Abstract
Objective
To study the temporal dynamics of tissue impedance after deep brain stimulation (DBS).
Background
DBS therapy commonly employs a constant voltage approach, and current delivery to the tissue is a function of electrode–tissue impedance. It is presumed that impedance fluctuates early postimplantation, with implications for variations in current delivery and therapeutic efficacy. We hypothesised that the largest variation will be recorded early after surgery, followed by stabilisation.
Methods
Review of impedance checks of implanted DBS systems at standard parameters during the first five months postimplantation. All measurement time points were binned into 1-week periods, and we used repeated measures analysis of variance with Tukey pairwise multiple comparisons correction. The analysis was repeated after normalising impedance values for each subject to that patient’s baseline value.
Results
There was an initial (non-significant) drop in impedance at week 1, followed by significant increase at week 3 (p=0.0002). There were no further significant differences in impedance values at subsequent time points. Analysis of normalised data showed a significant difference between the initial measurement in postoperative week 1 (normalised value 1) and week 3 (normalised value 1.73, p<0.0001), with no further difference among the subsequent weekly points during the 5-month follow-up. No significant hourly variations were found at any time points.
Conclusions
We found major changes in impedance within the first month postimplantation, with no further variation. This is an important confirmation in patients of this temporal dynamics of the impedance of implanted DBS hardware, with potential therapeutic implications.
INTRODUCTION
Deep brain stimulation (DBS) is an established, effective therapy for Parkinson’s disease and other movement disorders.1–3 The most widely used stimulation paradigm employs a constant voltage delivery, and the current delivered to the target tissue is a function of the electrode–tissue impedance. DBS impedance fluctuations may contribute to variations in response to stimulation. Little is known about the variations of electrical properties and tissue reactions to stimulation in humans, and the postoperative variations in impedance have received little systematic attention in a clinical setting. It is, however, widely presumed that the impedance in the implanted system fluctuates, particularly early postimplantation, and this is in turn responsible for fluctuations in current delivery and therapeutic efficacy.4
In this study, we examined the temporal macrodynamics (long-term postoperative follow-up of up to 5 months) and microdynamics (hourly variations at several postoperative time points) of tissue impedance after DBS implantation. This spontaneous variation pattern has potential relevance for determining therapeutic benefit and the development of future treatment paradigms.
Based on prior anecdotal data, we hypothesised that the impedance macrodynamics would follow a predictable pattern and that the largest variation would be recorded early after the surgery, followed by stabilisation. We further hypothesised that the microdynamics will exhibit limited evolution postimplantation.
METHODS
This is a retrospective study of recorded impedance characteristics of implanted DBS hardware using the standardised electrode impedance check functions of the Medtronic hardware. Anonymised data obtained from clinical observations were analysed, including electrode impedance measures of the DBS hardware. This study was approved by the NIH Office of Human Research Subjects Protection, exempt #11875.
Patients were implanted bilaterally with Medtronic model 3389 stimulating electrodes and Activa PC battery systems. Patient selection was based on the CAPSIT criteria.5 The surgical procedure and subsequent management followed best clinical practice standards. Briefly, the procedure entails placement of DBS electrodes connected subcutaneously to a connector lead and then to an impulse generator,6 which is then programmed to appropriate parameters. Subjects were included on a consecutive basis at two treatment centres: the National Institute of Neurological Disorders and Stroke Parkinson Clinic and the Washington Brain and Spine Institute, Bethesda, MD.
Regular checks of the impedance of the implanted system were performed as part of routine clinical care using the Medtronic N’Vision 8840 Clinician Programmer. These checks are performed as standard of care to ensure proper functionality of the hardware at each visit. The implanted system performs in-clinic checks of the impedance at the electrode–tissue interface in both pseudo-unipolar (‘unipolar’) and bipolar configurations. This check is performed using standard parameters: frequency of 100 Hz, pulse width of 80 microseconds and amplitude options of 0.25 V, 0.7 V, 1.5 V or 3.0 V. All analyses were performed at the standard 1.5 V amplitude. Data were retrospectively analysed for this purpose. Values reported as ‘out of range’ were excluded even if they were not representative of a true system malfunction.
