Abstract
Background:
The relationship between physical heart failure (HF) symptoms and pathophysiological mechanisms is unclear.
Objective:
To quantify the relationship between plasma β-adrenergic receptor kinase-1 (βARK1) and physical symptoms among adults with HF.
Methods:
We performed a secondary analysis of data collected from two studies of adults with HF. Plasma βARK1 was quantified using an enzyme-linked immunosorbent assay. Physical symptoms were measured with the HF Somatic Perception Scale (HFSPS). Generalized linear modeling was used to quantify the relationship between βARK1 and HFSPS scores.
Results:
The average age (n = 94) was 54.5±13.1 years, 76.6% were male, and a majority (83.0%) had Class III or IV HF. βARK1 was significantly associated with HFSPS scores (β = 0.22±0.10, p = 0.038), adjusting for other predictors of physical symptoms (model R2 = 0.250, F(7, 70) = 3.34, p = 0.004).
Conclusions:
Higher βARK1 is associated with worse physical HF symptoms, pinpointing a potential pathophysiologic underpinning.
Keywords: Heart Failure, Symptom, Biomarkers, beta-Adrenergic Receptor Kinases, G protein-coupled receptor kinase-2
Introduction
Many adults with heart failure (HF) experience significant physical symptoms like dyspnea and fatigue1 that impair quality-of-life2 and drive healthcare utilization.3 The biological underpinnings of symptoms in HF using common objective markers of heart function are unclear,4,5 however, and mismatches between what patients experience symptomatically and how they present hemodynamically are common.6 Novel markers related to pathophysiologic mechanisms, such as sympathetic overactivation, may be helpful in understanding the origin and propagation of physical symptoms in HF.
Chronic overactivation of the sympathetic nervous system is a well-known phenomenon in HF.7 More specifically, β-adrenergic receptors are essential molecules in the control of cardiac function and neurohormonal activation in HF.8 β-adrenergic receptor function is controlled by several molecular mechanisms, one of them being β-adrenergic receptor kinase-1 (βARK1; a.k.a. G protein-coupled receptor kinase-2). With chronic catecholamine stimulation, βARK1 desensitizes and causes internalization of β-adrenergic receptors.9 Decreased β-adrenergic receptor signaling reduces energy expenditure but also limits the capacity to acutely increase cardiac output. In HF, βARK1 is elevated in myocardial cells and lymphocytes10 and is detectable in plasma. Hence, the purpose of this study was to quantify the relationship between plasma βARK1 and physical symptoms among adults with HF.
Methods
Sample
We performed a secondary analysis of data collected from two merged studies of HF patients. The sample of 94 participants included two cohorts: community-dwelling adults with HF (n = 46) and adults with advanced HF awaiting left ventricular assist device placement (n = 48).11 Both cohorts were included in this analysis to capture a wide spectrum of symptoms and disease severity across HF patients. Key inclusion criteria for both cohorts were age 21 years or older and ability to read and comprehend 5th grade English or Spanish. The community-dwelling cohort was required to have current or past HF symptoms (i.e. New York Heart Association (NYHA) Class I-IV), and the advanced HF cohort had to be eligible for continuous-flow left ventricular assist device implantation as a bridge to transplantation or as destination therapy. Potential participants were excluded if they had a diagnosis of major cognitive impairment, prior heart transplantation or mechanical circulatory support, concomitant terminal illness, major psychiatric illness, or inability to complete the requirements of the study. Our Institutional Review Board approved both studies, and we obtained written informed consent from all participants.
Measurement
Sociodemographic and clinical data.
Data on age, gender, marital status, race, and education were obtained using a sociodemographic questionnaire. An attending HF cardiologist assessed functional status (i.e. NYHA Class) during the same visit as enrollment. Data on history, etiology, and treatment of HF were collected through an in-depth review of the electronic medical record. The Seattle Heart Failure Model (SHFM) score was calculated based on the model developed by Levy and colleagues12 and using the online calculator (https://depts.washington.edu/shfm). In this model, demographic and objective clinical variables and HF treatments are multiplied by respective slope coefficients to generate a single composite risk-prediction score with higher scores indicating worse prognosis. Clinical characteristics, including left ventricular ejection fraction and left ventricular internal end-diastolic diameter from echocardiographic assessments and pulmonary capillary wedge pressure, right atrial pressure, and cardiac index (calculated by the Fick equation) from right heart catheterization, were also collected. Comorbid conditions were summarized using the Charlson Comorbidity Index.13 Data collection procedures were identical for both studies.
