Abstract
An increasing number of individuals in our population are surviving to over 90 years and a subset is at risk for developing dementia. However, senile plaque and neurofibrillary tangle pathology does not consistently differentiate individuals with and without dementia. Synaptic protein loss is a feature of aging and dementia and may dissociate 90+ individuals with and without dementia. Synaptophysin (SYN), post synaptic density 95 (PSD-95) and growth associated protein 43 (GAP-43) were studied in the frontal cortex of an autopsy series of 32 prospectively followed individuals (92–105 years) with a range of cognitive function. SYN protein levels were decreased in individuals with dementia and increased in those with clinical signs of cognitive impairment insufficient for a diagnosis of dementia. SYN but not PSD-95 nor GAP-43 protein levels was significantly correlated with Mini Mental State Examination (MMSE) scores. Frontal cortex SYN protein levels may protect neuronal function in oldest-old individuals and reflect compensatory responses that may be involved with maintaining cognition.
Keywords: cognitively impaired not demented, MMSE, dementia, compensatory response, clinico-pathology correlation, growth associated protein-43, GAP-43, oldest-old, post synaptic density, PSD, synaptophysin
Introduction
Individuals surviving into very old age (>90 years) are one of the fastest growing segments of the United States population (US Census Bureau, 2004). The prevalence of Alzheimer’s disease (AD) increases with each decade over the age of 60, and several studies have estimated prevalence rates at 48–74% in nonagenarians[18,23,30,54]. In individuals under the age of 90 years, some studies show an association between the extent of senile plaque (SP) and/or neurofibrillary tangle (NFT) pathology and clinical severity of dementia [2,14,15,17,32,43] but others have also demonstrated significant AD pathology in nondemented elderly individuals [8,12,31]. The association between plaques and tangles may be weaker in adults over the age of 90 years (i.e., the oldest-old). Several clinico-pathology studies of the oldest old measuring the extent of SP and NFT do not show differences in the accumulation of AD pathology between cases with and without clinically-diagnosed dementia [20,21,48,49]. Some individuals with clinically diagnosed dementia show little AD pathology, and other clinically nondemented subjects do meet pathological criteria for AD [24,49]. Further, Braak & Braak (B&B) neurofibrillary tangle staging shows significant overlap for demented vs. nondemented oldest-old individuals [22,45]. The link between AD pathology and the presence of dementia in the oldest old is also complicated by a high frequency of individuals with dementia of unknown etiology [13].
A consistent observation in studies of non-AD brains in comparison to AD brains is the loss of synapses, which is also correlated with dementia severity [16,46,47]. In addition, several studies show a reduction in proteins associated with synapses using multiple methodologies [3,17,27–29,33,36,37,39,40,44,52].
The purpose of the current study was to test the hypothesis that there is an association between clinically-diagnosed dementia and synaptic protein loss in the oldest-old as measured by levels of : 1) presynaptic protein synaptophysin (SYN), 2) the post synaptic marker post synaptic density–95 (PSD-95), and 3) the synapse-associated growth molecule growth associated protein-43 (GAP-43). Second, we hypothesized that lower synapse-associated protein levels would be correlated with poorer performance on a measure of global cognition (MMSE). Third, we hypothesized that synaptic protein levels would be related to Braak & Braak neurofibrillary tangle and plaque stage.
Methods:
Subjects:
Subjects in this study were enrolled in The 90+ Study, a prospective longitudinal population-based study of aging and dementia in the oldest-old. The 32 subjects in this study (8 men, 24 women) were selected from the first 45 subjects that came to autopsy who had both frozen tissue available and a post mortem interval (PMI) of less than 8 hours. The 13 subjects that were not included in the study were 6 subjects without frozen tissue, 4 subjects with a PMI>8 hours, 2 subjects with no protein measurements, and 1 subject without education information.
Clinical Diagnosis and Neuropsychological Testing
Using all available information, clinical diagnoses were determined by a multidisciplinary consensus diagnostic conference. Dementia diagnosis was assigned using Diagnostic and Statistical Manual of Mental Disorders 4th edition (DSM-IV) criteria [5]. The diagnosis of cognitively impaired not demented (CIND) was assigned to participants with clinical evidence of cognitive or functional loss but not of sufficient severity to meet criteria for dementia. Participants with no cognitive or functional loss were classified as normal. Each individual in The 90+ Study was given a full neuropsychological test battery described by Whittle and colleagues (2007)[55]. The present study used the Mini-Mental Status Examination (MMSE; [19]) to represent the domain of global cognition. The MMSE is a short screening instrument for dementia with scores ranging from 0–30, and lower scores indicate poorer cognitive ability. The majority of subjects included in the autopsy study had multiple visits to the clinic as subjects were evaluated every 6 months. We selected the visit closest to the time of death and included the interval between the visit and death as a covariate in statistical analyses.