At the time of implantation, in one centre (Washington Brain and Spine Institute) impedance was measured using the External Neural Stimulator device, and in the other centre (NINDS) by using the Activa PC battery. To compare these measurements, we corrected for the difference in impedance attributable to the extension line (the only difference between the two systems) by adding 0.345 Ω/cm to the impedance value (the resistance of the extension cord, DBS Extension Kit for Deep Brain Stimulation (8–4) 37085 manual). The data were corrected by adding the above resistance to unipolar measurements and double this value for bipolar measurements, consistent with the current path in the two measurement types.
Statistical analysis was performed using SAS V.9.2 (SAS Institute Inc, Cary, North Carolina, USA). For the temporal macrodynamics, we used repeated measures analysis of variance (ANOVA) with Tukey pairwise multiple comparisons correction, evaluating the difference between different time points. Two types of analysis were performed: First, given the fact that measurements were not always obtained at the exact same time point postimplantation, we binned the time points into weekly intervals and considered each 1-week bin a data point. For each patient, we have averaged the impedance values of all contacts for unipolar and bipolar configurations, respectively. We treated each of these weekly binned averages as one time point. Given the variation in the exact time of measurement for each patient, the number of data points for each week bin was different. We have then analysed the variation in these weekly values, starting from the time of implantation. Unipolar and bipolar values were analysed separately. Second, we performed an analysis for subjects with values at more than one time points, after normalisation to the initial (baseline) value measured for that subject. This allowed