SUMMARY:
Progress in Ni/photoredox dual catalysis has enabled the construction of C(sp3)-hybridized centers under extremely mild reaction conditions in the presence of diverse functional groups. These strategies, however, are mainly restricted to the assembly of one C-C or C-heteroatom linkage because of the competitive two-component reactions and facile β-hydride elimination from alkylmetal complexes. Recently, photoinduced nickel-catalyzed 1,2-difunctionalizations of alkenes and alkynes have attracted extensive research efforts as they allow the construction of two sequential chemical bonds from inexpensive starting materials in one pot. Herein, we explore recent advances, state the current challenges, and discuss perspectives on the design of new catalytic systems.
Graphical Abstract

eTOC Blurb
Ni/photoredox dual catalysis has enabled the assembly of challenging bonds under mild reaction conditions. These synthetic processes, however, are typically restricted to the construction of one C-C or C-heteroatom bond. Recently, photoinduced Ni-catalyzed 1,2-difunctionalizations of alkenes and alkynes have emerged as valuable advances for the rapid diversification of simple organic molecules from abundant feedstock in a single synthetic step. Herein, we highlight recent progress, analyze the current challenges, and speculate upon future opportunities in this research arena.
INTRODUCTION
The pursuit of efficient synthetic tools to assemble complex molecular architectures is of longstanding interest in organic synthesis. The recent disclosure of metallaphotoredox catalysis has offered new avenues for unique C-C bond disconnections, especially in the context of biologically relevant targets.1–8 Through single-electron transfer (SET), a photoexcitable catalyst operates under oxidative or reductive quenching paradigms to generate radical intermediates that are subsequently intercepted by nickel. This allows the installation of C(sp3)-hybridized centers under mild reaction conditions (weak bases, room temperature, and visible light) with excellent functional group tolerance.1–8 Owing to the enhanced solubility and specificity in clinical candidates bearing C(sp3) centers, Ni/photoredox dual catalysis has found extensive application in the medicinal chemistry community.9,10 Although progress has been made, the vast majority of these catalytic processes proceed to forge exclusively one C-C or C-heteroatom bond. The development of one-pot conjunctive cross-couplings of C-C π-bonds yields greater atom economy, dramatically increases molecular complexity, eliminates sequential independent transformations, and enhances reaction simplicity.
To advance the field of synthesis, 1,2-difunctionalization has emerged as a powerful tool to construct two chemical bonds across an unsaturated system from readily available feedstocks (Figure 1).11,12,13,14,15 Despite recent advances in this arena, conventional two-electron difunctionalizations rely on the use of organometallic reagents, elevated temperatures, and expensive metal catalysts. A formidable challenge associated with this traditional manifold using transition-metal catalysts is β-hydride elimination from alkylmetal intermediates, particularly for unactivated alkenes, limiting the use of C(sp3)-hybridized reagents.16 In this context, conjunctive radical-based cross-couplings offer a new solution to these challenges. Seminal research from the groups of Baran,17 Zhang,18 Nevado,19,20 Giri,21 Chu,22 and Wang,23 among others,24 has showcased the potential of these processes. However, these transformations in large part operate using stoichiometric metal reductants, with associated characteristics that would benefit from complementary reactivity modes. Therefore, the continued development of photoinduced difunctionalizations represents a fruitful area for exploration.
Figure 1.

Dual photoredox and nickel-catalyzed 1,2-difunctionalizations.
In this Perspective, we examine recent advances in 1,2-difunctionalizations of C-C π-systems based on Ni/photoredox dual catalysis. Although visible-light-promoted, photocatalyst-free difunctionalizations with nickel have been reported,25 they are beyond the scope of this work and will not be discussed herein. Overall, the goal is to demonstrate the power of this catalytic approach while highlighting the current challenges. We also speculate upon other opportunities that might be feasible using similar photocatalytic strategies.
Ni-catalyzed multicomponent 1,2-carbodifunctionalization, 1,2-carbosulfonylation, and 1,2-carbosilylation of alkenes
In 2014, our group demonstrated the merger of photoredox and Ni catalysis to forge C(sp3)-C(sp2) bonds.1 Concurrent with our initial report that alkyl radicals stemming from organotrifluoroborates are viable partners in Ni-catalyzed cross-couplings, the MacMillan and Doyle groups, under a similar paradigm, disclosed a photoinduced decarboxylative cross-coupling and a direct C(sp3)-H arylation protocol of dimethylanilines.2 Over the past decade, we have showcased the utility of numerous families of radical precursors including silicates,26 dihydropyridines,27 alkylpyridinium salts,28 thiols,29 and sulfinates,30 among others.31 A more recent interest in the group has been the advancement of multicomponent reactions (MCRs) to deliver novel structural motifs with an accompanying increase in molecular complexity.
An attractive subset of multicomponent transformations are vicinal carbodifunctionalizations, enabling the installation of two carbon-based entities across C-C π-bonds in a single step.11 Recent strategies for alkene diarylation or alkylarylation have been studied using nickel- or palladium catalysis.32,33 Although these advancements presented milestones in their own right, these processes typically rely on forcing reactions conditions and remain in large part underexplored in the context of diverse C(sp3)-hybridized nucleophiles. This is in part because of the competing two-component coupling reactions that occur prior to engaging the desired unsaturated bond, facile β-hydride elimination and undesired dimerization. Efforts to bypass these complications in alkene difunctionalizations have benefited from the use of perfluoroalkyl radicals18,23,34 or electronically biased alkenes,35 limiting the widespread applicability of these conjunctive couplings.
