]
- 25.Stender S, Budinski D, Gosho M, et al. Pitavastatin shows greater lipid-lowering efficacy over 12 weeks than pravastatin in elderly patients with primary hypercholesterolaemia or combined (mixed) dyslipidaemia. Eur J Prev Cardiol. 2013;20(1):40–53. doi: 10.1177/2047487312451251. [DOI] [PubMed] [Google Scholar]
- 26.Sponseller CA, Morgan RE, Kryzhanovski VA, et al. Comparison of the lipid-lowering effects of pitavastatin 4 mg versus pravastatin 40 mg in adults with primary hyperlipidemia or mixed (combined) dyslipidemia: a Phase IV, prospective, US, multicenter, randomized, double-blind, superiority trial. Clin Ther. 2014;36(8):1211–1222. doi: 10.1016/j.clinthera.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Hoy SM. Pitavastatin: a review in hypercholesterolemia. Am J Cardiovasc Drugs. 2017;17(2):157–168. doi: 10.1007/s40256-017-0213-8. [DOI] [PubMed] [Google Scholar]
- 28.Yokote K, Shimano H, Urashima M, et al. Efficacy and safety of pitavastatin in Japanese patients with hypercholesterolemia: LIVES study and subanalysis. Expert Rev Cardiovasc Ther. 2011;9(5):555–562. doi: 10.1586/erc.11.47. [DOI] [PubMed] [Google Scholar]
- 29.Barrios V, Escobar C. Clinical benefits of pitavastatin: focus on patients with diabetes or at risk of developing diabetes. Future Cardiol. 2016;12(4):449–466. doi: 10.2217/fca-2016-0018. [DOI] [PubMed] [Google Scholar]
- 30.Mosepele M, Molefe-Baikai OJ, Grinspoon SK, Triant VA. Benefits and risks of statin therapy in the HIV-infected population. Curr Infect Dis Rep. 2018;20(8):20. doi: 10.1007/s11908-018-0628-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grinspoon SK, Fitch KV, Overton ET, et al. Rationale and design of the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE) Am Heart J. 2019;212:23–35. doi: 10.1016/j.ahj.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirota T, Ieiri I. Drug-drug interactions that interfere with statin metabolism. Expert Opin Drug Metab Toxicol. 2015;11(9):1435–1447. doi: 10.1517/17425255.2015.1056149. [DOI] [PubMed] [Google Scholar]
- 33.Madsen CM, Varbo A, Nordestgaard BG. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur Heart J. 2017;38(32):2478–2486. doi: 10.1093/eurheartj/ehx163. [DOI] [PubMed] [Google Scholar]
- 34.Nicholls SJ, Tuzcu EM, Sipahi I, et al. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA. 2007;297(5):499–508. doi: 10.1001/jama.297.5.499. [DOI] [PubMed] [Google Scholar]
- 35.Kontush A, Chapman MJ. Antiatherogenic small, dense HDL--guardian angel of the arterial wall? Nat Clin Pract Cardiovasc Med. 2006;3(3):144–153. doi: 10.1038/ncpcardio0500. [DOI] [PubMed] [Google Scholar]
- 36.Rosenson RS, Brewer HB, Jr, Ansell BJ, et al. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat Rev Cardiol. 2016;13(1):48–60. doi: 10.1038/nrcardio.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin HP, Baghdasarian S, Singer MR, et al. Dietary cholesterol, lipid levels, and cardiovascular risk among adults with diabetes or impaired fasting glucose in the Framingham Offspring Study. Nutrients. 2018;10(6) doi: 10.3390/nu10060770. pii: E770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kishida K, Funahashi T, Shimomura I. Importance of assessing the effect of statins on the function of high- density lipoproteins on coronary plaque. Cardiovasc Hematol Disord Drug Targets. 2012;12(1):28–34. doi: 10.2174/187152912801823156. [DOI] [PubMed] [Google Scholar]
- 39.Safai N, Suvitaival T, Ali A, et al. Effect of metformin on plasma metabolite profile in the Copenhagen Insulin and Metformin Therapy (CIMT) trial. Diabet Med. 2018;35(7):944–953. doi: 10.1111/dme.13636. [DOI] [PubMed] [Google Scholar]
- 40.Meikle PJ, Formosa MF, Mellett N, et al. HDL phospholipids, but not Cholesterol Distinguish Acute Coronary Syndrome from stable coronary artery disease. J Am Heart Assoc. 2019;8(11):e011792. doi: 10.1161/JAHA.118.011792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chapman MJ, Orsoni A, Robillard P, et al. Effect of high-dose pitavastatin on glucose homeostasis in patients at elevated risk of new-onset diabetes: insights from the CAPITAIN and PREVAIL-US studies. Curr Med Res Opin. 2014;30(5):775–784. doi: 10.1185/03007995.2013.874989. [DOI] [PubMed] [Google Scholar]
- 42.Matsubara T, Naruse K, Arakawa T, et al. Impact of pitavastatin on high-sensitivity C-reactive protein and adiponectin in hypercholesterolemic patients with the metabolic syndrome: the PREMIUM Study. J Cardiol. 2012;60(5):389–394. doi: 10.1016/j.jjcc.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 43.Carmena R, Betteridge DJ. Diabetogenic action of statins: mechanisms. Curr Atheroscler Rep. 2019;21(6):23. doi: 10.1007/s11883-019-0780-z. [DOI] [PubMed] [Google Scholar]