Standard Neuropathological Diagnostic Procedures:
At the University of California, Irvine – Alzheimer’s Disease Research Center, one hemisphere was selected based on a neurologist’s impression of any asymmetry in clinical features of each individual. The selected hemisphere was immersion fixed in 4% paraformaldehyde for 2 weeks. The contralateral hemisphere was coronally sectioned and frozen at −80°C. Multiple paraformaldehyde-fixed regions were subsequently dissected and paraffin embedded to establish a final neuropathologic diagnosis. These blocks were sectioned at 8μm. Thin sections were stained with the modified Bielschowsky procedure with hematoxylin-eosin to identify senile plaques and neurofibrillary tangles and the Klűver-Barrera stain, to visualize cell loss and white matter degeneration. Immunostaining for tau, synuclein, ubiquitin, glial fibrillary acidic protein and CD68 were used to detect Pick bodies, Lewy bodies and gliosis as described in a previous publication [26]. Braak & Braak neurofibrillary tangle and beta-amyloid plaque staging was based upon previously published criteria [7].
Western blotting.
Frozen samples from the midfrontal gyrus (Broadman Area 9) were homogenized in 2 % SDS/ PBS (150 mg/ml) plus protease inhibitors (ICN Pharmaceuticals, Costa Mesa, CA). Proteins were separated on a 10–20 % SDS-PAGE Criterion gel (Bio-Rad Laboratories, Hercules, CA) and transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories, Hercules, CA). Membranes were probed with anti-SYN (1:5000, Chemicon International., Temcula, CA), anti- PSD95 (1:100, Abcam Inc., Cambridge, MA), or anti-GAP-43 (1:2000; Novus Biologicals., Littleton, CO). GAPDH (anti-GAPDH ~36 kDa - 1:5000; Abcam Inc., Cambridge, MA) was used as a protein loading control. Detection was by incubating membranes in either anti-rabbit (1:2500–1:5000), or anti-mouse horseradish (1:2500–1:5000) peroxidase-conjugated secondary antibody and then visualized by chemiluminescence (ECL – Pierce, Rockford, IL). A subset of samples was pooled and each membrane included 4 lanes with increasing protein concentrations (2.5–20 μg or 10–60 μg) to ensure that optical densities were within the linear range of detection. Membranes were scanned and the optical densities of the band of interest were quantified. Values are expressed as percentage of loading control optical density. Western blot experiments for SYN were repeated in a replication study that showed high reliability between the two experiments (r=0.90 p<.0005)(data not shown).
Data Analysis:
We examined the relationship between synapse markers and age at death, post mortem interval, gender, and education to determine if these variables should be included as covariates in subsequent analyses. Pearson correlations were used to assess the relationship between protein levels and age at death and PMI, while a t-test was used to assess the relationship between protein levels and gender or education (≤some college vs. college graduate or higher). All remaining analyses were performed using multiple linear regression with synaptic protein levels as the outcome and adjusting for the appropriate covariates. To determine whether synaptic protein levels were associated with dementia, a dummy coded variable was used in the regression model to classify people as nondemented (normal and CIND) vs. demented. We then ran similar analyses that further subdivided the nondemented group, thus classifying participants into normal, CIND, or demented. Second, we looked at the association between synaptic proteins and global cognition by including MMSE in the regression model as a continuous variable and including the interval between MMSE and death as an additional covariate. Third, we analyzed the relationship between synaptic proteins and the extent of pathology based upon Braak & Braak neurofibrillary tangle and plaque stage in the entire sample. Finally, using dummy coded variables we categorized individuals into 4 groups according to the combination of clinical diagnosis (nondemented or demented) and B&B tangle stage that included: (1) nondemented, tangle stage ≤ III, (n=8); (2) nondemented, tangle stage > III, (n=3); (3) demented, tangle stage ≤ III, (n=5); and (4) demented, tangle stage > III, (n=16). Post-hoc pairwise comparisons of the means for all regression analyses were made using the Bonferroni method.
Results:
Of the 32 cases used in this study, there was a wide range of final neuropathology diagnoses. Of subjects that were not demented, 3 were characterized as being normal, 9 exhibited senile degenerative changes insufficient for a diagnosis of AD. Of individuals who were demented, 4 appeared normal (no AD changes), 5 exhibited senile degenerative changes insufficient for a diagnosis of AD, 7 had AD, 2 had a mixed AD and Lewy body disease diagnosis, 1 subject diagnosed clinically with FTD had a final diagnosis of corticobasal degeneration. Last, one individual with dementia exhibited nerve cell loss with astrocytosis.