To address these challenges, we recently reported a three-component carbodifunctionalization of vinyl boronates and other electronically deficient alkenes accommodating a wide array of aryl halides and alkyltrifluoroborates (Figure 2).36 Specifically, we envisioned a mechanistic scenario in which photoexcitation of the iridium photocatalyst {E1/2[Ir*III/IrII] = +1.32 V vs SCE for [Ir{dF(CF3)ppy}2(bpy)]PF6}37 engages the organotrifluoroborate (E1/2 = +1.26 V vs SCE for potassium tert-butyl trifluoroborate)38 in oxidative fragmentation to deliver an alkyl radical. This intermediate then undergoes a Giese-type addition, generating an α-boryl radical species that is funneled into the nickel cycle to forge the corresponding C(sp3)-C(sp2) bond through a single electron transmetalation/cross-coupling cycle. Strikingly, secondary organotrifluoroborates were viable substrates under this reaction manifold despite a competitive two-component arylation pathway. Unfortunately, efforts to incorporate stabilized radicals such as benzyl- or α-amino derivatives proved unsuccessful. This presumably stems from the competitive formation of direct C(sp2)-C(sp3) bond linkages prior to addition of radical species to the desired alkene. Notably, this process is highly selective for electron-deficient alkenes and tolerates a variety of terpenes and steroidal moieties.
Figure 2.

Three-component carbodifunctionalization of vinyl boronates.
Given the versatility of organoboron reagents in fundamental processes such as Suzuki-Miyaura and Chan-Lam cross-couplings,39 extensive research has been devoted toward the synthesis of these building blocks. Traditionally, boryl groups are introduced through transition metal-catalyzed transformations of halides40 or hydroboration of unsaturated bonds.41 The advancement of novel strategies to access this functional handle from readily-obtained organic feedstocks would expedite the discovery of drug candidates in pharmaceutical settings. Indeed, shortly after our report, the Martin group independently disclosed an elegant 1,2-carbodifunctionalization of vinyl boronates using commercially available organic halides with excellent chemo- and regioselectivity.42 Through careful optimization, the authors were able to identify a suitable terminal reductant (TMEDA) and an organic photosensitizer (4CzIPN) to overcome a precedented two-component reductive coupling between the two electrophilic counterparts.31,43 It is worth noting the mild nature of the amine reductant is key to preserving functional group tolerance. To probe the mechanism of this reaction, reductive quenching studies were conducted to support a single-electron-transfer event from the reduced state of the photocatalyst to the tertiary alkyl bromide. Interestingly, no Heck-type product was observed under the reaction conditions. Notably, the scope of the alkene acceptor was extended to olefins bearing phthalimides, esters, and phosphonates. Simultaneously, Aggarwal reported a similar strategy from carboxylic acid feedstocks.44 The electrophile coupling partner, however, was restricted to aryl iodides because of increased formation of Giese adducts when the corresponding bromide was utilized. Numerous primary, secondary, and tertiary α-amino acids were viable substrates. Of note, N-methylation was utilized to obtain higher yields in substrates where the NH group proved problematic. Finally, a wide range of α-oxy acids afforded the desired product in good yields.
While working on our manuscript, the Chu and Nevado groups independently published two additional carbodifunctionalization protocols using alkyl oxalates and silicates, respectively.45,46 In the Chu report, a wide array of unactivated, electron-deficient and electron-rich olefins, partnered with aryl halides and oxalates, reacted efficiently to deliver the desired product despite their reluctance to capture nucleophilic radicals. Other acceptors, including 1,1-disubstituted and 1,2-disubstituted alkenes, failed to react under the reaction conditions, presumably because of their sterically hindered nature. In a similar manner, the Nevado group described the use of secondary or tertiary alkylsilicates, aryl iodides, and a variety of alkenes (including acrylates, acrylonitriles, vinylboronic esters, and allylic acetates) to forge two C-C bonds simultaneously. In the same report, Nevado and co-workers studied the feasibility of 1,2-carbosulfonylation via single-electron oxidation of readily available sodium arenesulfinates.46 Under the optimized conditions, sulfinates with electronically distinct profiles were successfully engaged in this multicomponent reaction. The scope of the electrophilic partner was extended to both aryl iodides and -bromides, expanding the synthetic utility of this catalytic process.
Because of the high demand for ethylene derivatives in industrial settings, methods for the incorporation of this simple alkene in fine chemicals are highly desired.47 Commonly, catalytic strategies for ethylene derivatization are limited to monofunctionalization via hydroacylation, hydrovinylation, Wacker oxidation, and Heck reactions, among others.48 This is typically attributed to challenges stemming from β-hydride elimination, oligomerization, and polymerization.49 In 2019, Wu and co-workers demonstrated a dual photoredox and nickel-catalyzed three-component diarylation of ethylene feedstock using electronically diverse aryl halides as electrophiles.50 Utilizing a stop-flow micro-tubing reactor, the authors were able to optimize the photo-mediated difunctionalization of this flammable gas in a practical fashion. Mediating the redox potentials of various photocatalysts, selective formation of 1,2-diarylethanes, 1,4-diarylbutanes, or 2,3-diarylbutanes was observed. Finally, DFT calculations, cyclic voltammetry measurements, and radical trapping experiments were conducted to deduce the origin of this selectivity.
In addition to 1,2-carbodifunctionalizations, Hu and co-workers recently disclosed an arylsilylation protocol of electron-deficient alkenes under metallaphotoredox catalysis.51 Utilizing TMS3SiH as the silane source, the group demonstrated that a variety of electronically distinct aryl- and heteroaryl bromides were viable substrates. Mechanistically, the authors propose the intermediacy of a photogenerated bromine radical that abstracts a hydrogen atom from the silane to give a silyl radical. Addition of this species to an acrylate generates a nucleophilic β-silyl alkyl radical that gets funneled into a Ni-catalyzed cross-coupling cycle.
Ni-catalyzed intramolecular 1,2-carbodifunctionalization and 1,2-amidoarylation of alkenes
Recently, Overman and co-workers focused their attention on the synthesis of functionalized γ-butyrolactones as privileged structural scaffolds prevalent in bioactive molecules and pharmaceutical drugs.52,53 The authors exploited an elegant alkoxycarbonyl radical cyclization/cross-coupling cascade with aryl- and vinyl iodides.53 Harnessing an oxidizing Ir-based photocatalyst under visible light irradiation, a tertiary homoallylic oxalate participates in SET to deliver an alkoxycarbonyl radical that rapidly undergoes a 5-exo cyclization. The resulting primary carbon-centered radical is then intercepted by Ni to furnish a spirocyclic system. Of note, the choice of counterion of the oxalate radical precursor proved important, with cesium offering the optimal reactivity. Importantly, this coupling tolerates sensitive functional groups, including unprotected alcohols and enolizable ketones.