β-adrenergic receptor kinase-1.
Whole blood was collected from all participants, centrifuged at 2800 rpm for 10 minutes at 5°C to separate plasma, and stored at −80°C until ready to process. We used a commercially-available enzyme-linked immunosorbent assay (ELISA) (Cusabio, College Park, MD) to quantify plasma βARK1 based on a quantitative sandwich immunoassay technique according to manufacturer’s instructions. Samples were run in duplicate and a mean value obtained. A standard curve with known βARK1concentrations (18–600 pg/mL) was run in parallel. The intra- and inter-assay coefficients of variation were 3.6% and 1.8% respectively.
Physical symptoms.
Physical symptoms were measured with the 18-item HF Somatic Perception Scale (HFSPS).14 The HFSPS measures perceived severity of both nonspecific symptoms (e.g. fatigue) and acute symptoms (e.g. dyspnea) in HF. Scores on the HFSPS range from 0–90 (higher scores indicate worse perceived physical symptoms). The validity of the HFSPS has been established.14 Reliability (Cronbach’s α) of the HFSPS in our sample was 0.91.
Statistical Analysis
Standard descriptive statistics of frequency, central tendency, and dispersion were used to describe the sample. Raw values of plasma βARK1 were natural log-transformed to achieve normality; both values were used in analyses, and raw values were reported to support clinical translation. Generalized linear modeling was used to quantify the relationship between βARK1 and HFSPS scores. Specifically, we used stepwise modeling using backward selection (p < 0.20 retention) to examine the influence of plasma βARK1 on physical symptoms, while controlling for other significant predictors of physical symptoms and with the goal of identifying a parsimonious multivariate model that was not saturated with nonsignificant factors. Informed by our prior research,15 variables added into the stepwise model (in addition to plasma βARK1) included age, gender, ischemic versus non-ischemic etiology, SHFM score, Charlson Comorbidity Index, atrial fibrillation, treatment with an angiotensin-converting enzyme inhibitor or angiotensin receptor block, right atrial pressure, cardiac index, left ventricular internal end-diastolic diameter, and blood urea nitrogen to creatinine ratio. We controlled for beta-blocker use in the final model. Additionally, in order to ascertain the value of plasma βARK1 in addition to SHFM scores (as a composite indicator of worse prognosis) in predicting physical symptoms, we used moderation analysis to quantify the interaction of plasma βARK1 and SHFM score in explaining HFSPS scores. All analyses were performed using Stata/MP v.13 (College Station, TX).
Results
The young, mostly male, and mostly Non-Hispanic Caucasian sample had primarily NYHA Class III or IV HF and non-ischemic etiology (Table 1). The median time since HF diagnosis was approximately 6 years. On average, participants had high filling pressures and low ejection fraction, and the majority were treated with evidence-based therapies. Plasma βARK1 ranged from 0.34 to 126.9 pg/mL.
Table 1:
Characteristics of the Sample (n = 94)
| M±SD, N(%), or Median [IQR] | |
|---|---|
| Age (years) | 54.5±13.1 |
| Male | 72 (76.6) |
| Non-Hispanic Caucasian | 78 (83.0) |
| Education level | |
| ≤ High school degree | 30 (31.9) |
| > High school but < college degree | 39 (41.5) |
| ≥ College degree | 25 (26.6) |
| Body Mass Index (kg/m2) | 29.9±6.9 |
| Charlson Comorbidity Index (weighted) | 2.5±1.7 |
| Atrial Fibrillation | 48 (51.1) |
| General Heart Failure Characteristics: | |
| Time with Heart Failure (years) | 6.1 [1.5–12.0] |
| NYHA Functional Class | |
| Class I | 3 (3.2) |
| Class II | 13 (13.8) |
| Class III | 55 (58.5) |
| Class IV | 23 (24.5) |
| Non-ischemic Etiology | 59 (62.8) |
| Prescribed a β-blocker | 50 (53.2) |
| Prescribed an ACE-I or ARB | 69 (73.4) |
| Serum sodium (mEq/L) | 136.3±4.3 |
| Serum hematocrit (%) | 38.6±5.5 |
| Serum BUN/creatinine ratio (mg/dL:1) | 20.3 [16.3–25.7] |
| SHFM Score | 2.7±1.2 |
| Left ventricular internal end-diastolic diameter (cm) | 6.9±1.3 |
| Left ventricular ejection fraction (%) | 23.2±9.8 |
| Pulmonary capillary wedge pressure (mm/Hg) | 20.6±9.7 |
| Right atrial pressure (mm/Hg) | 8.4±4.4 |
| Cardiac index (L/min/m2 by Fick equation) | 2.0±0.5 |
| Plasma Biomarker: | |
| βARK-1 (raw values; pg/mL) | 10.7 [4.2–28.9] |
| Symptoms: | |
| Physical symptoms (HFSPS; 0–90) | 35.2±18.6 |
Abbreviations: ACE-I, Angiotensin Converting Enzyme-Inhibitor; ARB, Angiotensin Receptor Blocker; βARK1, β-adrenergic receptor kinase-1; BUN, blood urea nitrogen; HFSPS, Heart Failure Somatic Perception Scale; IQR, interquartile range; M, mean; NYHA, New York Heart Association; SD, standard deviation; SHFM, Seattle Heart Failure Model
The combination of plasma βARK1 and clinical characteristics significantly explained 25.0% of the variance in HFSPS scores (F(7, 70) = 3.34, p = 0.004) (Table 2). In addition to other clinical characteristics retained in the final model, βARK1 was independently and significantly associated with HFSPS scores (β = 0.22 ± 0.10, p = 0.038). Substituting the log-transformed value of βARK1 demonstrated similar significant results (data not shown).