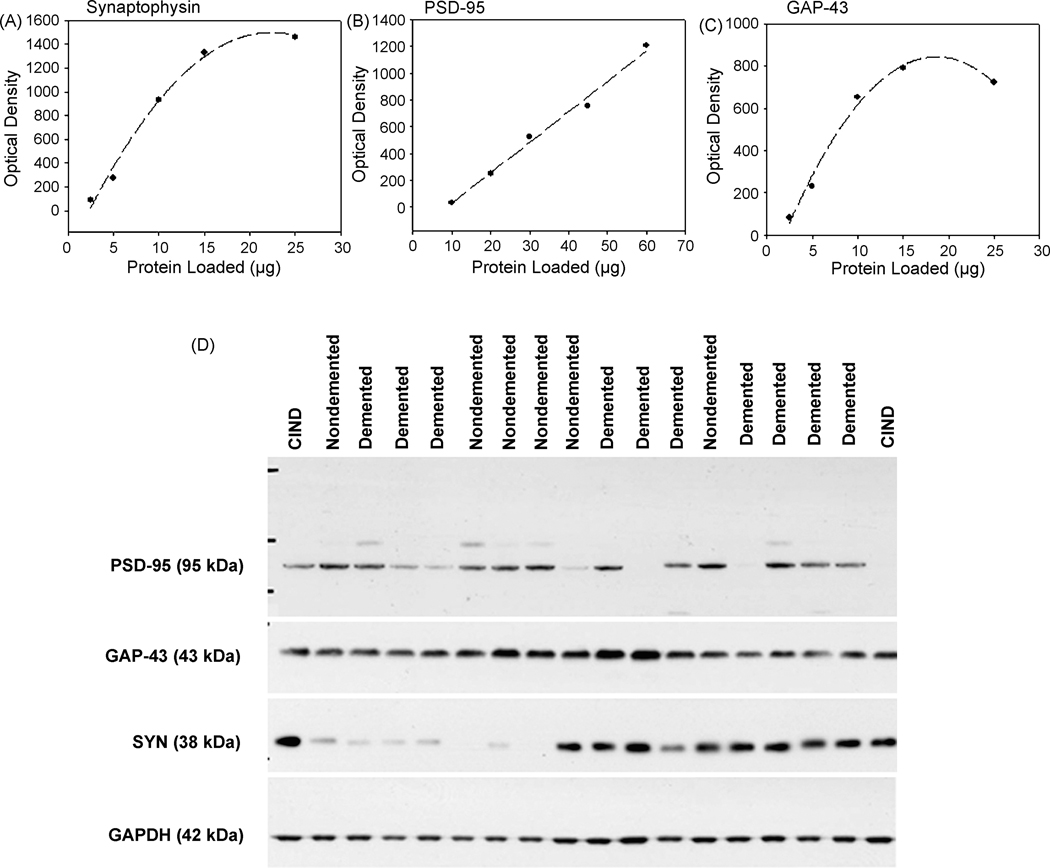
To ensure that densitometric measurements of protein levels of SYN, PSD-95, and GAP-43 were not saturated, a standard curve was derived for each. Fig 1 shows that the minimal amount of sample necessary for detection that was also within the linear range of the standard curve was 5 μg for SYN (Fig 1A), 15 μg for PSD-95 (Fig 1B) and 5 μg for GAP-43 (Fig 1C). In Western blot experiments, a single band representing SYN was observed at ~38 kDa (Fig. 1D), two bands were observed representing PSD-95, a prominent band at ~95 kDa and one slightly higher band at ~98 kDa (Fig 1D), and a single band representing GAP-43 was observed at ~43 kDa (Fig 1D).
Figure 1.
Quantification of frontal cortex synapse proteins in the oldest-old. All protein levels for individual synapse markers were established based on loading increasing concentrations of pooled homogenates of frontal cortex tissue to establish optimal amounts for quantifying synaptophysin (SYN)</P/> (A) PSD-95 (B) and for GAP-43 (C). Lines indicate either linear or logistic regression. Representative western blots of PSD-95, GAP-43 and SYN are shown for a subset of oldest-old cases used in the current study along with a protein loading control (GAPDH) (D).
Table 1 shows characteristics of the 32 participants included in the study. Participants had an average age at death of 96 years (range 92–105), were mostly women (75%), had primarily at least a college education (69%), and a majority were clinically diagnosed as demented (62.5%). Pearson correlation coefficients did not show any significant correlations between the three proteins (SYN and PSD-95 r=- 0.10, p=0.57; SYN and GAP-43 r=0.27, p=0.14; PSD-95 and GAP-43 r=−0.29, p=0.10).
Table 1.
Characteristics of 90+ Study Participants Included in The Study*
| Characteristic | Normal Cognition (N = 7) | CIND (N = 5) | Demented (N = 20) | Total (N = 32) |
|---|---|---|---|---|
| Mean (Range) | ||||
| Age at Death (yrs) | 95.7 (93 – 99) | 96.4 (94 – 100) | 96.1 (92 – 105) | 96.1 (92 – 105) |
| Brain weight (g) | 1225.1 (1127 – 1390) | 1105.4 (921 – 1253) | 1088.1 (871 – 1290) | 1120.8 (871 – 1390) |
| PMI (hrs) | 4.5 (2.2 – 7.0) | 4.7 (3.8 – 6.0) | 4.1 (2 – 7) | 4.3 (2 – 7) |
| BBNFT (stage) | 3.0 (2 – 4) | 2.8 (1 – 4) | 4.3 (2 – 6) | 3.8 (1 – 6) |
| BBSP (stage) † | 0.9 (0 – 2) | 1.8 (1 – 2) | 1.5 (0 – 3) | 1.4 (0 – 3) |
| MMSE | 26.0 (21 – 29) | 26.0 (21 – 29) | 14.3 (0 – 27) | 18.9 (0 – 29) |
| Interval from exam to death (months) | 8.3 (2.8 – 12.9) | 7.6 (3.5 – 12.3) | 6.8 (1.7 – 16.1) | 7.2 (1.7 – 16.1) |
| SYN (% of GAPDH) | 106.0 (51.2 – 177.5) | 177.7 (117.7 – 241.5) | 72.3 (0 – 194.2) | 96.1 (0.0 – 241.5) |
| PSD-95 (% of GAPDH) | 24.9 (2.4 – 71.2) | 17.4 (0.0 – 54.7) | 26.8 (0.0 – 79.0) | 24.9 (0.0 – 79.0) |
| GAP-43 (% of GAPDH) | 98.3 (59.3 – 147.5) | 124.6 (52.6 – 258.8) | 71.8 (27.2 – 156.6) | 85.8 (27.2 – 258.8) |
| Number (%) | ||||
| Female (%) | 4 (57) | 4 (80) | 16 (80) | 24 (75) |
| Education (%) | ||||
| less than college | 1 (14) | 2 (40) | 7 (35) | 10 (31) |
| college or higher | 6 (86) | 3 (60) | 13 (65) | 22 (69) |
CIND = Cognitively impaired not demented
PMI = Post-mortem interval; BBNFT = Braak & Braak neurofibrillary tangle; BBSP = Braak & Braak senile plaque; MMSE = Mini-mental state exam; SYN = synaptophysin; PSD = post synaptic density; GAP = growth associated protein
Average BBSP stage was obtained by assigning a numerical score to each stage as follows: 0=0, A=1, B=2, C=3.