In another report, Wang and co-workers developed an asymmetric carbodifunctionalization of carbamoyl chloride-tethered alkenes and abundant aldehydes through a photoinduced HAT process (Figure 3).54 Mechanistically, tetrabutylammonium decatungstate (TBADT) is irradiated at 390 nm to form a potent excited state *[W]4−• that engages the aldehyde in hydrogen atom abstraction (HAT). The active Ni(0) catalyst is obtained through two sequential single-electron reduction processes from [W]6−2H+, a species stemming from a disproportionation event during the course of the reaction. Addition of the acyl radical followed by oxidative addition of the carbamoyl chloride gives rise to a high-valent Ni(III) acyl complex. Notably, an enantio-determining migratory insertion with the tethered olefin takes place, followed by reductive elimination, to deliver the oxindole with the desired quaternary stereogenic center. The feasibility of a facile oxidative addition of the carbamoyl chloride to a low-valent nickel species prior to combination of the acyl radical is likely and cannot be ruled out without compelling mechanistic evidence. To achieve high levels of enantiocontrol, a phosphinooxazoline (PHOX) ligand scaffold was identified. Both aliphatic and (hetero)aromatic aldehydes could be employed with higher loading of TBADT under longer irradiation time.
Figure 3.
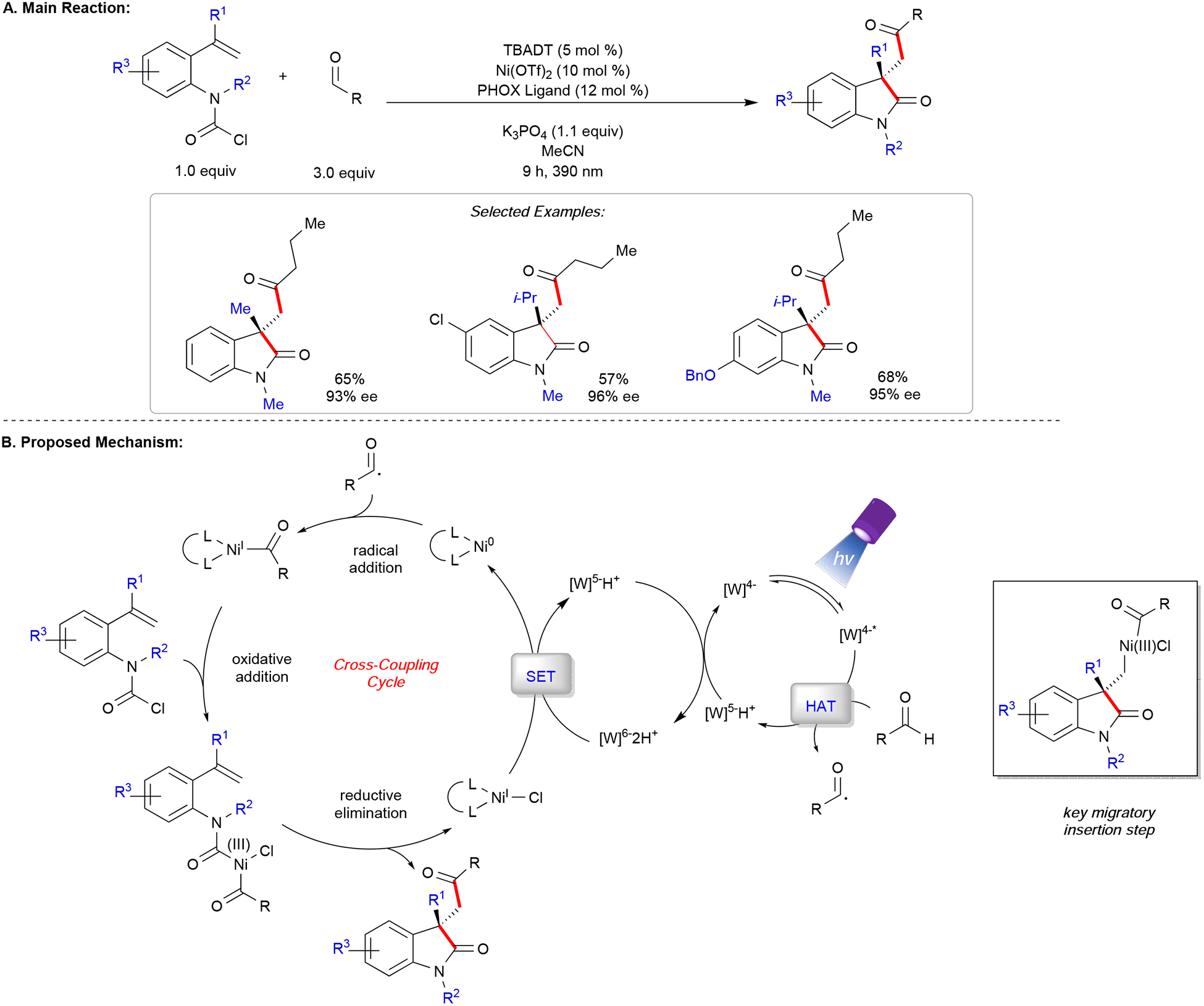
Asymmetric acyl-carbamoylation of alkenes.
