Abstract
Introduction:
The largest Ebola virus (EBOV) outbreak occurred from 2013 – 2016 in West Africa and consequently resulted in the largest cohort of Ebola virus disease (EVD) survivors to date. Ocular disease is among the most common sequelae reported in EVD survivors. This review discusses the prevalence, manifestations, pathogenesis, diagnosis and management of EVD-related ocular disease.
Areas covered:
An extensive review of the literature was performed to detail the prevalence and manifestations of EVD-related ocular disease. We also review current eye screening and treatment strategies and our current understanding and approach to invasive ophthalmic procedures including surgery.
Expert opinion:
The ocular sequelae of EVD can lead to vision impairment or blindness, if untreated. Keys to the prevention of such an outcome include timely evaluation and access to appropriate ophthalmic care. The persistence of EBOV in the eye and other immune-privileged sites is the subject of ongoing investigation, but should not be a barrier to care if appropriate screening and biosafety measures are taken. Improved understanding of the pathogenesis of this condition and ongoing clinical care are needed for EVD survivors at-risk for ocular complications.
Keywords: Ebola, Ebola virus, Ebola virus disease, Ebola sequalae, uveitis
Introduction
1. Clinical presentation of acute Ebola virus infection
1.1. Background
Since Ebola virus disease (EVD) first was identified in 1976, there have been 36 recorded outbreaks, the most recent of which is currently ongoing in the Democratic Republic of the Congo (DRC) and has claimed over 1000 lives, the largest documented EVD outbreak within DRC to date [1]. The EVD outbreak in West Africa of 2013 – 2016, which was concentrated in Sierra Leone, Guinea and Liberia, was the largest in history with over 28, 600 cases resulting in approximately 11,300 deaths[2]. While the loss of life from this outbreak is staggering, the sheer volume of survivors has drawn attention to a variety of symptoms that develop during EVD convalescence and sequelae, including neuropsychiatric disorders, arthritis, abdominal pain, hearing and vision loss[3–7]. Taken together, this spectrum of findings has been termed the post Ebola virus disease syndrome (PEVDS). Ocular complications in EVD patients have been reported in both the acute phase of the disease as well as part of PEVDS resulting in blindness in up to 38% of affected individuals[8]. Given the impact of these ocular sequelae on vision, continued care and improved understanding of these findings for EVD survivors is warranted.
1.2. Clinical disease
Ebola virus (EBOV) is an enveloped, non-segmented single stranded RNA-virus of the Filoviridae family. There are six different species of the EBOV including Zaire, Sudan, Taï Forest, Reston and Bundibugyo; recently, the Bombali strain of EBOV was identified in bat reservoirs and was named after the northern Bombali region where it was identified. While this strain has been found to be capable of infecting human cells, it is unknown whether this strain can lead to human EVD[9]. The Zaire EBOV strain is the deadliest of these six strains with the highest case fatality rate, and was the strain implicated in West African outbreak. Natural animal reservoirs of the disease include fruit bats, chimpanzees, gorillas and duikers. Animal-human transmission may occur via direct contact or consumption of animal carriers while human-human transmission may be caused by exposure to bodily fluids including blood, saliva, sweat, urine, semen, breast milk, vomit and feces.
EBOV has an incubation period of 2 – 21 days and begins initially with non-specific symptoms including fever, headache, malaise and diarrhea. During this phase of the disease, the EBOV impairs the host immune response by disabling dendritic cell function thereby decreasing T-cell activation and cytokine release. Simultaneously, macrophages are infected and induced to secrete high levels of cytokines which lead to the expression of pro-inflammatory modulators by both infected and non-infected macrophages. The resultant ‘cytokine storm’ leads to increased vascular permeability, hypovolemic shock, disseminated intravascular coagulation, diffuse hemorrhage and multi-organ failure leading to death 6 – 16 days after the onset of symptoms[10]. Ocular findings during the acute phase of the disease include bilateral conjunctivitis with or without subconjunctival hemorrhage. Acute vision loss of yet unknown etiology has also been reported[11].
The gold standard for diagnosis of EBOV is by reverse transcriptase polymerase chain reaction (RT-PCR). Other methods include the detection of immunoglobulin M (IgM) and G (IgG) antibodies and detection of EBOV antigens by antigen detection tests. Additionally, during this most recent outbreak, the GeneXpert Ebola Assay (Cepheid, Sunnyvale, CA, USA) was tested in the field with high sensitivity and specificity on both whole blood and buccal swabs. This technology decreases sampling processing time and takes out the multiple processing steps in traditional RT-PCR. Drawbacks include the need for continuous electricity and storage of reagents in cool environments, which may be a challenge in resource-limited settings[12]. A Biosafety Level 4 laboratory is required for testing of suspected EBOV specimens.