Table 2:
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| β±SE | p | β±SE | p | |
| 0.11±0.10 | 0.256 | 0.22±0.10 | 0.038 | |
| Male | −8.17±4.57 | 0.078 | −9.15±4.54 | 0.048 |
| Age | −0.26±0.15 | 0.079 | −0.41±0.15 | 0.009 |
| ACE-I/ARB | 4.01±4.43 | 0.368 | 10.36±4.64 | 0.029 |
| SHFM score | 0.17±1.72 | 0.920 | 4.93±2.22 | 0.030 |
| Right atrial pressure | −0.70±0.46 | 0.127 | −1.66±0.50 | 0.001 |
| Beta-blocker | −2.14±3.92 | 0.586 | 0.68±4.48 | 0.880 |
| Model R2/Adjusted R2 | 0.250/0.175 | |||
Physical symptoms were measured with the Heart Failure Somatic Perception Scale
Results reported were factors retained in stepwise modeling using backward selection that included: age, gender, ischemic versus non-ischemic etiology, Seattle Heart Failure Model score, Charlson Comorbidity Index, atrial fibrillation, treatment with an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, right atrial pressure, cardiac index, left ventricular internal end-diastolic diameter, blood urea nitrogen to creatinine ratio, and plasma β-adrenergic receptor kinase-1. Treatment with a beta-blocker was held in the final model as a lockterm.
Abbreviations: ACE-I, Angiotensin Converting Enzyme-Inhibitor; ARB, Angiotensin Receptor Blocker; βARK1, β-adrenergic receptor kinase-1; SE, standard error; SHFM, Seattle Heart Failure Model.
In moderation analysis, plasma βARK1 (β = 1.42 ± 0.31, p <0.001) and SHFM scores (β = 12.40 ± 1.58, p < 0.001) were independently associated with HFSPS scores. There also was a significant interaction effect of plasma βARK1 and SHFM scores in predicting HFSPS scores (Figure; interaction effect: β = −0.42 ± 0.09, p < 0.001; model: F(5,86) = 49.26, p < 0.001), indicating that the combination of plasma βARK1 and SHFM scores is better at explaining the gradient of HFSPS scores as opposed to either measure alone. Indeed, the spectrum of HFSPS scores more closely followed the range of plasma βARK1 levels compared with the range of SHFM scores. As an example, patients with the worse physical symptoms had higher levels of plasma βARK1 but not higher SHFM scores.
Figure.