In addition to 1,2-carbodifunctionalizations, the Jamison group reported a regioselective annulation reaction of 2-iodoaniline derivatives and terminal alkenes under Ni/photoredox dual catalysis.55 In this protocol, 3-substituted indoline derivatives are synthesized in good yields with excellent functional group tolerance. Mechanistic studies suggest the intermediacy of a Ni(III) complex is necessary to facilitate a direct C(sp3)-N reductive elimination. The resulting Ni(0) active species is obtained via a single-electron-reduction event. Later, our group disclosed a cascade amidoarylation of unactivated olefins in 2019 (Figure 4).56 Inspired by an earlier report from the Knowles laboratory on the generation of amidyl radicals through concerted proton-coupled electron transfer (PCET) under photoredox catalysis,57 we envisioned that a rapid 5-exo-trig cyclization (k ~ 105 s−1) across a pendent alkene would generate a primary nucleophilic radical that gets funneled into a nickel-catalyzed cross-coupling cycle. In this manner, the synthesis of privileged N-heterocyclic scaffolds can be accomplished from alkenyl amides and (hetero)aryl halides. A variety of electron-neutral and electron-rich anilines, as well as carbamates and ureas, are efficiently incorporated. To provide evidence for an operating concerted PCET mechanism, experiments including Stern-Volmer, NMR titration, and cyclic voltammetry were conducted. Notably, relative cyclization rates for carbamates were reported for the first time, shedding light on new avenues in chemical synthesis.
Figure 4.
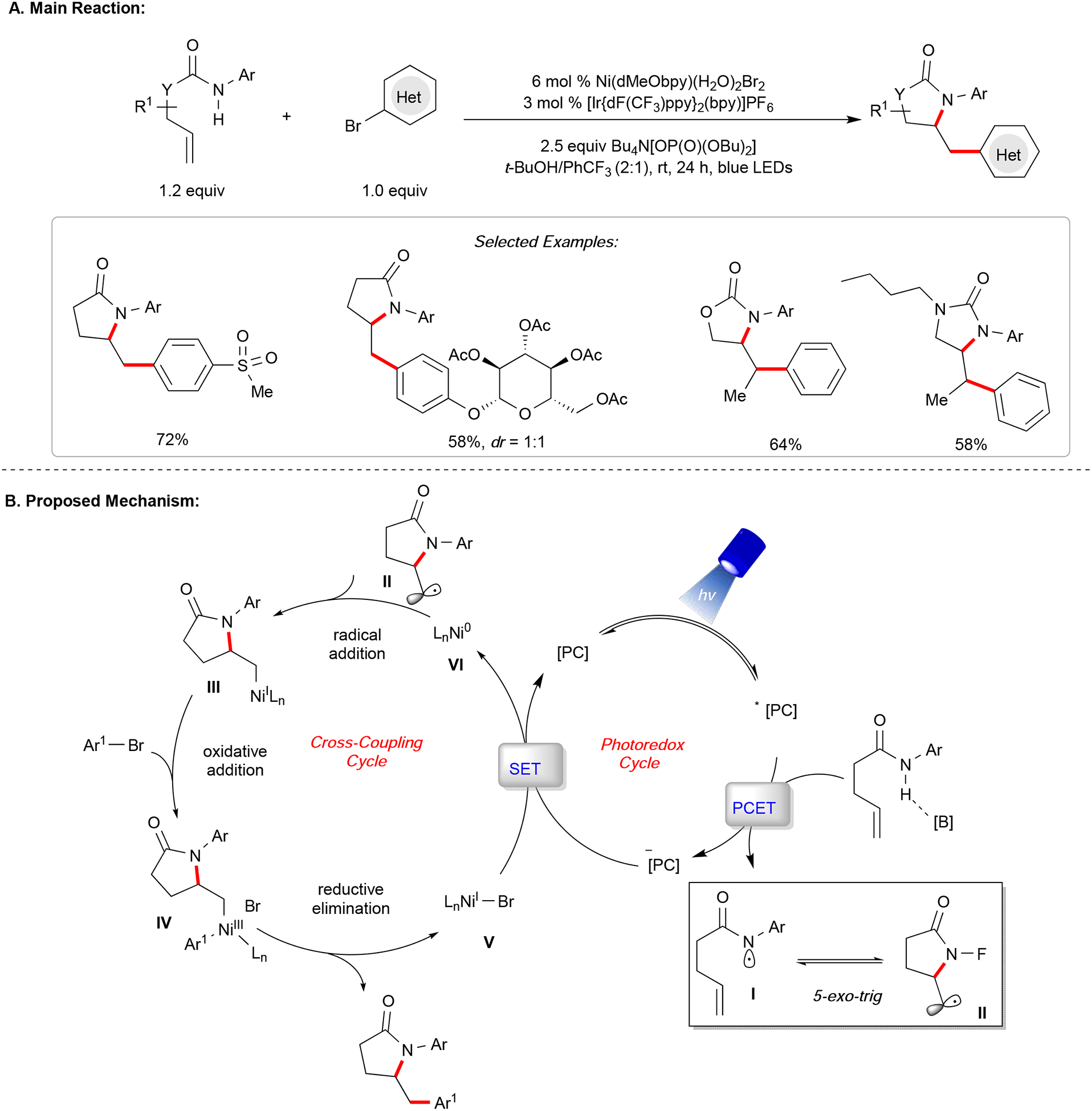
Amidoarylation of unactivated olefins.