The current mainstay of treatment of acute cases of EVD is supportive care though there is ongoing research into the role of antivirals, small-interfering RNA and convalescent plasma[13]. There have also been exhaustive efforts to develop an effective vaccine against EBOV – specifically the Zaire strain.
2. Post Ebola virus disease syndrome
Many short-term and long-term health problems have been reported following infection with all species of EBOV. Common symptoms include musculoskeletal pain, headache, mood disorders, abdominal pain and ocular disorders. In the largest study of EVD survivors to date, Etard and colleagues found that 606 of 802 patients had PEVDS symptoms at the time of enrollment including general (40%), musculoskeletal (38%), neurosensory (37%) and ocular (18%) complaints [14]. Other large cohort studies have reported a similar range of findings[3, 15–17].
The exact pathogenesis of these findings is yet to be determined but may be attributed to residual dysfunction from the acute phase of the disease, sustained immune response, hypersensitivity-type reaction(s) and/or viral persistence in immune-privileged sites. Mattia and colleagues found that higher viral load at acute presentation was an independent risk factor for development of uveitis[3]. Similarly, the presence of surrogate markers of severe acute disease – melena and seizures - have been associated with a higher risk of developing PEVDS[15].
2.1. Viral persistence in the eye and other immune-privileged sites
While there was limited historical evidence that EBOV replicated in immune-privileged sites (eyes, brain and testes), the recent outbreak in West Africa produced a number of confirmatory cases and provided an increased understanding of the potential for long-term EBOV persistence in these sites[18, 19]. Moreover, recent work in rhesus monkey survivors has confirmed that EBOV may progressively disseminate to and persist in these sites via CD68+ (macrophage/monocyte) cells[20]. These findings have significant public health implications with regard to recrudescence, but also may help provide insight into the pathogenesis of PEVDS.
The detection of viable EBOV in the aqueous humor of an EVD survivor was first described in 2015 by Varkey et al.[21]. This patient developed severe, acute, unilateral uveitis during the convalescent phase of EVD, 9 weeks after clearance of viremia. At 14 weeks after the diagnosis of EVD, an aqueous humor sample tested positive for EBOV RNA on quantitative RT-PCR assay with a high concentration of EBOV within the aqueous humor. A peripheral blood specimen tested negative for EBOV RNA by quantitative RT-PCR assay. To investigate the pathogenesis of EVD-associated uveitis, Smith et al. performed in vitro inoculation of human retinal pigment epithelial cells (ARPE-19 cell line) with EBOV[22]. They found that RPE cells were permissive to infection with EBOV and that EBOV-infected cells showed down-regulation of intrinsic apoptotic signaling pathways which would be one explanation for EBOV persistence in ocular fluids.
The potential for EBOV to persist within the aqueous humor of other EVD survivors and ongoing need to address vision impairment due to cataract in West African EVD survivors led to the Ebola Virus Persistence in Ocular Tissues and Fluids (EVICT) study – a large cross-sectional study described by Shantha and colleagues to evaluate the safety of cataract surgery in EVD survivors[23]. In this study, 50 EVD survivors with visually significant cataracts were enrolled and aqueous humor samples were tested for EBOV by quantitative RT-PCR assay. For logistical reasons, all patients could not be tested at the same time so were divided in two separate sessions - phase 1 and phase 2. All 50 ocular fluid specimens tested negative for EBOV RT-PCR with a median time from EVD diagnosis to anterior chamber paracentesis of 19 months in phase 1 and 34 months in phase 2 of the study.
Besides concerns to ophthalmologists related to EBOV persistence within the eye, the persistence of EBOV in other immune-privileged sites (i.e. reproductive organs) pose public health risk due to the potential for sexual transmission during EVD convalescence. Viral persistence has also been reported in the central nervous system (i.e. cerebrospinal fluid leading to meningocephalitis) and delayed clearance has been reported in breast milk[24]. The presence of viable EBOV in semen has been reported as late as 565 days (18.5 months) from time of discharge from the Ebola Treatment Unit (ETU)[25]. Further, sexual transmission of EBOV 470 days (15.4 months) after onset of symptoms was determined to be the cause of a new cluster of EVD in Guinea and Liberia in 2016[26]. In a large observational cohort of 220 male EVD survivors, Deen et al. found evidence of EBOV RNA in all 7 male participants tested within 3 months of ETU discharge, 26 of 42 (62%) tested between 4 to 6 months, 4 of 38 (11%) tested between 13–15 months, 1 of 25 (4%) tested between 16 to 18 months, and none of 12 at 19 months or later[27]. There is at least one confirmed case of EBOV detected in breast milk and one case with genomic evidence of mother-child transmission via breast milk[19, 28] Further understanding of the dynamics of EBOV entry into immune privileged sites (i.e. eye, central nervous system, reproductive organs), the kinetics of its clearance and relationship of viral persistence in various immune privileged sites are the subject of ongoing basic and clinical sciences investigation.