building a curve for each subject’s temporal dynamics, followed by testing across time points. For the micro-dynamics, we performed hourly measurements of the impedance characteristics at various postoperative time points when patients had a long enough clinic stay for other purposes. Three to eight hourly time points were collected, and we employed a repeated measures ANOVA. We also analysed the different impedance dynamics in two brain targets, subthalamic nucleus (STN) and globus pallidus interna (GPi), the two sides of the brain, and between the two centres by adding these factors as covariates in the analysis.
RESULTS
Temporal macrodynamics
Twenty patients were included in the analysis. The impedance dynamics was first analysed in weekly detail during the first month postimplantation. A total of five measurements were available for time 0 (day of implantation), 17 for week 1, 6 for week 3 and 5 for week 4, respectively. As shown in figure 1, there was a trend for a decrease in values from time 0 to week 1 (1987 Ω vs 1530 Ω), and a significant increase in impedance from week 1 to week 3 (1530 Ω vs 2530 Ω, p=0.0002). We then extended the analysis including all time bins with at least two values. No further significant differences were recorded from week 3 onward, indicating stabilisation in impedance. The data presented are for bipolar impedance measures; the impedance dynamics for unipolar measurements were similar.
Figure 1.
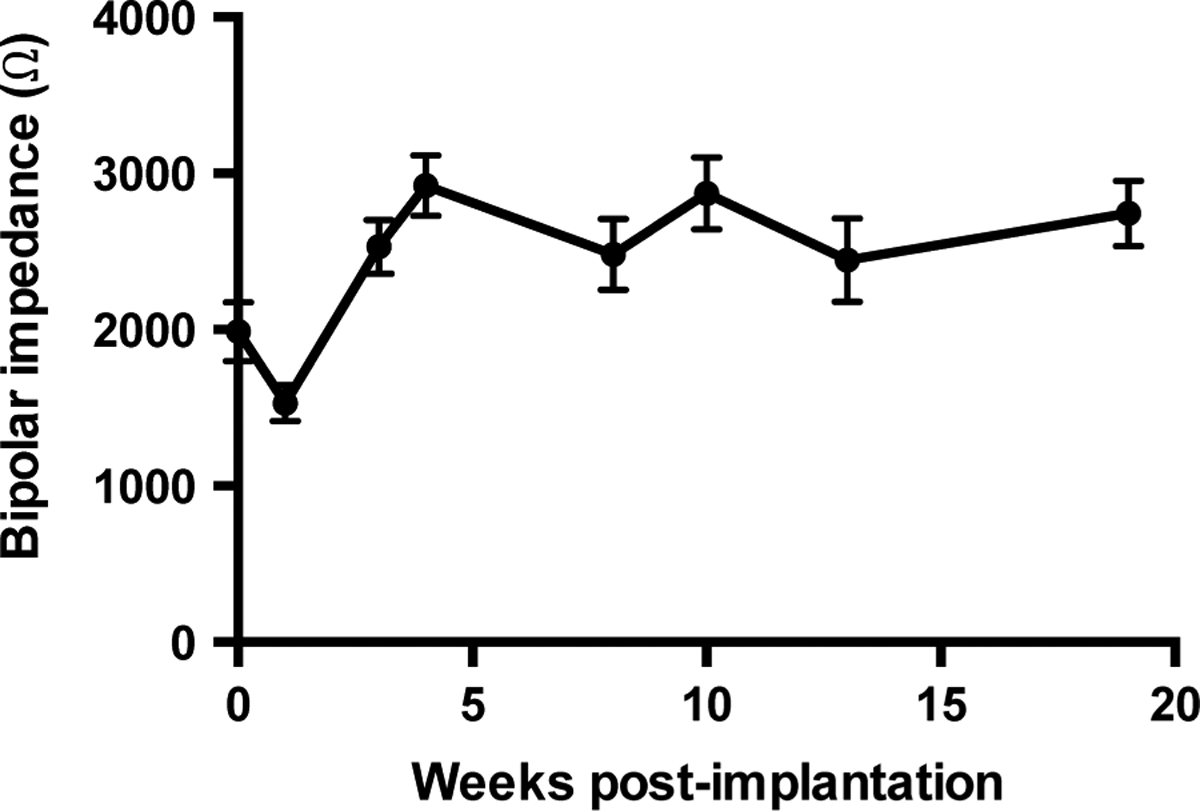
Bipolar impedance dynamics binned by week postimplantation. There was a trend for a decrease in values from time 0 (implantation) to week 1 (1987Ω vs 1530Ω) and a significant increase in impedance from week 1 to week 3 (1530Ω vs 2530Ω, p=0.0002). Error bars represent standard error of mean.