Ni-catalyzed multicomponent 1,2-carbodifunctionalization and 1,2-carbosulfonylation of alkynes
Substituted alkenes are prevalent in nature and serve as key structural motifs in pharmaceuticals, agrochemicals, and medicinal targets. Although transition-metal-catalyzed functionalization of alkynes has been extensively studied, the majority of these transformations rely on the formation of syn-substituted alkenylmetal intermediates governed by strict stereoelectronic requirements.58 Other reactions that yield olefins proceed via radical addition and subsequent cross-coupling in favor of the anti-addition product.59 The geometry of the corresponding C-C double bond is dictated by steric elements of the highly reactive alkenyl radical.60 Although numerous cascade processes have been devoted to address the challenges associated with regio- and steroselectivity outcomes, carbodifunctionaliztion of alkynes based on metallaphotoredox catalysis had not been realized until recently reported by Chu and co-workers in 2018 (Figure 5).61 Using visible light irradiation, this conjunctive cross coupling exploits a contra-thermodynamic E → Z isomerization event to give rise to stereodefined trisubstituted alkenes under mild reaction conditions. Specifically, the group envisioned an alkylarylation protocol employing aryl bromides, terminal alkynes, and tertiary oxalates, readily prepared from commercially available alcohols, as alkyl radical feedstocks. The authors propose that a single-electron oxidation of the tertiary oxalate (E1/2 = +1.28 V vs SCE in MeCN for tert-BuOCOCO2Cs)61 by the photoexcited state of [Ir{dF(CF3)ppy}2(dtbbpy)]PF6 (E1/2[Ir*III/IrII] = + 1.21 V vs SCE in MeCN)38 would deliver an alkyl radical that undergoes a regioselective addition to the alkyne, giving rise to an alkenyl radical. This is followed by anti-addition to generate the corresponding (E)-alkenyl-Ni(I) species. Oxidative addition of the aryl bromide results in a high-valent Ni(III) complex. Reductive elimination and subsequent photochemical isomerization furnishes the (Z)-alkene. Finally, single-electron-transfer (SET) from the ground state of the photocatalyst (E1/2[IrIII/IrII] = −1.37 V vs SCE in MeCN)37 to Ni(I) (E1/2[NiII/Ni°] = −1.2 V vs SCE in DMF)1 concurrently terminates both catalytic cycles.
Figure 5.

Three-component carbodifunctionalization of alkynes.
Another recent example was disclosed by Rueping, who demonstrated the synthesis of trisubstituted olefins with modest syn-selectivity from aliphatic carboxylic acids under blue light irradiation.62 This protocol, however, was restricted to Boc- and Cbz-protected secondary amino acids, with a single example of a tertiary α-amino acid. A variety of aryl bromides derived from pharmaceutically relevant compounds were coupled efficiently under the reaction conditions. An excess of the alkyl radical precursor is not required to achieve synthetically useful yields in this process, despite the ability of radicals derived from secondary α-amino acids via oxidative decarboxylation to undergo direct C(sp2)-C(sp3) arylation in metallaphotoredox catalysis.2
In addition to constructing two carbon-based moieties across an unsaturated system, Rueping and co-workers further described an elegant aryl-sulfonylation of alkynes using sodium sulfinates and aryl halides with excellent chemo-, regio- and stereoselectivity.63 Mechanistically, the authors propose a key 1,2-migratory insertion of the alkyne with a Ni(I) complex followed by a Ni-assisted anti/syn isomerization to deliver the anti-addition product. Remarkably, the choice of photocatalyst proved essential to promote a photoinduced isomerization event of the newly formed alkene product. To obtain better Z/E selectivity, the authors selected photocatalysts with triplet state energies that exceed that of the excited state of the anti-addition product but are less efficient than that of the isomeric product. These protocols present new opportunities for the rapid diversification of abundant chemical feedstock under mild conditions.
CONCLUSION AND OUTLOOK
In the last decade, Ni/photoredox dual catalysis has emerged as a valuable advance for forging unique C(sp3)-C(sp2) linkages under remarkably mild conditions through a single-electron-transfer (SET) mechanism. In contrast to traditional two-electron transmetalation modes, this strategy does not require forcing conditions or reactive pyrophoric reagents. More recently, photoinduced difunctionalizations of unsaturated bonds have attracted significant attention as they allow the assembly of two consecutive chemical entities in a single transformation. However, numerous challenges persist in this research arena, with many opportunities for further development. These include the following: (1) To date, the scope of the radical acceptor is largely limited to activated terminal alkenes. This is because of sluggish addition rates and increased β-hydride elimination associated with the more highly substituted, unactivated olefins. To exploit the power of 1,2-difunctionalizations to a greater extent, the merger of highly substituted C-C π-systems, allenes, and other allylic derivatives would be advantageous. (2) With the emergence of photocatalysts harboring distinct redox potentials and triplet state energies,64 additional studies are needed to explore the implementation of novel radical precursors in difunctionalizations, especially heteroatom-centered species. (3) Currently, there are few examples of enantiocontrolled Ni/photoredox dual catalytic systems, particularly in the context of difunctionalizations. The development and application of novel ligand scaffolds through high-throughput screening efforts, for example, to achieve absolute stereochemical control of radical processes would push the boundaries of this field. Further advancement of 1,2-difunctionalizations of alkynes with defined stereochemical outcomes, particularly in the presence of additional unsaturated systems, is highly desired. (4) To enhance access to new chemical space, the formation of two C(sp3)-C(sp3) linkages would be beneficial. This would require a careful design of reaction parameters to achieve chemo- and regioselectivity. Specifically, identifying the appropriate nickel precatalyst-to-photocatalyst ratios is essential to the formation of reactive radical species in a regulated fashion. (5) Finally, the incorporation of emerging base metals including cobalt,65 chromium,66 and iron67 under photoredox catalysis presents exciting opportunities for novel reactivity trends that should be carefully examined. With the adequate chemical tools devoted to address these synthetic gaps, 1,2-difunctionalizations can be fully exploited in the context of industrial and medicinal applications. One such opportunity is the synthesis of biomolecules with a high density of pendent functional groups such as those in DNA-encoded library (DEL) synthesis.31,68 This innovative technology is used in pharmaceutical settings to identify drug-like candidates with high affinity binding to therapeutic targets through sampling uncharted chemical space (>106 to 1012 library members).69 In this vein, the incorporation of multicomponent reactions under metallaphotoredox catalysis in DELs would allow a higher content of C(sp3) carbons and expedite drug discovery. We anticipate this mode of catalysis will soon serve as a pivotal platform for accessing complex molecular targets from commodity chemicals.
Challenges and opportunities.
Nickel/photoredox dual catalysis offers a new paradigm to construct challenging linkages under mild reaction conditions with high functional group tolerance
Photoinduced Ni-catalyzed 1,2-difunctionalizations of alkenes and alkynes allow for rapid access to molecular complexity and diversity in one synthetic step
Further development of stereocontrolled Ni/photoredox dual catalytic systems would grant access to uncharted chemical space. This requires a deeper mechanistic understanding with respect to the origin of stereoinduction in dual catalysis.