3. Ocular findings
Ocular sequelae are common among EVD survivors with vision loss, eye redness and eye pain being the most frequently reported symptoms (Table 1). A variety of ophthalmic findings have been reported including uveitis, conjunctivitis, scleritis, cataract, ocular hypertension, extraocular motility disorders, and one reported case of optic neuropathy[3, 6, 11, 21, 23, 29]. Uveitis is the most common ophthalmic manifestation of PEVDS and has been reported in 13% - 34% of EVD survivors[3, 8, 11, 16, 29]. A wide spectrum of active uveitic findings has been reported including scleritis, keratic precipitates, anterior chamber inflammation (cells/flare), vitritis and engorged blood vessels[3, 8, 11, 16, 29–31]. A similar range of uveitic sequelae has also been described including posterior synechiae, iris heterochromia, cataract (Figure 1), chorioretinal scarring (Figure 2) and optic disc pallor[3, 8, 16, 31].
Table 1.
Signs, Symptoms and Clinical Findings. Table shows signs and symptoms reported by Ebola virus disease survivors and clinical features described in prior reports of Ebola virus disease survivors in the United States and West Africa.
| Signs/Symptoms | Clinical Findings |
|---|---|
| Eye redness Eye discharge / Tearing Photophobia Eye pain Blurry vision Floaters |
External -Conjunctival injection -Subconjunctival hemorrhage |
|
Cornea -Band keratopathy -Keratic precipitates | |
|
Anterior Chamber -Inflammatory cells and flare -Hyphema | |
|
Iris -Posterior synechiae -Neovascularization -Seclusio pupillae -Iris heterochromia | |
|
Lens -Dense white cataract -Posterior subcapsular cataract -Capsular fibrosis | |
|
Posterior Segment / Globe -Vitreous cells -Optic disc edema -Macular edema -Wedge-shaped chorioretinal scars - Phthisis bulbi (Cosmetic deforming of the globe due to chronic low intraocular pressure, often multifactorial in nature including inflammation and scarring) |
Figure 1.
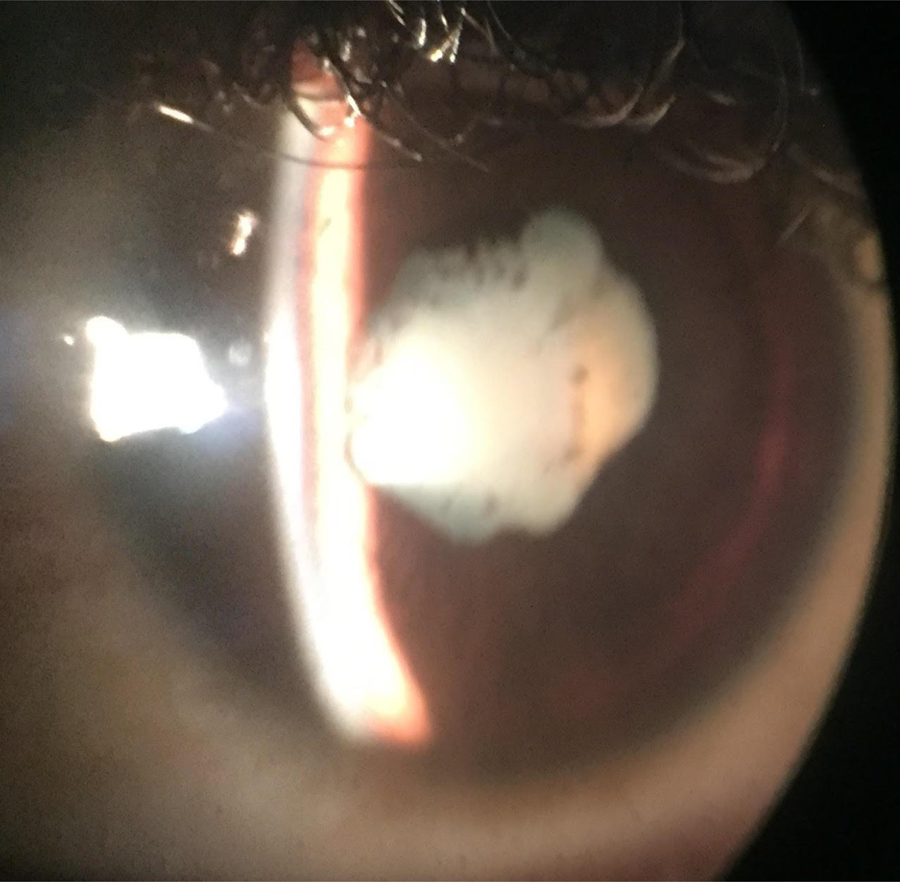
Slit lamp photograph with an iPhone shows a dense uveitic white cataract with posterior synechiae (i.e. adhesions from iris to the lens giving a misshapen pupil). There are pigment granules on the lens capsule indicative of prior inflammation.