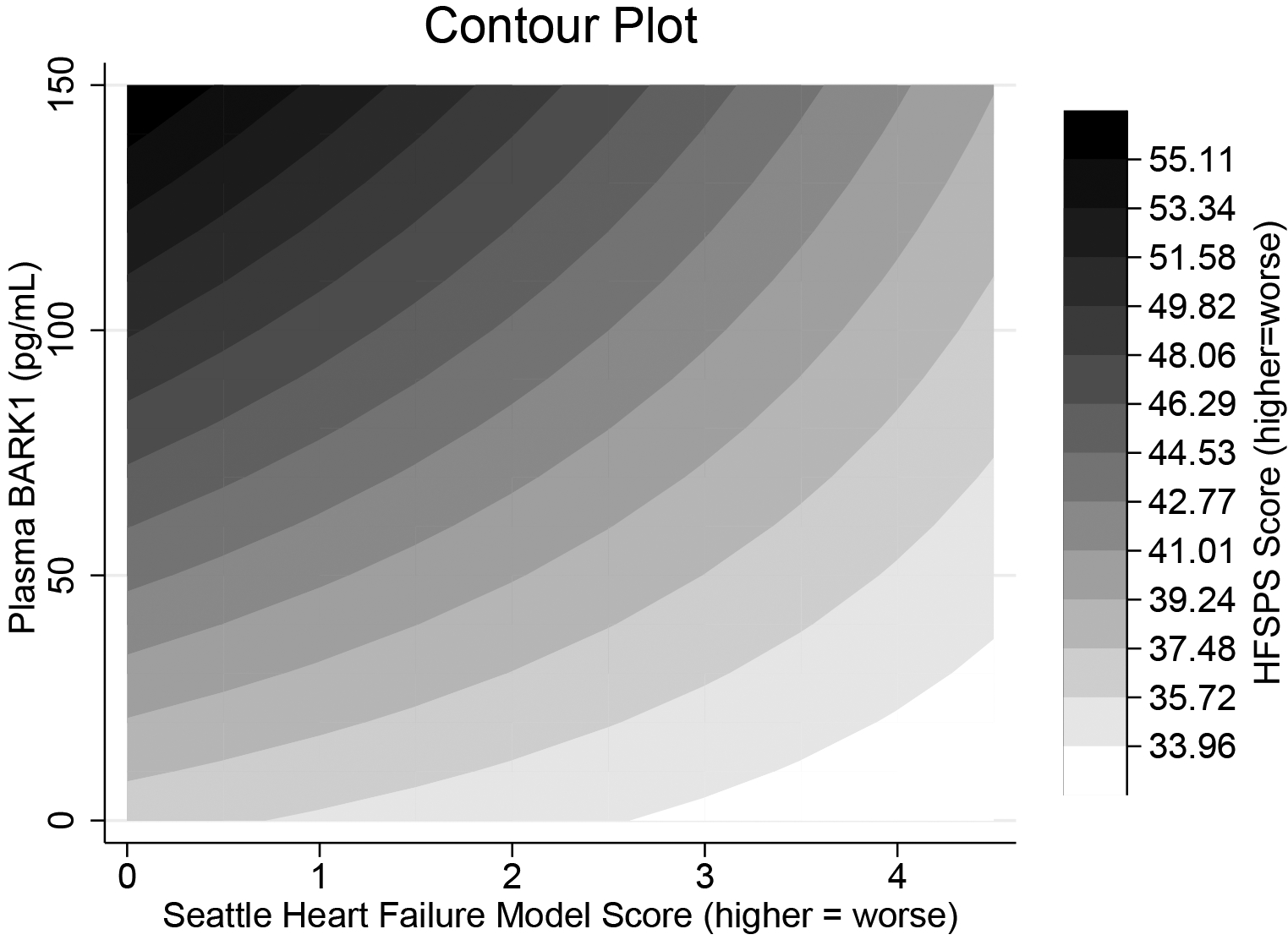
Contour plot of the interaction between plasma βARK1 and SHFM score in explaining physical symptoms measured by the HFSPS. The plot shows that HFSPS scores are dependent on both βARK1 and SHFM scores, but the range of HFSPS scores more closely follows the gradient of βARK1 levels than the gradient of SHFM scores. For example, those with the worst physical symptoms (black) have higher levels of βARK1 but better SHFM scores. Conversely, those with the least physical symptoms (white) have worse SHFM scores and relatively low plasma βARK1. Abbreviations: βARK1, β-adrenergic receptor kinase-1; HFSPS, Heart Failure Somatic Perception Scale; SHFM, Seattle Heart Failure Model.
Discussion
With recently improved understanding of the role of βARK1 in the progression of HF9 and as a potential therapeutic target in HF,16 we explored the relationship between plasma βARK1, as a marker of chronic sympathetic overactivation, and physical symptoms in HF. Our main findings were 1) elevated plasma βARK1 was significantly associated with worse physical symptoms after adjusting for other clinical characteristics, and 2) plasma βARK1 provides more information in differentiating physical symptoms in HF compared with the SHFM.