Age at death was significantly correlated with PSD-95 levels (r=0.57 p<0.001) and higher PSD protein level was associated with increasing age. There was no correlation between age and SYN (r=−0.09 p=0.63) or GAP-43 (r=−0.02 p=0.93). There was a trend toward higher SYN levels among people with higher education (t=−1.85, p=0.07) but there was no differences in education and PSD-95 (t(30)=0.18 p=0.86) or GAP-43 (t(30)=0.96 p=0.35). Conversely, post mortem interval was not correlated with SYN (r=0.20 p=0.26), PSD-95 (r=−0.05 p=0.77) or GAP-43 (r=0.08 p=0.67) protein levels and gender was not significantly related to levels of any of the synaptic proteins. Therefore, we decided to only adjust for age at death and education in all analyses.
Protein levels and cognitive diagnosis
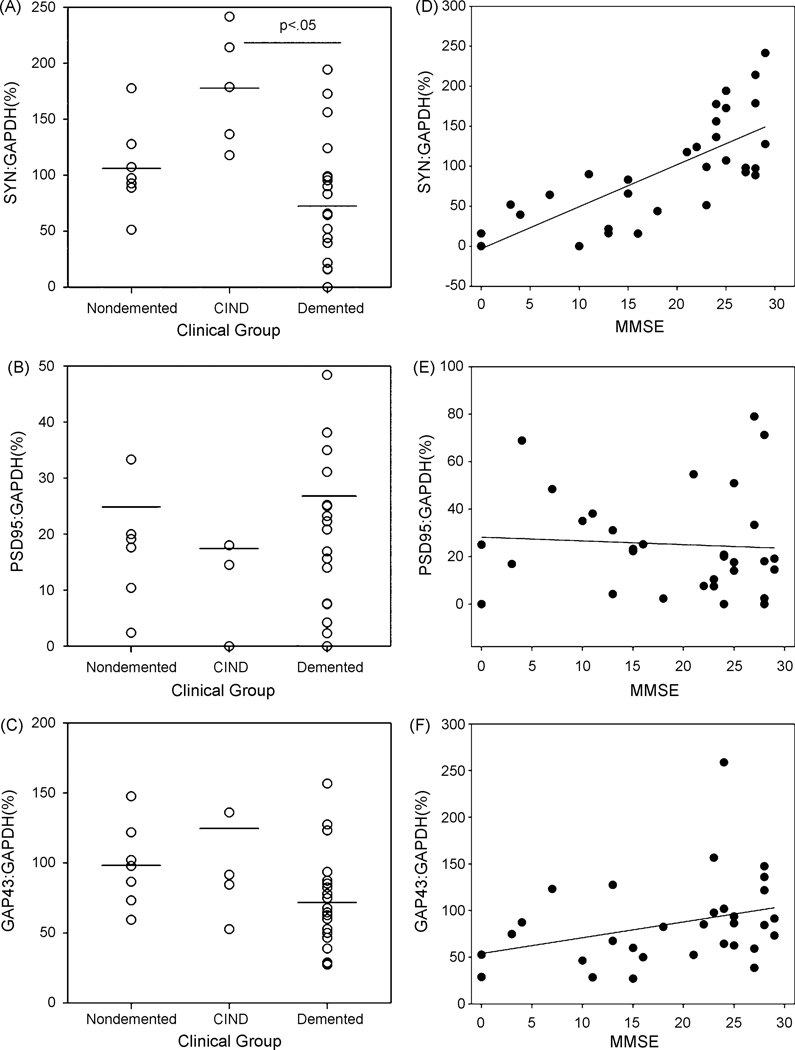
In a regression model that adjusted for age at death and education level, SYN protein levels were significantly lower in demented compared to nondemented participants (F(1,28)=8.6, p=0.007). When we further subdivided the participants into 3 clinical groups, normal (n=7), CIND (participants with clinical evidence of cognitive or functional loss but not of sufficient severity to meet criteria for dementia, n=5) or demented (n=20), SYN also showed a significant association with clinical grouping (F(2,27)=9.6, p=<0.001). Post hoc tests revealed that SYN levels were significantly higher in the CIND group compared to the demented group (Fig 2A). No other comparisons were significant. PSD-95 levels were not associated with clinical diagnosis, either when classifying participants as demented vs. not demented (F(1,28)=0.5, p=0.51) or when classifying participants as normal, CIND, and demented (F(2,27)=0.70, p=0.51)(Fig. 2B). When exploring the association between GAP-43 and cognitive diagnosis we found that demented participants had significantly lower levels of GAP-43 compared to nondemented participants (F(1,28)=5.0, p=0.03), and levels were slightly higher in CIND than normal and demented (F(2,27)=3.2, p=0.06)(Fig. 2C). However, when an outlier with a very high GAP-43 level was excluded, the association only trended towards significance in the demented vs. not demented analysis (F(1,27)=3.3, p=0.08) and was not significant in the three cognitive groups analysis (F(2,26)=1.7, p=0.21).