We then normalised all impedance values for each subject to that subject’s initial value for further comparison of the temporal dynamic curves. Ten patients were included in the analysis — for consistency we included only those subjects whose initial value was within the first week from implantation. There is a significant difference between the first postoperative week (normalised value 1) and the next time point (week 3, normalised value 1.73, p<0.0001), with no further significant variation up to the 5 months follow-up (1.76) (figure 2).
Figure 2.
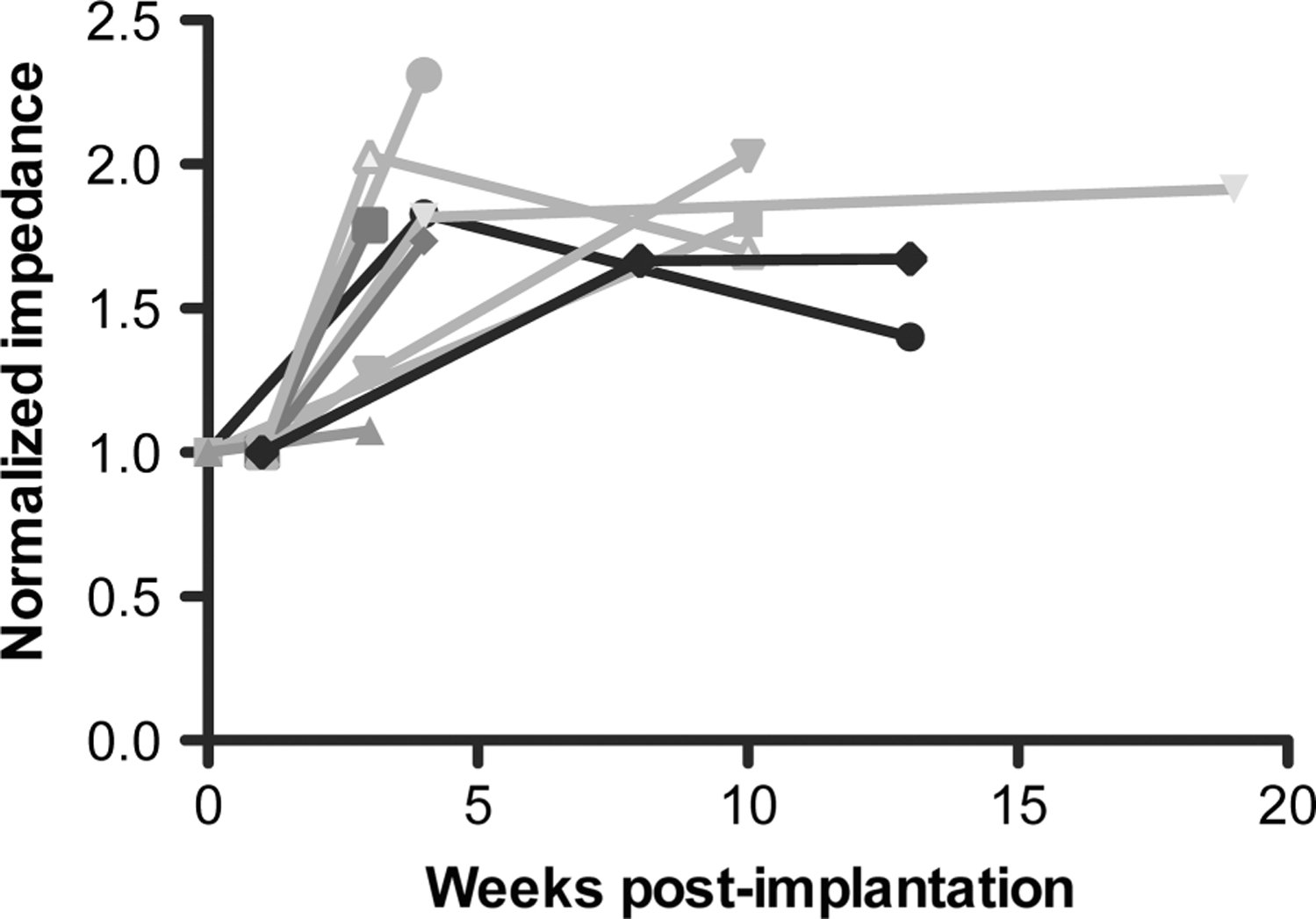
Impedance curves for each subject, normalised to each subject’s initial impedance value. There is a significant difference between the first week (normalised value 1) and subsequent time points (1.73 at week 3), p<0.0001, with no further significant variation up to the 5 months follow-up.
Temporal microdynamics
Twelve patients were included in the analysis. Hourly impedance measurements were recorded in patients ranging from 22 to 342 days postimplantation. The hourly variation in impedance was not significant at any of the tested time points (figure 3).
Figure 3.
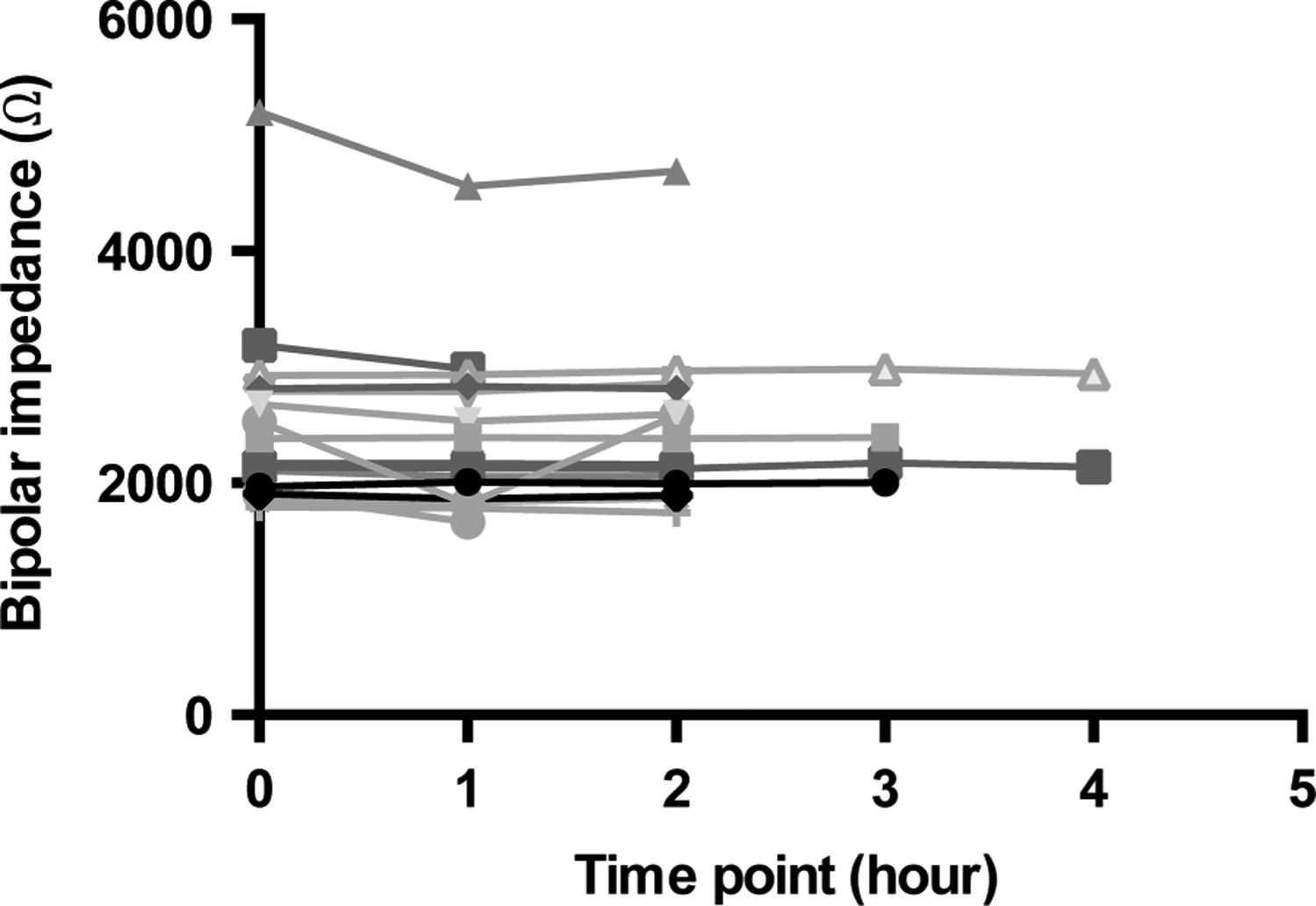
Impedance microdynamics measured at hourly time points. Each curve plots the hourly impedance of a single patient. The hourly variation in impedance was not significant at any of the tested time points.
Differences between STN and GPi targets and influence of side and collection centre
No significant difference in impedance values was found between the two participating centres and between the left and right side of the brain. For STN and GPi, the values were similar in magnitude and exhibited identical temporal trends.
For all the above measurements, the unipolar and bipolar values followed a similar trend, and no significant difference was found between the two dynamic patterns. All figures show bipolar values and trends for clarity.
DISCUSSION