The observed relationship provides preliminary evidence of the role of sympathetic overactivation, as measured by plasma βARK1, in explaining the biological underpinnings of physical symptoms in HF. βARK1 has been shown to improve (i.e. decrease) among patients implanted with a ventricular assist device17 and following cardiac transplantation,18 suggesting that a decrease in βARK1 would track with improved symptoms. As chronic sympathetic overactivation is a hallmark of HF pathophysiology, it seems logical that decreased sympathetic activity would result in better symptoms perhaps through better exercise capacity or ability to respond acutely to catecholamines; however, more research using a longitudinal design and multimarker approach is needed to fully understand this relationship. Specifically, it will be important to understand autonomic balance by integrating additional sympathetic and parasympathetic markers and how these change in relation to symptoms and exercise capacity, particularly when followed by interventions.
The interactive effect of plasma βARK1 and SHFM scores indicates the combination of plasma βARK1 and a common prognostication model tells us more about physical symptoms in HF than either alone. In fact, the gradient in plasma βARK1 more closely followed a gradient in physical symptoms than the range of SHFM scores, a composite indicator of worse prognosis based on clinical and treatment parameters. This finding indicates that to understand physical symptoms in HF we have to look beyond the current list of clinical parameters included in prognostication models and consider alternative markers. In other words, if we want to differentiate who will have better or worse physical symptoms in HF, it is necessary identify markers that more closely track with patient-reported outcomes compared with markers that predict survival alone.
A clinical implication from this study is that sympathetic overactivation is one pathophysiologic mechanism that underlies physical symptom burden in HF. Given the little-to-no association between what we measure objectively (e.g. hemodynamics) and what patients experience symptomatically,4–6 this is an important next step in identifying a biological underpinning of physical symptoms in HF. These findings, coupled with other advances in HF symptom biology,19,20 could inform conversations in clinical settings, especially when eliciting information regarding symptom burden. Moreover, these results could provide an amenable target for ameliorating symptom burden through interventions directed at reducing sympathetic overdrive, including a combination of pharmacological, exercise, and self-care interventions.7,21,22
A few limitations are noted. We had a relatively small sample that was young, racially homogeneous, and mostly non-ischemic with moderate to advanced HF, which limits the generalizability of our findings. Moreover, because this was a cross-sectional analysis, we are unable to draw conclusions about the temporal relationship between plasma βARK1 and physical symptoms in HF. Finally, while we included both stable and advanced HF patients in this analysis, we may not have captured the full spectrum of symptom manifestation in HF.
Future research is needed to integrate additional sympathetic measures (e.g. plasma catecholamines), as well as autonomic measures (e.g. heart rate variability), with plasma βARK1 to fully profile the role of sympathetic overactivation in explaining variations in physical symptoms in HF. Similarly, longitudinal research is needed to understand changes in both plasma βARK1 and symptoms in both stable and advanced HF patients. Finally, the value of plasma βARK1 in predicting additional clinical and patient-oriented outcomes would allow us to quantify the clinical relevance of plasma βARK1 in HF.
Conclusions
Higher plasma βARK1, as a marker of chronic sympathetic overactivation, is associated with worse physical symptoms in HF. Given the significant symptom burden in HF, there is a need to understand the biological underpinnings of symptoms in HF. We have provided preliminary evidence of the role of βARK1 in explaining the biological underpinnings of physical symptoms in HF, but more research is needed to fully understand this relationship.