ACKNOWLEDGMENTS
The authors are grateful for the financial support provided by NIGMS (R35 GM 131680 to G.M.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Tellis JC, Primer DN, and Molander GA (2014). Single-electron transmetalation in organoboron cross-coupling by photoredox/ nickel dual catalysis. Science 345, 433–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuo Z, Ahneman DT, Chu L, Terrett JA, Doyle AG, and MacMillan DWC (2014). Merging photoredox with nickel catalysis: coupling of a-carboxyl sp3-carbons with aryl halides. Science 345, 437–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Primer DN, Karakaya I, Tellis JC, and Molander GA (2015). Single-electron transmetalation: an enabling technology for secondary alkylboron cross-coupling. J. Am. Chem. Soc 137, 2195–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shields BJ, and Doyle AG (2016). Direct C(sp3)-H cross coupling enabled by catalytic generation of chlorine radicals. J. Am. Chem. Soc 138, 12719–12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heitz DR, Tellis JC, and Molander GA (2016). Photochemical nickel-catalyzed C-H arylation: synthetic scope and mechanistic investigations. J. Am. Chem. Soc 138, 12715–12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tellis JC, Kelly CB, Primer DN, Jouffroy M, Patel NR, and Molander GA (2016). Single-electron transmetalation via photoredox/nickel dual catalysis: unlocking a new paradigm for sp3-sp2 cross-coupling. Acc. Chem. Res 49, 1429–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsui JK, Lang SB, Heitz DR, and Molander GA (2017). Photoredox-mediated routes to radicals: the value of catalytic radical generation in synthetic methods development. ACS. Catal 7, 2563–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milligan JA, Phelan JP, Badir SO, and Molander GA (2019). Alkyl Carbon-Carbon Bond Formation by Nickel/Photoredox Cross-Coupling. Angew. Chem. Int. Ed 58, 6152–6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown DG, and Bostrom J (2016). Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? J. Med. Chem 59, 4443–4458. [DOI] [PubMed] [Google Scholar]
- 10.Schneider N, Lowe DM, Sayle RA, Tarselli MA, and Landrum GA (2016). Big data from pharmaceutical patents: a computational analysis of medicinal chemists’ bread and butter. J. Med. Chem 59, 4385–4402. [DOI] [PubMed] [Google Scholar]
- 11.Derosa J, Apolinar O, Kang T, and Engle KM (2020). Recent developments in nickel-catalyzed intermolecular dicarbofunctionalization of alkenes. Chem. Sci Accepted Manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tu H-Y, Zhu S, Qing F-L, and Chu L (2020). Recent advances in nickel-catalyzed three-component difunctionalization of unactivated alkenes. Synthesis. 52, A–K. [Google Scholar]
- 13.For a selected review on asymmetric 1,2-difunctionalizations using copper catalysis, please see:; Li Z-L, Fang G-C, Gu Q-S, and Liu X-Y (2020). Recent advances in copper-catalysed radical-involved asymmetric 1,2-difunctionalization of alkenes. Chem. Soc. Rev 49, 32–48. [DOI] [PubMed] [Google Scholar]
- 14.For a recent review on cascade reactions, please see:; Zhu C, Yue H, Chu L, Rueping M (2020). Recent advances in photoredox and nickel dual-catalyzed cascade reactions: pushing the boundaries of complexity. Chem. Sci, 11, 4051–4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.For a selected review on 1,2-difunctionalizations using palladium catalysis, please see:; Yin G, Mu X, and Liu G (2016). Palladium(II)-catalyzed oxidative difunctionalization of alkenes: bond forming at a high-valent palladium center. Acc. Chem. Res 49, 2413–2423. [DOI] [PubMed] [Google Scholar]
- 16.Tasker SZ, Standley EA, and Jamison TF (2014). Recent advances in homogeneous nickel catalysis. Nature 509, 299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin T, Cornella J, Li C, Malins LR, Edwards JT, Kawamura S, Maxwell BD, Eastgate MD, and Baran PS (2016). A general alkyl-alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents. Science 352, 801–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu J-W, Min Q-Q, Yu L-C, and Zhang X (2016). Tandem difluoroalkylation-arylation of enamides catalyzed by nickel. Angew. Chem. Int. Ed 55, 12270–12274. [DOI] [PubMed] [Google Scholar]
- 19.García-Domínguez A, Li Z, and Nevado C (2017). Nickel-catalyzed reductive dicarbofunctionalization of alkenes. J. Am. Chem. Soc 139, 6835–6838. [DOI] [PubMed] [Google Scholar]
- 20.Shu W, García-Domínguez A, Quirós MT, Mondal R, Cárdenas DJ, and Nevado C (2019). Ni-catalyzed reductive dicarbofunctionalization of nonactivated alkenes: scope and mechanistic insights. J. Am. Chem. Soc 141, 13812–13821. [DOI] [PubMed] [Google Scholar]
- 21.KC S, Dhungana RK, Shrestha B, Thapa S, Khanal N, Basnet P, Lebrun RW, and Giri R (2018). Ni-catalyzed regioselective alkylarylation of vinylarenes via C(sp3)-C(sp3)/C(sp3)-C(sp2) bond formation and mechanistic studies. J. Am. Chem. Soc 140, 9801–9805. [DOI] [PubMed] [Google Scholar]
- 22.Zhao X, Tu H-Y, Guo L, Zhu S, Qing F-L, and Chu L (2018). Intermolecular selective carboacylation of alkenes via nickel-catalyzed reductive radical relay. Nat. Commun 9, 3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang K-F, Bian K-J, Li C, Sheng J, Li Y, and Wang X-S (2019). Nickel-catalyzed carbofluoroalkylation of 1,3-enynes to access structurally diverse fluoroalkylated allenes. Angew. Chem. Int. Ed 58, 5069–5074. [DOI] [PubMed] [Google Scholar]