Figure 2.
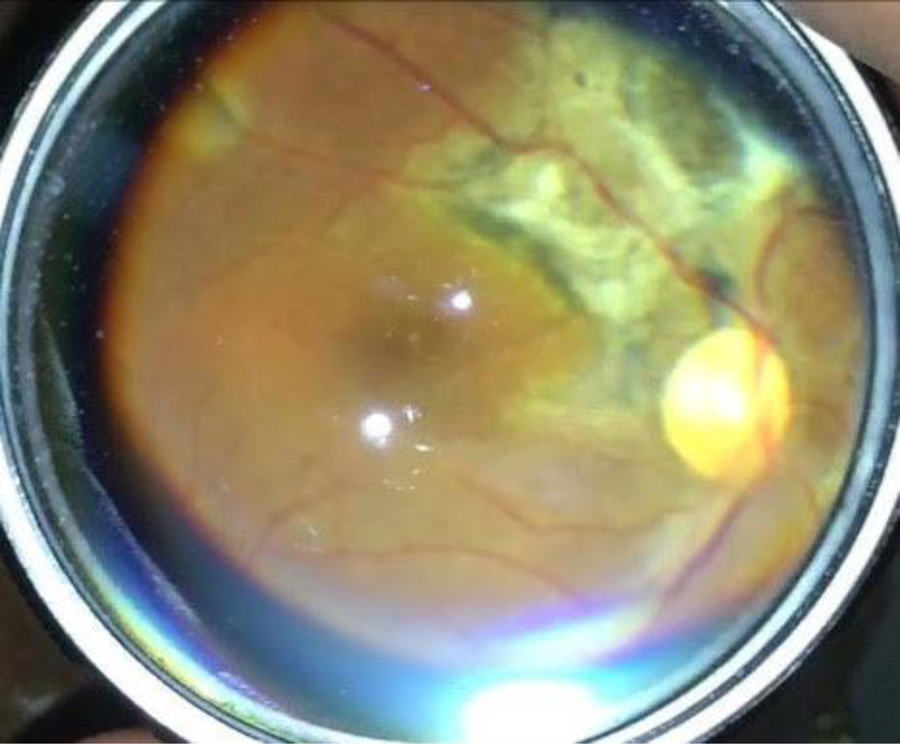
Fundus photograph taken with an iPhone and condensing lens shows optic nerve pallor and chorioretinal scar with subretinal fibrosis along the superotemporal vascular arcade indicating posterior uveitis.
Follow-up from the 1995 Kikwit outbreak provided the first reports of uveitis in 4 of 21 survivors between 42 and 72 days following acute EVD[11]. The recent outbreak in West Africa produced a large survivor population from which several large studies have further investigated ocular sequelae of EVD. While these reports have largely studied uncontrolled cohorts, they nevertheless have provided valuable information regarding the incidence, natural history and risk factors contributing to the development of ocular sequelae.
Of 277 Sierra Leone EVD survivors, Mattia et al. reported uveitis in 18% including anterior (46%), posterior (26%) and panuveitis (25%) with a median time of presentation of 3 weeks following discharge from the ETU[3]. In another large, retrospective cohort study from Sierra Leone, 58 of 166 (35%) evaluated survivors were found to have uveitis with anterior (62%) and panuveitis (21%) being the most common anatomic locations and 59% of cases being bilateral [16]. In a case-control study by Steptoe et al., 82 Sierra Leone EVD survivors and 105 controls underwent ophthalmic examination with a median time of examination of 411 days following discharge from the ETU[30]. Among the EVD survivor group, evidence of prior uveitis by location was found 10% (anterior) and 9.8% (intermediate). The authors also reported novel retinal lesions seen in 14 of the 82 EVD survivors which they propose represent sequelae of retrograde neuronal transmission of the virus[6].
In the largest study of ocular complications among Liberian EVD survivors, Shantha et al. observed uveitis in 21 of 96 (22%) patients, including 5 individuals with bilateral disease[8]. By anatomic location, 15% were anterior, 58% posterior and 27% were described as panuveitis. Of the 22 eyes with posterior or panuveitis, peripheral chorioretinal scarring was observed in 14 eyes (64%) and macular chorioretinal scarring was observed in 2 eyes (9%). A recent study by Wilson et al. reported that among 242 EVD survivors from Liberia, 47% reported eye complaints that persisted for 10–12 months following discharge from the ETU[32].