Funding Acknowledgment
The work reported in this study was funded by the National Institutes of Health/National Institute of Nursing Research (NIH/NINR) (F31NR015936; Denfeld) and through support from the Achievement Rewards for College Scientists program. The parent study was supported by grants from the NIH/NINR (R01NR013492; Lee), the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR000128), and the Oregon Health & Science University Hartford Center. Dr. Denfeld is currently supported as a Scholar of the Oregon Building Interdisciplinary Research Careers in Women’s Health K12 Program funded by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the NIH under Award Number K12HD043488. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Declaration of Conflicting Interests
None Declared
References
- 1.Alpert CM, Smith MA, Hummel SL, Hummel EK. Symptom burden in heart failure: Assessment, impact on outcomes, and management. Heart Fail Rev. 2017;22(1):25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zambroski CH, Moser DK, Bhat G, Ziegler C. Impact of symptom prevalence and symptom burden on quality of life in patients with heart failure. Eur J Cardiovasc Nurs. 2005;4(3):198–206. [DOI] [PubMed] [Google Scholar]
- 3.Moser DK, Doering LV, Chung ML. Vulnerabilities of patients recovering from an exacerbation of chronic heart failure. Am Heart J. 2005;150(5):984.e987–984.e913. [DOI] [PubMed] [Google Scholar]
- 4.Guglin M, Patel T, Darbinyan N. Symptoms in heart failure correlate poorly with objective haemodynamic parameters. Int J Clin Pract. 2012;66(12):1224–1229. [DOI] [PubMed] [Google Scholar]
- 5.Shah MR, Hasselblad V, Stinnett SS, et al. Dissociation between hemodynamic changes and symptom improvement in patients with advanced congestive heart failure. Eur J Heart Fail. 2002;4(3):297–304. [DOI] [PubMed] [Google Scholar]
- 6.Lee CS, Hiatt SO, Denfeld QE, Mudd JO, Chien C, Gelow JM. Symptom-hemodynamic mismatch and heart failure event risk. J Cardiovasc Nurs. 2015;30(5):394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Floras JS. Sympathetic Nervous System Activation in Human Heart Failure. Clinical Implications of an Updated Model. J Am Coll Cardiol. 2009;54(5):375–385. [DOI] [PubMed] [Google Scholar]
- 8.Lee CS, Tkacs NC. Current concepts of neurohormonal activation in heart failure: mediators and mechanisms. AACN Adv Crit Care. 2008;19(4):364–385. [DOI] [PubMed] [Google Scholar]
- 9.Hullmann J, Traynham CJ, Coleman RC, Koch WJ. The expanding GRK interactome: Implications in cardiovascular disease and potential for therapeutic development. Pharmacol Res. 2016;110:52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iaccarino G, Barbato E, Cipolletta E, et al. Elevated myocardial and lymphocyte GRK2 expression and activity in human heart failure. Eur Heart J. 2005;26(17):1752–1758. [DOI] [PubMed] [Google Scholar]
- 11.Lee CS, Mudd JO, Gelow JM, et al. Background and design of the profiling biobehavioral responses to mechanical support in advanced heart failure study. J Cardiovasc Nurs. 2014;29(5):405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levy WC, Mozaffarian D, Linker DT, et al. The Seattle Heart Failure Model: Prediction of survival in heart failure. Circulation. 2006;113(11):1424–1433. [DOI] [PubMed] [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 14.Jurgens CY, Lee CS, Riegel B. Psychometric analysis of the Heart Failure Somatic Perception Scale as a measure of patient symptom perception. J Cardiovasc Nurs. 2017;32(2):140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denfeld QE, Mudd JO, Gelow JM, Chien C, Hiatt SO, Lee CS. Physical and psychological symptom biomechanics in moderate to advanced heart failure. J Cardiovasc Nurs. 2015;30(4):346–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cannavo A, Komici K, Bencivenga L, et al. GRK2 as a therapeutic target for heart failure. Expert Opin Ther Targets. 2018;22(1):75–83. [DOI] [PubMed] [Google Scholar]
- 17.Hata JA, Williams ML, Schroder JN, et al. Lymphocyte levels of GRK2 (βARK1) mirror changes in the LVAD-supported failing human heart: Lower GRK2 associated with improved β-adrenergic signaling after mechanical unloading. J Card Fail. 2006;12(5):360–368. [DOI] [PubMed] [Google Scholar]
- 18.Bonita RE, Raake PW, Otis NJ, et al. Dynamic changes in lymphocyte GRK2 levels in cardiac transplant patients: A biomarker for left ventricular function. Clin Transl Sci. 2010;3(1):14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee CS, Hiatt SO, Denfeld QE, Chien CV, Mudd JO, Gelow JM. Gender-specific physical symptom biology in heart failure. J Cardiovasc Nurs. 2015;30(6):517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heo S, Moser DK, Pressler SJ, Dunbar SB, Dekker RL, Lennie TA. Depressive Symptoms and the Relationship of Inflammation to Physical Signs and Symptoms in Heart Failure Patients. Am J Crit Care. 2014;23(5):404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gademan MGJ, Swenne CA, Verwey HF, et al. Effect of Exercise Training on Autonomic Derangement and Neurohumoral Activation in Chronic Heart Failure. J Card Fail. 2007;13(4):294–303. [DOI] [PubMed] [Google Scholar]
- 22.Lee CS, Tkacs NC, Riegel B. The influence of heart failure self-care on health outcomes: Hypothetical Cardioprotective mechanisms. J Cardiovasc Nurs. 2009;24(3):179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]