- 24.For a selected example on 1,2-difunctionalizations involving radical intermediates, please see:; Anthony D, Lin Q, Baudet J, and Diao T (2019). Nickel-catalyzed asymmetric reductive diarylation of vinylarenes. Angew. Chem. Int. Ed 58, 3198–3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao X, Li B, and Xia W (2020). Visible-light-promoted photocatalyst-free hydroacylation and diacylation of alkenes tuned by NiCl2.DME. Org. Lett 22, 1056–1061. [DOI] [PubMed] [Google Scholar]
- 26.Jouffroy M, Primer DN, and Molander GA (2016). Base-free photoredox/nickel dual-catalytic cross-coupling of ammonium alkylsilicates. J. Am. Chem. Soc 138, 475–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Badir SO, Dumoulin A, Matsui JK, and Molander GA (2018). Synthesis of reversed C-acyl glycosides through Ni/photoredox dual catalysis. Angew. Chem. Int. Ed 57, 6610–6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yi J, Badir SO, Kammer LM, Ribagorda M, and Molander GA (2019). Deaminative reductive arylation enabled by nickel/photoredox dual catalysis. Org. Lett 21, 3346–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jouffroy M, Kelly CB, and Molander GA (2016). Thioetherification via photoredox/nickel dual catalysis. Org. Lett 18, 876–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cabrera-Afonso MJ, Lu Z-P, Kelly CB, Lang SB, Dykstra R, Gutierrez O, and Molander GA (2018). Engaging sulfinate salts via Ni/photoredox dual catalysis enables facile Csp2-SO2R coupling. Chem. Sci 9, 3186–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.For another example utilizing alkyl bromides and alpha-silylamines as radical precursors, please see:; Badir SO, Sim J, Billings K, Csakai A, Zhang X, Dong W, and Molander GA (2020). Multifunctional building blocks compatible with photoredox-mediated alkylation for DNA-encoded library synthesis. Org. Lett 22, 1046–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.For a selected example on 1,2-diarylation, please see:; Derosa J, Kleinmans R, Tran VT, Karunananda MK, Wisniewski SR, Eastgate MD, and Engle KM (2018). Nickel-catalyzed 1,2-diarylation of simple alkenyl amides. J. Am. Chem. Soc 140, 17878–17883. [DOI] [PubMed] [Google Scholar]
- 33.For a selected example of 1,2-alkylarylation, please see:; Wu X, Lin H-C, Li M-L, Li L-L, Han Z-Y, and Gong L-Z (2015). Enantioselective 1,2-difunctionalization of dienes enabled by chiral palladium complex-catalyzed cascade arylation/allylic alkylation reaction. J. Am. Chem. Soc 137, 13476–13479. [DOI] [PubMed] [Google Scholar]
- 34.For a selected example using a radical-polar cross-over reaction, please see:; Carboni A, Dagousset G, Magnier E, and Masson G (2014). One pot and selective intermolecular aryl- and heteroaryl-trifluoromethylation of alkenes by photoredox catalysis. Chem. Commun 50, 14197–14200. [DOI] [PubMed] [Google Scholar]
- 35.For a selected example using copper catalysis, please see:; Wang F, Wang D, Mu X, Chen P, and Liu G (2014). Copper-catalyzed intermolecular trifluoromethylarylation of alkenes: mutual activation of arylboronic acid and CF3+ reagent. J. Am. Chem. Soc 136, 10202–10205. [DOI] [PubMed] [Google Scholar]
- 36.Campbell MW, Compton JS, Kelly CB, and Molander GA (2019). Three-component olefin dicarbofunctionalization enabled by nickel/photoredox dual catalysis. J. Am. Chem. Soc 141, 20069–20078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelly CB, Patel NR, Primer DN, Jouffroy M, Tellis JC, and Molander GA (2017). Preparation of visible-light-activated metal complexes and their use in photoredox/nickel dual catalysis. Nat. Protoc 12, 472–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Primer DN, and Molander GA (2017). Enabling the cross-coupling of tertiary organoboron nucleophiles through radical-mediated alkyl transfer. J. Am. Chem. Soc 139, 9847–9850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fyfe JWB, and Watson AJB (2017). Recent developments in organoboron chemistry: old dogs, new tricks. Chem 3, 31–55. [Google Scholar]
- 40.For a selected example on transition-metal-catalyzed borylation of alkyl halides, please see:; Atack TC, and Cook SP (2016). Manganese-catalyzed borylation of unactivated alkyl chlorides. J. Am. Chem. Soc 138, 6139–6142. [DOI] [PubMed] [Google Scholar]
- 41.For a selected review on transition-metal promoted hydroboration of olefins, please see:; Burgess K, and Ohlmeyer MJ (1991). Transition-metal-promoted hydroborations of alkenes, emerging methodology for organic transformations. Chem. Rev 91, 1179–1191. [Google Scholar]
- 42.Sun S-Z, Duan Y, Mega RS, Somerville RJ, and Martin R (2020). Site-selective 1,2-dicarbofunctionalization of vinyl boronates through dual catalysis. Angew. Chem. Int. Ed 59, 4370–4374. [DOI] [PubMed] [Google Scholar]
- 43.Duan Z, Li W, and Lei A (2016). Nickel-catalyzed reductive cross-coupling of aryl bromides with alkyl bromides: Et3N as the terminal reductant. Org. Lett 18, 4012–4015. [DOI] [PubMed] [Google Scholar]
- 44.Mega RS, Duong VK, Noble A, and Aggarwal VK (2020). Decarboxylative conjunctive cross-coupling of vinyl boronic esters using metallaphotoredox catalysis. Angew. Chem. Int. Ed 59, 4375–4379. [DOI] [PubMed] [Google Scholar]
- 45.Guo L, Tu H-Y, Zhu SQ, and Chu L (2019). Selective, intermolecular alkylarylation of alkenes via photoredox/nickel dual catalysis. Org. Lett 21, 4771–4776. [DOI] [PubMed] [Google Scholar]
- 46.García-Domínguez A, Mondal R, and Nevado C (2019). Dual photoredox/nickel-catalyzed three-component carbofunctionalization of alkenes. Angew. Chem. Int. Ed 58, 12286–12290. [DOI] [PubMed] [Google Scholar]
- 47.Independent Chemical Information Service (2007). Ethylene uses and market data. https://www.icis.com/explore/resources/news/2007/11/05/9075777/ethylene-uses-and-market-data.