It is well known that DBS efficacy can vary in patients, particularly in the first weeks to months after surgery.7 As a result, it is common to delay initiation of stimulation by approximately 1 month. It has been speculated that this could at least partly be due to changes in the tissue–electrode impedance. This observation has also prompted the development of constant-current stimulation paradigms in an attempt to stabilise current delivery and ameliorate this variation.4,8 In the present study, we report the detailed dynamics of impedance measurements of implanted DBS systems in patients. Since this dynamic can significantly impact therapeutic efficacy, it has great potential implications for therapy and research.
The electrical impedance in the electrode–tissue system can be conceptualised as the potential ability to resist the passage of current. The electrode–tissue interface impedance may be operating in a non-linear regime at stimulation parameters used in clinical practice.9 This impedance is also significantly influenced by electrical stimulation.10 The reasons for the variability in impedance of the implanted electrode–tissue system are not fully known. It is apparent that the substrate for this variability rests in the characteristics of the tissue and those of the electrode–tissue interface.11,12 It has been hypothesised that local anatomic changes can be responsible for the variability of electrical properties, given that tissue changes occur locally after implantation, including fluid accumulation and encapsulation.13 The encapsulation has in turn been shown to alter the resistivity of the local environment. The electrode impedance measurements are a good proxy for this electrode–tissue interface impedance, which in turn determines the amount of current delivered to the tissue. We have explored here the variation in this impedance and the possibility that this variation could explain variable or unpredictable response to therapy.