In a prospective study of 341 EVD survivors from Guinea, uveitis was observed in 46 patients (13.5%) - of which 75% of cases were identified within the first 2 months following ETU discharge[29]. The anatomic location of uveitis included anterior in 22 (48%), at least anterior but no visibility of fundus due to cataract in 7 (15%), posterior in 13 (28%) and panuveitis in 4 (9%). Notably, relapses were observed in 4 (9%) of 46 patients, occurring between 10 and 13 months after discharge from the ETU.
Similar to the broad spectrum of manifestations, vision loss in EVD-related ocular disease can also range from minimal to severe. Hereth et al. reported visual acuity (VA) of ≤ 6/12 (Snellen equivalent 20/40) in 15 of 341 patients examined within 2 months of discharge from the ETU[29]. Among 21 EVD survivors found to have uveitis, Shantha et al. found 17 of these to have VA ≤ 20/40 with 10 of the 17 having VA ≤ 20/400[8].
4. Management of Ebola-associated ocular disease
While there are no prospective or controlled studies evaluating the efficacy of treatment of EVD-related uveitis, successful management has been anecdotally reported using topical corticosteroids as the mainstay of treatment. Oral corticosteroids have also been employed in aggressive cases[33]. In one patient, aggressive panuveitis with iris heterochromia and recalcitrant vitritis were treated with a 21-day course of the antiviral favipiravir, in addition to topical, periocular, and oral corticosteroids[21, 34]. At day 45 following presentation, the patient had near complete resolution of inflammation and visual acuity had improved to 20/15. Lastly, when considering treatment options for uveitis, practitioners should be reminded of the high prevalence of other infectious causes of uveitis in EBOV-endemic countries such as tuberculosis, herpes viruses and syphilis, which also require laboratory testing and appropriate anti-infective therapy.
The reported prevalence of cataracts in EVD survivors ranges from 6.7 – 10%[3, 8, 29]. Given that EBOV has been reported to persist in the aqueous humor, there is the potential risk of EBOV exposure and transmission to health care workers during invasive ophthalmic surgery such as cataract extraction. As such, the aforementioned EVICT study was undertaken to evaluate safe and effective measures of performing invasive ophthalmic surgery in EVD survivors[23]. All had previously undergone rigorous pre-surgical screening including testing of aqueous humor and conjunctiva for EBOV RNA by RT-PCR. All testing and surgical procedures were performed in a special facility was designed adhering to World Health Organization (WHO) guidelines and all practitioners donned full personal protective equipment (PPE) for aqueous humor sampling, followed by modified-PPE for surgery. 34 patients who tested negative for EBOV underwent manual small incision cataract surgery. Median presenting VA was hand motions with improvement to a median VA of 20/30 at three-months postoperative follow-up[23]. While these findings are promising, further research is needed to establish the safety of surgery at earlier time points in the recovery period.
Timely medical and surgical treatment of EVD-related ocular disease may help prevent permanent vision loss but detection of eye disease hinges on regular ophthalmic examination and patient awareness related to signs and symptoms of ocular disease. The WHO has published interim guidelines for the care of EVD survivors which includes ophthalmic examination within 1 month of discharge from the ETU as well as education related to uveitis symptoms[35]. Efforts by multiple partners including non-governmental organizations, academic institutions and government are ongoing in all three countries affected by the 2013 – 2016 outbreak to better screen and provide eye care to EVD survivors[36, 37].
5. Other emerging infectious diseases
The recent EVD outbreak in West Africa was a stark reminder that, despite therapeutic advances in medicine, rapidly emerging infectious diseases have the potential to lead to devastating epidemics with tragic loss of life, tremendous socioeconomic cost, and widespread damage to already fragile health systems. While progress has been made related to EBOV-specific diagnostic, treatment and prevention strategies, it is vitally important that the lessons learned are applied broadly to the management of future outbreaks of other etiologies. A combination of factors, including global population growth and increasing global travel, favor the emergence and potential spread of new pathogens. In 2018 alone, the WHO has identified 42 outbreaks in 32 countries due to a variety of causes, including both well-characterized infectious pathogens (Cholera, Yellow Fever) and emerging infectious diseases (EVD, Chikungunya, Zika)[38]. Virus surveillance and predictive modeling will play a crucial role in a proactive approach to preparing for the next epidemic through identification of high priority diseases within an extremely broad spectrum of infectious threats. [39].
Ocular involvement has been reported in several emerging infectious diseases in addition to EVD. Specifically, anterior and posterior uveitis are known manifestations of the West Nile Virus, Chikungunya, Zika and the Human T lymphocyte virus type 1 (HTLV-1), among many others[40, 41]. This underscores the importance of ophthalmic examination in the evaluation of patients who are affected by or are survivors of such diseases.