- 48.Saini V, Stokes BJ, and Sigman MS (2013). Transition-metal-catalyzed laboratory-scale carbon-carbon bond-forming reactions of ethylene. Angew. Chem. Int. Ed 52, 11206–11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mirbach MF, Mirbach MJ, and Saus A (1982). High-pressure photochemistry and UV spectroscopy in gas-liquid systems. Chem. Rev 82, 59–76. [Google Scholar]
- 50.Li J, Luo Y, Cheo HW, Lan Y, and Wu J (2019). Photoredox-catalysis-modulated, nickel-catalyzed divergent difunctionalization of ethylene. Chem 5, 192–203. [Google Scholar]
- 51.Zhang Z, and Hu X (2020). Arylsilylation of electron-deficient alkenes via cooperative photoredox and nickel catalysis. ACS Catal. 10, 777–7882. [Google Scholar]
- 52.Mao B, Fañanás-Mastral M, and Feringa BL (2017). Catalytic asymmetric synthesis of butenolides and butyrolactones. Chem Rev. 117, 10502–10566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weires NA, Slutskyy Y, and Overman LE (2019). Facile preparation of spirolactones by an alkoxycarbonyl radical cyclization-cross-coupling cascade. Angew. Chem. Int. Ed 58, 8561–8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fan P, Lan Y, Zhang C, and Wang C (2020). Nickel/photo-cocatalyzed asymmetric acyl-carbamoylation of alkenes. J. Am. Chem. Soc 142, 2180–2186. [DOI] [PubMed] [Google Scholar]
- 55.Tasker SZ, and Jamison TF (2015). Highly regioselective indoline synthesis under nickel/photoredox dual catalysis. J. Am. Chem. Soc 137, 9531–9534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang S, Gutiérrez-Bonet Á, and Molander GA (2019). Merging photoredox PCET with Ni-catalyzed cross-coupling: cascade amidoarylation of unactivated olefins. Chem 5, 339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choi GJ, and Knowles RR (2015). Catalytic alkene carboaminations enabled by oxidative proton-coupled electron transfer. J. Am. Chem. Soc 137, 9226–9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.For a selected example, please see:; Chinchilla R, and Nájera C (2014). Chemicals from alkynes with palladium catalysts. Chem. Rev 114, 1783–1826. [DOI] [PubMed] [Google Scholar]
- 59.For a selected example with palladium catalysis, please see:; Domański S, and Chaładaj W (2016). A broadly applicable method for Pd-catalyzed carboperfluoro-alkylation of terminal and internal alkynes: a convenient route to tri- and tetrasubstituted olefins. ACS Catal. 6, 3452–3456. [Google Scholar]
- 60.For a seclected example with copper catalysis, please see:; Xu T, and Hu X (2015). Copper-catalyzed 1,2-addition of α-carbonyl iodides to alkynes. Angew. Chem. Int. Ed 54, 1307–1311. [DOI] [PubMed] [Google Scholar]
- 61.Guo L, Song F, Zhu S, Li H, and Chu L (2018). syn-Selective alkylarylation of terminal alkynes via the combination of photoredox and nickel catalysis. Nat. Comm 9, 4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yue H, Zhu C, Kancherla R, Liu F, and Rueping M (2020). Regioselective hydroalkylation and arylalkylation of alkynes by photoredox/nickel dual catalysis: application and mechanism. Angew. Chem. Int. Ed 59, 2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu C, Yue H, Maity B, Atodiresei I, Cavallo L, and Rueping M (2019). A multicomponent synthesis of stereodefined olefins via nickel catalysis and single electron/triplet energy transfer. Nat. Catal 2, 678–687. [Google Scholar]
- 64.For a selected example, please see:; Kim T, McCarver SJ, Lee C, and MacMillan DWC (2018). Sulfonamidation of aryl and heteroaryl halides through photosensitized nickel catalysis. Angew. Chem. Int. Ed 57, 3488–3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.For a selected example, please see:; Thullen SM, and Rovis T (2017). A mild hydroaminoalkylation of conjugated dienes using a unified cobalt and photoredox catalytic system. J. Am. Chem. Soc 139, 15504–15508. [DOI] [PubMed] [Google Scholar]
- 66.For a selected example, please see:; Schwarz JL, Kleinmans R, Paulisch TO, and Glorius F (2020). 1,2-Amino alcohols via Cr/photoredox dual-catalyzed addition of alpha-amino carbanion equivalents to carbonyls. J. Am. Chem. Soc 142, 2168–2174. [DOI] [PubMed] [Google Scholar]
- 67.For a selected example, please see:; Ouyang X-H, Li Y, Song R-J, Hu M, Luo S, and Li J-H (2019). Intermolecular dialkylation of alkenes with two distinct C(sp3) -H bonds enabled by synergistic photoredox catalysis and iron catalysis. Sci. Adv 5, eaav9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Phelan JP, Lang SB, Sim J, Berritt S, Peat AJ, Billings K, Fan L, and Molander GA (2019). Open-air alkylation reactions in photoredox-catalyzed DNA-encoded library synthesis. J. Am. Chem. Soc 141, 3723–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harris PA, King BW, Bandyopadhyay D, Berger SB, Campobasso N, Capriotti CA, Cox JA, Dare L, Dong X, Finger JN, et al. (2016). DNA-encoded library screening identifies benzo[b][1,4]oxazepin-4-ones as highly potent and monoselective receptor interacting protein 1 kinase inhibitors. J. Med. Chem 59, 2163–2178. [DOI] [PubMed] [Google Scholar]