Our study adds to the limited literature exploring the impedance dynamics in a clinical context and provides information previously unavailable. An important recent paper addressed this question by employing a different approach and using the older generation Medtronic hardware.14 In this study, the authors found some additional variability on longer follow-up, but this could have been partly due to a group of outliers in their population. We have instead focused more closely on the early postoperative period using the newer generation Activa system and explored the hourly variation as well. Another relatively large retrospective study analysed therapeutic impedance (the tissue impedance at stimulation parameters, as opposed to the standardised impedance checks used here) dynamics in patients with STN stimulation only.15 This limits any conclusions, given that therapeutic impedance is influenced by the variable frequency of stimulation and other factors. One study explored the correlations of impedance characteristics with outcome in dystonia patients only, with a relatively short follow-up period and with limited conclusions regarding temporal impedance dynamics.16 A smaller study investigated electrical properties during a shorter postoperative period using a direct measurement with externalised leads and did not find significant changes.11 The study by Abosch and colleagues focused on local field potential recordings and measured impedance as a secondary measure, but used an external construct and only measured impedance at the time of implantation or battery change.17
Further, impedance microdynamics were characterised by analysing the evolution of impedance at successive hourly time points. Our results reflect measured variations in a realistic clinical setting rather than relying on models with limited clinical relevance and application.
Prior research suggested that impedance can be affected by chronic stimulation and the activity status of the electrodes.14,17 While this is an important feature, in this study we did not focus on this aspect but rather on the longitudinal temporal dynamics of impedance with direct clinical relevance. By focusing on the values of the electrode impedance checks performed at preset parameters, we obtained proxy measurements of the impedance values presented at the tissue–hardware interface that are not dependent on the stimulation parameters. This allows an estimate of the tissue-specific factors influencing therapeutic efficacy.
Our results suggest that the major fluctuations in tissue impedance occur within the first month postimplantation, and with further refinement, within the first week postimplantation. This is consistent with the anecdotal accounts that report variable and unreliable response to stimulation early postoperatively, a reason often cited for delaying initiation of stimulation.7 Our findings are corroborated by some studies indicating an early drop in impedance postoperatively,14,18 but they differ from those of some prior studies using therapeutic impedance values to assess variability,15 where no significant variation was found.
Our analysis of the microdynamics of tissue impedance suggests that there is no significant hourly variability in impedance at any time points. This has implications for the expectations of benefit and therapeutic approach in day-to-day practice and research. If the impedance does not vary throughout the day, changes in current delivery due to this factor cannot explain unreliable response to therapy, as has been described in some conditions.
There are several limitations to our study. This is a retrospective study, and we had to account for some heterogeneity in the measurements in our analysis. Given this retrospective nature, we employed two different types of analyses to validate our results. First, since our data points did not always fall on the same day postoperatively, we binned the data to achieve a more even distribution and to capture clinically relevant week-long time intervals. Also, in order to compare the temporal variations across subjects, we employed a normalisation method to the baseline impedance value. This allowed comparison of the temporal curves for all subjects within the same amplitude parameters. Given that no differences were found between the two sides of the brain in our population, we have treated the different side electrodes as individual points in the analysis. The sample size was limited by our choice to analyse only the data obtained at the standard 1.5 V impedance check. We have excluded all measurements where out-of-range results were found, which differs from other recent studies,14 but we believe this makes our results more reliable. This did, however, reduce the statistical power.
In spite of these limitations, our design allows preliminary conclusions about the temporal dynamics of tissue–hardware interfaces in DBS patients, placing this factor in perspective for therapy considerations in patients in a clinical context. Further studies are needed and prospective exploration in a standardised fashion of the tissue–electrode impedance should be planned, preferably with a longer time frame.