- 44.Navarese EP, Buffon A, Andreotti F, et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol. 2013;111(8):1123–1130. doi: 10.1016/j.amjcard.2012.12.037. [DOI] [PubMed] [Google Scholar]
- 45.Kosmas CE, Silverio D, Sourlas A, et al. Impact of lipid-lowering therapy on glycemic control and the risk for new-onset diabetes mellitus. Drugs Context. 2018;7:212562. doi: 10.7573/dic.212562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamakawa T, Takano T, Tanaka S, et al. Influence of pitavastatin on glucose tolerance in patients with type 2 diabetes mellitus. J Atheroscler Thromb. 2008;15(5):269–275. doi: 10.5551/jat.e562. [DOI] [PubMed] [Google Scholar]
- 47.Yokote K, Saito Y CHIBA. Influence of statins on glucose tolerance in patients with type 2 diabetes mellitus: subanalysis of the collaborative study on hypercholesterolemia drug intervention and their benefits for atherosclerosis prevention (CHIBA study) J Atheroscler Thromb. 2009;16(3):297–298. doi: 10.5551/jat.e1008. [DOI] [PubMed] [Google Scholar]
- 48.Teramoto T, Shimano H, Yokote K, et al. New evidence on pitavastatin: efficacy and safety in clinical studies. Expert Opin Pharmacother. 2010;11(5):817–828. doi: 10.1517/14656561003641990. [DOI] [PubMed] [Google Scholar]
- 49.Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 50.Singh A, Gupta A, Collins BL, et al. Familial hypercholesterolemia among young adults with myocardial infarction. J Am Coll Cardiol. 2019;73(19):2439–2450. doi: 10.1016/j.jacc.2019.02.059. [DOI] [PubMed] [Google Scholar]
- 51.Bubnova M, Aronov DM, Deev AD. Statin prescription patterns, adherence, and attainment of cholesterol treatment goals in routine clinical care in elderly with hyperlipidemia and coronary heart disease: a Russian observational study. Atherosclerosis. 2016;252:e46. doi: 10.1016/j.atherosclerosis.2016.07.380. [DOI] [Google Scholar]
- 52.Rodriguez F, Maron DJ, Knowles JW, et al. Association of statin adherence with mortality in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2019;4(3):206–213. doi: 10.1001/jamacardio.2018.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barter PJ. High- Versus low-dose statin: effects on cardiovascular events and all-cause death. Circulation. 2018;137(19):2013–2015. doi: 10.1161/CIRCULATIONAHA.118.034407. [DOI] [PubMed] [Google Scholar]
- 54.Taguchi I, Iimuro S, Iwata H, et al. High-Dose versus low-dose pitavastatin in Japanese patients with stable Coronary Artery Disease (REAL-CAD): a randomized superiority trial. Circulation. 2018;137(19):1997–2009. doi: 10.1161/CIRCULATIONAHA.117.032615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kimura K, Kimura T, Ishihara M, et al. JCS 2018 guideline on diagnosis and treatment of acute coronary syndrome. Circ J. 2019;83(5):1085–1196. doi: 10.1253/circj.CJ-19-0133. [DOI] [PubMed] [Google Scholar]
- 56.Kinoshita M, Yokote K, Arai H, et al. Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J Atheroscler Thromb. 2018;25(9):846–984. doi: 10.5551/jat.GL2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okazaki S, Yokoyama T, Miyauchi K, et al. Early statin treatment in patients with acute coronary syndrome: demonstration of the beneficial effect on atherosclerotic lesions by serial volumetric intravascular ultrasound analysis during half a year after coronary event: the ESTABLISH Study. Circulation. 2004;110(9):1061–1068. doi: 10.1161/01.CIR.0000140261.58966.A4. [DOI] [PubMed] [Google Scholar]
- 58.Hiro T, Kimura T, Morimoto T, et al. Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: a multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study) J Am Coll Cardiol. 2009;54(4):293–302. doi: 10.1016/j.jacc.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 59.Dohi T, Miyauchi K, Okazaki S, et al. Higher baseline LDL-C levels amplify the short-term benefit of early intensive statin treatment in acute coronary syndrome. J Atheroscler Thromb. 2011;18(1):42–48. doi: 10.5551/jat.5587. [DOI] [PubMed] [Google Scholar]
- 60.Hattori K, Ozaki Y, Ismail TF, et al. Impact of statin therapy on plaque characteristics as assessed by serial OCT, grayscale and integrated backscatter-IVUS. JACC Cardiovasc Imaging. 2012;5(2):169–177. doi: 10.1016/j.jcmg.2011.11.012. [DOI] [PubMed] [Google Scholar]