Figure 2.
Protein levels as a function of clinical diagnosis in the oldest-old. SYN protein levels were highest in individuals that were cognitively impaired but not demented (CIND) relative to cases with dementia (A). There was no difference in PSD-95 levels in the three clinical groups (B) and there was a trend towards higher GAP-43 in CIND cases (C) This trend was primarily due to one subject with very high GAP-43 protein levels. SYN protein levels in the frontal cortex were also significantly correlated with overall cognitive function measured by the MMSE (r=0.73)(D). However, there was no association between MMSE and PSD-95 (r=0.08) (E) or GAP-43 (r=0.31)(F) protein levels. (F). Lines at the top of graphs in A, B, and C, indicate Bonferroni corrected post hoc comparisons. Horizontal lines in A,B,C represent group means. Individual data points are shown as open circles. Lines in D, E, and F represent linear regression analyses.
Protein levels and global cognition score
Demented individuals showed a wide range of SYN, PSD95 and GAP43 protein levels and we hypothesized this may reflect the severity of dementia. We analyzed the association between protein levels and MMSE score with a regression analysis that included age at death and education level as covariates. The interval between last examination and death was not significant in any of the models and was dropped from analyses. Higher MMSE score was significantly associated with higher SYN levels (r=0.73 p<0.001)(Fig 2D). MMSE was not associated with PSD-95 levels (r=0.08 p=0.68)(Fig 2E) or GAP-43 levels (r=0.31 p=0.11) (Fig. 2F).
Protein levels and Braak and Braak Tangle and Plaque Staging
After adjusting for age at death and education level, there was a significant positive association between Braak & Braak tangle stage and SYN levels (F(5,24)=5.2, p=0.002). No association was seen between tangle stage and PSD-95 (F(5,24)=0.04, p=0.99) or GAP-43 (F(5,24)=1.5, p=0.23). We did not find an association between plaque staging and levels of SYN (F(3,26)=2.0, p=0.13) or PSD-95 (F(3,26)=1.0, p=0.40). There was a trend towards an association between plaque staging and GAP-43 levels (F(3,26)=2.6, p=0.07) which disappeared after exclusion of an outlier with a very high GAP-43 level (F(3,25)=1.8, p=0.17).
Protein levels comparison of High vs. Low Pathology in Nondemented vs. Demented
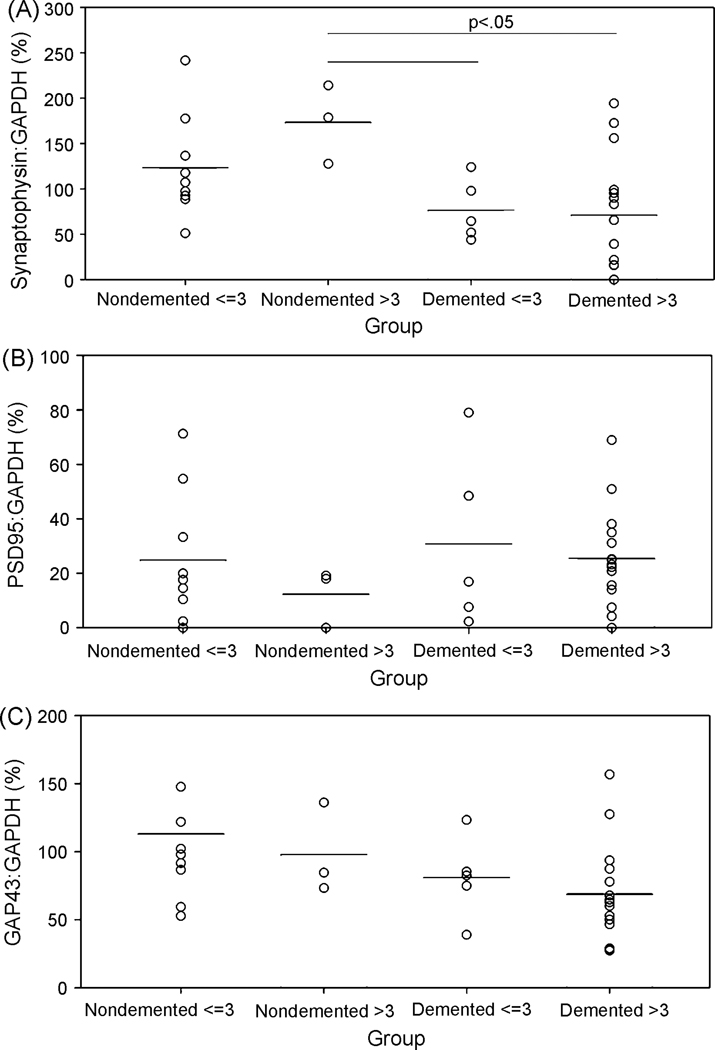
Increased levels of SYN and a trend toward increasing GAP-43 protein levels in individuals with CIND suggested a compensatory response in nondemented individuals with significant AD pathology. To address this, we used a post hoc exploratory analysis and categorized individuals in the study into 4 groups according to a combination of clinical diagnosis and B&B tangle stage that included: (1) nondemented, tangle stage ≤ III, (n=8); (2) nondemented, tangle stage > III, (n=3); (3) demented, tangle stage ≤ III, (n=5); and (4) demented, tangle stage > III, (n=16). Thus, group 2 serves as a high pathology control group. SYN protein varied significantly across groups (F(3,26)=3.6, p=0.03) and this was due to nondemented individuals with high pathology having significantly higher SYN than demented individuals with high pathology (Fig. 3A). Average SYN levels were very similar in the two demented groups (low and high pathology). Group differences for PSD-95 (F(3,26)=0.8, p=0.50) (Fig. 3B) and GAP-43 (F(3,26)=1.7, p=0.19) (Fig. 3C) were not significant. However, GAP-43 appears to linearly decline from being high in nondemented subjects with low tangle pathology to subjects with dementia and high tangle pathology.
Figure 3.
Comparison of synaptic protein levels between nondemented cases with and without tangle pathology to demented cases with and without tangle pathology. (A) SYN levels were highest overall in nondemented individuals with B&B stage > III. (B) PSD-95, however, was lowest overall only in cases with dementia and a B&B stage > III. (C) In contrast, GAP-43 was lowest overall in demented subjects with a B&B Stage < III. Lines at the top of the graphs indicate significant group differences using Bonferroni post hoc comparisons. Individual data points are represented as open circles and means of each group are shown as horizontal lines.