Acknowledgements
We would like to thank Justin Kemp and Jon Giftakis of Medtronic, Inc. who provided valuable technical advice.
Footnotes
Competing interests MH and KZ are parties to Clinical Trial Agreements with Medtronic, Inc. ZL is on the speaker board for Medtronic, Inc. CL, PM, PG, BM, TW and TP have no conflicts of interest to declare.
Ethics approval NIH Office of Human Research Subjects Protection.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Krack P, Batir A, Van Blercom N, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med 2003;349:1925–34. [DOI] [PubMed] [Google Scholar]
- 2.Fasano A, Daniele A, Albanese A. Treatment of motor and non-motor features of Parkinson’s disease with deep brain stimulation. Lancet Neurol 2012;11:429–42. [DOI] [PubMed] [Google Scholar]
- 3.Ostrem JL, Starr PA. Treatment of dystonia with deep brain stimulation. Neurotherapeutics 2008;5:320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lempka SF, Johnson MD, Miocinovic S, et al. Current-controlled deep brain stimulation reduces in vivo voltage fluctuations observed during voltage-controlled stimulation. Clin Neurophysiol 2010;121:2128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Defer GL, Widner H, Marie RM, et al. Core assessment program for surgical interventional therapies in Parkinson’s disease (CAPSIT-PD). Mov Disord 1999;14:572–84. [DOI] [PubMed] [Google Scholar]
- 6.Okun MS. Deep-brain stimulation for Parkinson’s disease. N Engl J Med 2012;367:1529–38. [DOI] [PubMed] [Google Scholar]
- 7.Deuschl G, Herzog J, Kleiner-Fisman G, et al. Deep brain stimulation: postoperative issues. Mov Disord 2006;21(Suppl 14):S219–37. [DOI] [PubMed] [Google Scholar]
- 8.Okun MS, Gallo BV, Mandybur G, et al. Subthalamic deep brain stimulation with a constant-current device in Parkinson’s disease: an open-label randomised controlled trial. Lancet Neurol 2012;11:140–9. [DOI] [PubMed] [Google Scholar]
- 9.Wei XF, Grill WM. Impedance characteristics of deep brain stimulation electrodes in vitro and in vivo. J Neural Eng 2009;6:046008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lempka SF, Miocinovic S, Johnson MD, et al. In vivo impedance spectroscopy of deep brain stimulation electrodes. J Neural Eng 2009;6:046001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Back C, Alesch F, Lanmuller H. Postoperative monitoring of the electrical properties of tissue and electrodes in deep brain stimulation. Neuromodulation 2003;6:248–53. [DOI] [PubMed] [Google Scholar]
- 12.Butson CR, Maks CB, McIntyre CC. Sources and effects of electrode impedance during deep brain stimulation. Clin Neurophysiol 2006;117:447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grill WM, Mortimer JT. Electrical properties of implant encapsulation tissue. Ann Biomed Eng 1994;22:23–33. [DOI] [PubMed] [Google Scholar]
- 14.Cheung T, Nuno M, Hoffman M, et al. Longitudinal impedance variability in patients with chronically implanted DBS devices. Brain Stimul 2013;6:746–51. [DOI] [PubMed] [Google Scholar]
- 15.Sillay KA, Chen JC, Montgomery EB. Long-term measurement of therapeutic electrode impedance in deep brain stimulation. Neuromodulation 2010;13:195–200. [DOI] [PubMed] [Google Scholar]
- 16.Hemm S, Vayssiere N, Mennessier G, et al. Evolution of brain impedance in dystonic patients treated by GPI electrical stimulation. Neuromodulation 2004;7:67–75. [DOI] [PubMed] [Google Scholar]
- 17.Abosch A, Lanctin D, Onaran I, et al. Long-term recordings of local field potentials from implanted deep brain stimulation electrodes. Neurosurgery 2012;71:804–14. [DOI] [PubMed] [Google Scholar]
- 18.Rosa M, Marceglia S, Servello D, et al. Time dependent subthalamic local field potential changes after DBS surgery in Parkinson’s disease. Exp Neurol 2010;222:184–90. [DOI] [PubMed] [Google Scholar]