6. Expert opinion
Since the initial observations of ocular manifestations of EVD were made two decades ago, much progress has been made in our understanding of the breadth of acute and chronic eye findings, though many questions remain with regard to pathogenesis and management. It is now well-established that EVD-related uveitis is among the most common sequelae and can have devastating consequences if left untreated. Identification and diagnosis of EVD-related uveitis remain a challenge, primarily due to gaps in both the patients’ understanding of their disease and access to ophthalmic care. Fortunately, most cases of EVD-related uveitis that are seen by trained providers respond to local and systemic corticosteroids, both of which are usually obtainable in resource-limited settings. The specific role of antiviral therapy for uveitis associated with EVD is unknown and warrants further investigation given the prior identification of EBOV in the aqueous humor.
The discovery of EBOV persistence in the aqueous humor has offered insights into possible mechanisms of uveitis in EVD survivors but also has had public health ramifications related to the risk of transmission via invasive ophthalmic procedures. Subsequent investigations in cell culture and animal models have identified several cellular and molecular mechanisms by which EBOV can persist and possibly reactivate within immune-privileged sites such as the eye[20, 22, 42]. From a more clinical perspective, the ongoing EVICT study has been able to delineate safe and effective screening and protocol measures for patients needing invasive ophthalmic procedures and short-term outcomes show dramatic improvement in VA following cataract surgery in EVD survivors. Continued efforts in both the laboratory and for affected patients will be necessary to provide ongoing care for EVD survivors.
EVD outbreaks remain ongoing with 1264 cases and 814 deaths reported in the most recent outbreak in the DRC which began in August 2018[1]. As such, rapid disease surveillance, contact tracing, and supportive care of patients with EVD are of paramount importance to reduce mortality and quell ongoing transmission during EVD outbreaks. Moreover, it is also vital that we continue to develop the paradigms of care for EVD survivors following care of acute EVD, in order to manage systemic and ophthalmic sequelae. Additional research is particularly needed with regard to management of EVD-associated uveitis, including therapy for acute cases of uveitis, as well as in recurrent and recalcitrant cases.
The barriers to survivor care for resource-limited settings include access to resources and providers, as well as education of survivors regarding the symptoms for which they are to return for ongoing care. Recommendations were made via interim guidance in the WHO Clinical Care for EVD survivors, and improved understanding of EVD sequelae will inform paradigms for future survivor care. Collaboration between WHO, local ministries of health, local and national non-governmental organizations, have coordinated efforts to screen and monitor EVD survivors and are needed. For example, the Sierra Leone National Eye Care Program launched a nationwide campaign with the Ministry of Health and screened approximately 2700 EVD-survivors for eye disease in the months following the West African EVD outbreak. Similar efforts are needed in other EVD-affected countries to provide the necessary evaluation and treatment of EVD-related sequelae. Finally, it is imperative that we use lessons learned from the 2013 – 2016 West African EVD outbreak to build a framework for the management of future outbreaks of EVD and other emerging infectious diseases.
Article highlights.
EVD has a high prevalence of ocular involvement, particularly uveitis; continued study is needed to determine pathogenesis and natural history.
Topical and oral steroids are effective strategies for intraocular inflammation, but more research is needed to establish definitive guidelines and treatment strategies, particularly for recurrent cases.
The EVICT study has resulted in a model for safe and feasible cataract surgery resulting in vision restoration for a cohort of EVD survivors.
Continued resources and funding are needed to address ophthalmology clinical care and education gaps, medication and equipment limitations, and other health care disparities that present barriers to evaluation and treatment of eye care in EVD and other emerging infectious diseases.
Acknowledgements
The authors gratefully acknowledge Ebola virus disease survivors and their families, as well as their many partners in Sierra Leone and Liberia with whom they have worked. Specifically, the authors thank the Lowell and Ruth Gess/Kissy United Methodist Church Eye Hospital, Dr. Lowell Gess and the Gess family, Mr. Roger and Mrs. Melanie Reiners, Mr. Ibrahim Conteh, Central Global Vision Fund and Christian Blind Mission International. We also thank the Sierra Leone Association of Ebola Survivors, Bishop John Yambasu, Mr. Rahm and Mrs. Radha Sitaraman, Partners in Health, Santen, Inc, Alcon Research Institute, Bayer Global Ophthalmology Awards Program, the Marcus Foundation, Emory University School of Medicine, Emory Global Health Institute, Emory Retina and Uveitis Services, and the Emory Eye Center leadership for their support.