Discussion
Levels of the presynaptic protein SYN in the frontal cortex of individuals aged 90 and over were significantly lower in cases with dementia and were associated with MMSE scores and the extent of neurofibrillary tangle pathology (controlling for both age at death and education level). SYN levels were also higher in nondemented participants with significant neurofibrillary tangle pathology as compared to participants with clinical dementia with and without neurofibrillary tangle pathology. PSD-95, a marker of postsynaptic densities, was not related to the presence or absence of dementia, MMSE score, or the extent of plaque and tangle pathology. The growth molecule GAP-43 showed similar levels in demented and nondemented individuals, and did not correlate with MMSE scores nor with plaque or tangle pathology
Synaptophysin is a 38 kDa integral membrane glycoprotein located in presynaptic vesicles [56]. Previous studies in younger individuals with AD (typically under 90 years of age) reported synaptic protein losses (e.g. [39,40,44,53]. Consistent with previous reports, participants with clinically diagnosed dementia in the present study had significantly lower frontal SYN than those without dementia. Further, increasingly severe neurofibrillary tangle pathology based on B&B staging was associated with lower SYN protein levels. Higher NFT pathology and reduced synaptophysin protein is consistent with a report of a decrease in mRNA for synaptophysin specifically in tangle-bearing neurons [9] but interestingly, this was independent of whether an individual was diagnosed with AD or clinically normal. In this previous study, the authors suggest that synaptophysin protein decreases may reflect the number of neurons differentially affected by disease with NFT pathology in dementia as compared with controls [9]. In the present study, SYN protein levels were also lower in those individuals with lower MMSE scores, which is consistent with previous reports in younger individuals [53]. Further, the correlation between MMSE scores and SYN protein level was 0.73, which is similar to previous reports [52,53] and also observed when counting synapses [16], thus SYN protein can account for ~53% of the variance in MMSE scores. This supports the hypothesis that SYN protein loss is associated with clinically diagnosed dementia and has been extended to a cohort of the oldest-old.
When considering cognitive status on a continuum from normal to mild cognitive impairment (MCI) to dementia, an increase in presynaptic protein levels in those with CIND (participants with clinical evidence of cognitive or functional loss but not of sufficient severity to meet criteria for dementia), as in the current study, may be considered as a compensatory response to incipient pathology. For instance, findings from the Religious Orders Study demonstrated higher levels of frontal SYN in the superior frontal cortex of participants with MCI as compared to participants with normal cognition or dementia [11]. Similarly, the present study noted significantly higher SYN levels in CIND cases than in dementia cases, suggesting a possible compensatory mechanism. However, we would like to note that the sample size is relatively small and additional studies would be helpful for confirming this result. Other studies demonstrated a potential compensatory response with observations of significantly higher SYN levels in elderly participants with high levels of pathology but normal cognition as compared to patients diagnosed with clinical and pathological AD [35,36]. Those findings were repeated in the present study. Clinically nondemented participants with high tangle pathology had the highest SYN protein levels overall and significantly higher SYN levels than the nondemented with low pathology group and demented groups (both high and low pathology). The pattern of elevated SYN levels in the present study of 90+ year olds suggests a presynaptic compensatory mechanism that is activated during the early stage of cognitive decline that might account for the relatively preserved cognitive abilities of participants in the presence of moderate to severe neuropathology.