Funding
This project was supported by unrestricted departmental grant from Research to Prevent Blindness, Inc. (New York, NY), NIH/NEI core grant P30-EY06360 (Department of Ophthalmology, Emory University School of Medicine), Building Interdisciplinary Careers in Women’s Health of the National Institutes of Health K12HD085850 ( J Shantha), an Alcon Research Institute Young Investigator Grant (S Yeh), Bayer Global Ophthalmology Awards Program (J Shantha), Marcus Foundation Combating Childhood Illness Seed Grant to Emory Global Health Institute (S Yeh), Emory University Research Committee Grant (S Yeh) and an unrestricted grant from Santen, Inc. (S Yeh, J Shantha) and the Rahm Sitaraman family.
Footnotes
Declaration of interest
S Yeh reports being a consultant for Santen, Inc. and Clearside Biomedical. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
Articles of special interest have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1.World Health Organization. Ebola situation reports: Democratic Republic of the Congo. https://www.who.int/ebola/situation-reports/drc-2018/en/. April 21 2019.
- 2.World Health Organization. Ebola Virus Disease: Fact Sheet. http://www.who.int/news-room/fact-sheets/detail/ebola-virus-disease. November 13 2018.
- 3.Mattia JG, Vandy MJ, Chang JC et al. Early clinical sequelae of Ebola virus disease in Sierra Leone: a cross-sectional study. Lancet Infect Dis 2016; 16:331–338. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nanyonga M, Saidu J, Ramsay A et al. Sequelae of Ebola Virus Disease, Kenema District, Sierra Leone. Clin Infect Dis 2016; 62:125–126. [DOI] [PubMed] [Google Scholar]
- 5.Vetter P, Kaiser L, Schibler M et al. Sequelae of Ebola virus disease: the emergency within the emergency. Lancet Infect Dis 2016; 16:e82–e91. * [DOI] [PubMed] [Google Scholar]
- 6.Steptoe PJ, Scott JT, Baxter JM et al. Novel Retinal Lesion in Ebola Survivors, Sierra Leone, 2016. Emerg Infect Dis 2017; 23:1102–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howlett PJ, Walder AR, Lisk DR et al. Case Series of Severe Neurologic Sequelae of Ebola Virus Disease during Epidemic, Sierra Leone. Emerg Infect Dis 2018; 24:1412–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shantha JG, Crozier I, Hayek BR et al. Ophthalmic Manifestations and Causes of Vision Impairment in Ebola Virus Disease Survivors in Monrovia, Liberia. Ophthalmology 2017; 124:170–177. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein T, Anthony SJ, Gbakima A et al. The discovery of Bombali virus adds further support for bats as hosts of ebolaviruses. Nat Microbiol 2018; 3:1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vine V, Scott DP and Feldmann H. Ebolavirus: An Overview of Molecular and Clinical Pathogenesis. Methods Mol Biol 2017; 1628:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kibadi K, Mupapa K, Kuvula K et al. Late ophthalmologic manifestations in survivors of the 1995 Ebola virus epidemic in Kikwit, Democratic Republic of the Congo. J Infect Dis 1999; 179 Suppl 1:S13–14. [DOI] [PubMed] [Google Scholar]
- 12.Semper AE, Broadhurst MJ, Richards J et al. Performance of the GeneXpert Ebola Assay for Diagnosis of Ebola Virus Disease in Sierra Leone: A Field Evaluation Study. PLoS Med 2016; 13:e1001980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayden FG, Friede M and Bausch DG. Experimental Therapies for Ebola Virus Disease: What Have We Learned? J Infect Dis 2017; 215:167–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Etard JF, Sow MS, Leroy S et al. Multidisciplinary assessment of post-Ebola sequelae in Guinea (Postebogui): an observational cohort study. Lancet Infect Dis 2017; 17:545–552. [DOI] [PubMed] [Google Scholar]
- 15.Clark DV, Kibuuka H, Millard M et al. Long-term sequelae after Ebola virus disease in Bundibugyo, Uganda: a retrospective cohort study. Lancet Infect Dis 2015; 15:905–912. [DOI] [PubMed] [Google Scholar]
- 16.Tiffany A, Vetter P, Mattia J et al. Ebola Virus Disease Complications as Experienced by Survivors in Sierra Leone. Clin Infect Dis 2016; 62:1360–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohammed H, Vandy AO, Stretch R et al. Sequelae and Other Conditions in Ebola Virus Disease Survivors, Sierra Leone, 2015. Emerg Infect Dis 2017; 23:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez LL, De Roo A, Guimard Y et al. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis 1999; 179 Suppl 1:S170–176. [DOI] [PubMed] [Google Scholar]
- 19.Bausch DG, Towner JS, Dowell SF et al. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis 2007; 196 Suppl 2:S142–147. [DOI] [PubMed] [Google Scholar]
- 20.Zeng X, Blancett CD, Koistinen KA et al. Identification and pathological characterization of persistent asymptomatic Ebola virus infection in rhesus monkeys. Nat Microbiol 2017; 2:17113. * [DOI] [PubMed] [Google Scholar]
- 21.Varkey JB, Shantha JG, Crozier I et al. Persistence of Ebola Virus in Ocular Fluid during Convalescence. N Engl J Med 2015; 372:2423–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith JR, Todd S, Ashander LM et al. Retinal Pigment Epithelial Cells are a Potential Reservoir for Ebola Virus in the Human Eye. Transl Vis Sci Technol 2017; 6:12. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shantha JG, Mattia JG, Goba A et al. Ebola Virus Persistence in Ocular Tissues and Fluids (EVICT) Study: Reverse Transcription-Polymerase Chain Reaction and Cataract Surgery Outcomes of Ebola Survivors in Sierra Leone. EBioMedicine 2018; 30:217–224. ** [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobs M, Rodger A, Bell DJ et al. Late Ebola virus relapse causing meningoencephalitis: a case report. Lancet 2016; 388:498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Purpura LJ, Rogers E, Baller A et al. Ebola Virus RNA in Semen from an HIV-Positive Survivor of Ebola. Emerg Infect Dis 2017; 23:714–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diallo B, Sissoko D, Loman NJ et al. Resurgence of Ebola Virus Disease in Guinea Linked to a Survivor With Virus Persistence in Seminal Fluid for More Than 500 Days. Clin Infect Dis 2016; 63:1353–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deen GF, Broutet N, Xu W et al. Ebola RNA Persistence in Semen of Ebola Virus Disease Survivors - Final Report. N Engl J Med 2017; 377:1428–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sissoko D, Keita M, Diallo B et al. Ebola Virus Persistence in Breast Milk After No Reported Illness: A Likely Source of Virus Transmission From Mother to Child. Clin Infect Dis 2017; 64:513–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hereth-Hebert E, Bah MO, Etard JF et al. Ocular Complications in Survivors of the Ebola Outbreak in Guinea. Am J Ophthalmol 2017; 175:114–121. [DOI] [PubMed] [Google Scholar]
- 30.Steptoe PJ, Scott JT, Harding SP et al. Ocular Complications in Survivors of the Ebola Outbreak in Guinea. Am J Ophthalmol 2017; 181:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steptoe PJ, Momorie F, Fornah AD et al. Multimodal Imaging and Spatial Analysis of Ebola Retinal Lesions in 14 Survivors of Ebola Virus Disease. JAMA Ophthalmol 2018; 136:689–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson HW, Amo-Addae M, Kenu E et al. Post-Ebola Syndrome among Ebola Virus Disease Survivors in Montserrado County, Liberia 2016. Biomed Res Int 2018; 2018:1909410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chancellor JR, Padmanabhan SP, Greenough TC et al. Uveitis and Systemic Inflammatory Markers in Convalescent Phase of Ebola Virus Disease. Emerg Infect Dis 2016; 22:295–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shantha JG, Crozier I, Varkey JB et al. Long-term Management of Panuveitis and Iris Heterochromia in an Ebola Survivor. Ophthalmology 2016; 123:2626–2628 e2622. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. Clinical care for survivors of Ebola virus disease. http://www.who.int/csr/resources/publications/ebola/guidance-survivors/en/. November 4 2018.
- 36.Yeh S, Shantha JG, Hayek B et al. Clinical Manifestations and Pathogenesis of Uveitis in Ebola Virus Disease Survivors. Ocul Immunol Inflamm 2018; 26:1128–1134. [DOI] [PubMed] [Google Scholar]
- 37.de St Maurice A, Ervin E, Orone R et al. Care of Ebola Survivors and Factors Associated With Clinical Sequelae-Monrovia, Liberia. Open Forum Infect Dis 2018; 5:ofy239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organization. DIsease outbreaks by year. http://www.who.int/csr/don/archive/year/2018/en/. November 6 2018.
- 39.Woolhouse ME, Brierley L, McCaffery C and Lycett S. Assessing the Epidemic Potential of RNA and DNA Viruses. Emerg Infect Dis 2016; 22:2037–2044. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Groot-Mijnes JDF, Chan ASY, Chee SP and Verjans G. Immunopathology of Virus-Induced Anterior Uveitis. Ocul Immunol Inflamm 2018; 26:338–346. [DOI] [PubMed] [Google Scholar]
- 41.Connors DB, Shantha JG and Yeh S. Emerging causes of viral-associated uveitis. Int Ophthalmol Clin 2015; 55:103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strong JE, Wong G, Jones SE et al. Stimulation of Ebola virus production from persistent infection through activation of the Ras/MAPK pathway. Proc Natl Acad Sci U S A 2008; 105:17982–17987. [DOI] [PMC free article] [PubMed] [Google Scholar]