Proposed models of the brain’s compensatory molecular mechanisms must take into account that increased or decreased SYN protein levels may not directly reflect changes in synapse number. In ultrastructural studies of AD biopsy and autopsy material, the number of synapses may decrease but the apposition size of remaining synapses may increase (reviewed by [46]. These observations have led to the hypothesis that initial neuron and synapse losses stimulate synaptic enlargement to maintain a stable total synaptic contact length during the progression of AD [46]. During this time of compensation, relative level of synaptic proteins may be predicted to stay the same or possibly increase, with a decline in levels of synaptic proteins beginning in early AD. An increase in SYN levels in CIND individuals may reflect an increase in synapse number or the size of individual synapses. However, since protein levels of PSD95, a post synaptic marker did not correlate with SYN protein our current results may suggest that synapse number or size per se was unchanged in our study. Increased SYN may better represent higher numbers of presynaptic vesicles or larger vesicles reflecting a change in synaptic function rather than synapse structure or number. Further studies should look more closely at the association between synaptic markers and clinically-determined cases of CIND, as well as neuropathological equivalents to CIND to distinguish between changes in the number of presynaptic vesicles and synapses versus a lengthening of the synapse. Longitudinal data sets that are able to clinically characterize and differentiate CIND cases who do convert to dementia as compared to those who maintain cognitive function will be important sources of information on how synaptic function and compensatory responses may discriminate between converters vs. non-converters.
A second measure of synapses in the current study was a postsynaptic protein, PSD-95, that is involved with learning and memory [42]. In Tg2576 mice, a mouse model of human brain aging associated with A® deposition, PSD-95 positive synaptic compartments are fewer and smaller in size by immunofluorescence and Aβ specifically reduces protein levels [4]. A similar association between PSD-95 and Aβ is observed in cortical synaptosome preparations from AD patients [25]. Further, PSD-95 is lower in the temporal cortex of AD cases relative to controls [34]. In the present study, we observed little overall clinical group differences in PSD-95 protein level by Western blotting nor a correlation with MMSE or with Braak & Braak tangle stage. This was somewhat unexpected given previous reports of synaptophysin and PSD-95 being colocalized in mouse models of AD [51]. Further, other measures of post-synaptic markers such as spinophilin, particularly when taking into account measures of NFT number in a regression analyses can account for up to 61.9% of the variability in MMSE scores in human frontal cortex [1]. The lack of group differences in PSD-95 and correlation with other pathological and clinical markers in the current study may be due to the use of total protein levels as an outcome measure that may obscure losses in vulnerable neuronal populations. Additional studies using immunohistochemistry and unbiased counting measures as described previously [1] would help to refine these initial studies.
The third marker of synapse associated proteins used in the current study, GAP-43, is involved with neurite outgrowth [6], synaptogenesis, and axonal sprouting [50]. In sporadic AD, the extent of GAP-43 immunolabeling increases in the hippocampus but is reduced in the frontal cortex ([37])[38], suggesting a region-specific compensatory response. Further, message levels of GAP-43 decrease with increasing tangle pathology [10]. Initially we observed a trend towards lower GAP-43 protein in demented cases and higher protein levels in those with CIND, a finding consistent with previous research. However, one individual with CIND had very high levels of GAP-43 and was a significant contributor to these effects. When this case was removed from the analysis, GAP-43 was not significantly lower in cases with dementia or higher in CIND brains. Further, GAP-43 was not associated with MMSE scores or Braak and Braak tangle stage. Levels of GAP-43 in the frontal cortex may not be a significant contributor to the presence or absence of dementia and associated brain changes in the oldest-old. However, similar methodological limitations as those discussed for the PSD-95 measures would also apply to GAP-43. Further, increased or decreased GAP-43 may be more robust if measured in the hippocampus due to the higher levels of expression in this vulnerable brain region.
Several caveats should be considered when evaluating the results of this study. First, correlations between SYN protein level and dementia status or MMSE may be relatively conservative because measuring synapse-associated proteins by Western blots does not take into account possible differences in synapse loss within select cortical layers. Further, there most likely will be differences in the amount or density of grey matter and the cell constituents (i.e. neurons, glial cells). Despite these potential contributors to SYN, PSD95 and GAP-43 protein levels, the differences in total protein were still able to differentiate our 3 clinical groups. Second, the participants in the study have a mix of pathology and extent of cognitive dysfunction and were grouped into a general dementia clinical category in the analyses instead of looking at specific subtypes of dementia (e.g., AD, vascular dementia, Lewy body dementia). This limits an interpretation of how synaptic protein levels may vary by type of dementia. This method of categorization was due in part to the small number of available participants representative of each group. Furthermore, clinical criteria for AD, such as the NINCDS-ADRDA criteria [41], were not developed to include patients over the age of 90 years. A third caveat concerns the small number of individuals with a clinical diagnosis of CIND (which in itself may be subject to variability in the definition used) and those characterized as being high pathology controls (which may vary depending on the criteria used for specific studies). An extension of these results to larger sample sizes may confirm possible compensatory events. This study provided preliminary evidence of a possible adaptation to plaque and tangle pathology via increased SYN levels in the frontal cortex of the oldest-old, suggesting continued brain plasticity into the 10th and 11th decades of life.
Acknowledgments:
The 90+ Study is supported by the National Institute on Aging grant R01AG21055 (CK), the Al and Trish Nichols Chair in Clinical Neuroscience (CK), ADRC P50 AG16573, and P50 AG000658. Preliminary portions of this study were presented at the 2006 Annual Conference of the American Academy of Neurology. The authors extend their sincere thanks to The 90+ Study participants, families, and caregivers for their time and effort and to the dedicated staff members of The 90+ Study for their continued hard work.
Footnotes
Disclosure Statement: The authors have no actual or potential conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Akram A, Christoffel D, Rocher AB, Bouras C, Kovari E, Perl DP, Morrison JH, Herrmann FR, Haroutunian V, Giannakopoulos P, Hof PR. Stereologic estimates of total spinophilin-immunoreactive spine number in area 9 and the CA1 field: Relationship with the progression of Alzheimer’s disease. Neurobiol Aging 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Alafuzoff L, Iqbal K, Friden H, Adolfsson R and Winblad B Histopathological criteria for progressive dementia disorders: clinical-pathological correlation and classification by multivariate analysis. Acta Neuropathologica (Berlin) 1987;74:209–25. [DOI] [PubMed] [Google Scholar]
- [3].Alford MF, Masliah E, Hansen LA, Terry RD. A simple dot-immunobinding assay for quantification of synaptophysin-like immunoreactivity in human brain. J Histochem Cytochem 1994;42(2):283–7. [DOI] [PubMed] [Google Scholar]
- [4].Almeida CG, Tampellini D, Takahashi RH, Greengard P, Lin MT, Snyder EM, Gouras GK. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol Dis 2005;20(2):187–98. [DOI] [PubMed] [Google Scholar]
- [5].Association AP. Diagnostic and statistical manual of mental disorders. 4th ed Washington, DC.: American Psychiatric Association; 1994. [Google Scholar]
- [6].Benowitz LI, Perrone-Bizzozero NI, Finklestein SP. Molecular properties of the growth-associated protein GAP-43 (B-50). J Neurochem 1987;48(5):1640–7. [DOI] [PubMed] [Google Scholar]
- [7].Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82(4):239–59. [DOI] [PubMed] [Google Scholar]
- [8].Caccamo A, Oddo S, Billings LM, Green KN, Martinez-Coria H, Fisher A, LaFerla FM. M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron 2006;49(5):671–82. [DOI] [PubMed] [Google Scholar]
- [9].Callahan LM, Vaules WA, Coleman PD. Progressive reduction of synaptophysin message in single neurons in Alzheimer disease. J Neuropathol Exp Neurol 2002;61(5):384–95. [DOI] [PubMed] [Google Scholar]
- [10].Coleman PD, Kazee AM, Lapham L, Eskin T, Rogers K. Reduced GAP-43 message levels are associated with increased neurofibrillary tangle density in the frontal association cortex (area 9) in Alzheimer’s disease. Neurobiol Aging 1992;13(6):631–9. [DOI] [PubMed] [Google Scholar]
- [11].Counts SE, Nadeem M, Lad SP, Wuu J, Mufson EJ. Differential expression of synaptic proteins in the frontal and temporal cortex of elderly subjects with mild cognitive impairment. J Neuropathol Exp Neurol 2006;65(6):592–601. [DOI] [PubMed] [Google Scholar]
- [12].Crystal H, Dickson D, Fuld P, Masur D, Scott R, Mehler M, Masdeu J, Kawas C, Aronson M, Wolfson L Clinico-pathologic studies in dementia: Nondemented subjects with pathologically confirmed Alzheimer’s disease. Neurology 1988;38:1682–7. [DOI] [PubMed] [Google Scholar]
- [13].Crystal HA, Dickson D, Davies P, Masur D, Grober E, Lipton RB. The relative frequency of “dementia of unknown etiology” increases with age and is nearly 50% in nonagenarians. Arch Neurol 2000;57(5):713–9. [DOI] [PubMed] [Google Scholar]
- [14].Cummings BJ, Pike CJ, Shankle R and Cotman CW Beta-amyloid deposition and other measures of neuropathology predict cognitive status in Alzheimer’s disease. Neurobiology of Aging 1996;17(6):921–33. [DOI] [PubMed] [Google Scholar]
- [15].Dayan AD. Quantitative histological studies on the aged human brain II. Senile plaques and neurofibrillary tangles in senile dementia (with an Appendix on the occurrence in cases of carcinoma). Acta Neuropathologica (Berlin) 1970;16:95–102. [DOI] [PubMed] [Google Scholar]
- [16].DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol 1990;27(5):457–64. [DOI] [PubMed] [Google Scholar]
- [17].Dickson DW, Crystal HA,Bevona C, Honer W, Vincent I and Davies P Correlations of synaptic and pathological markers with cognition of the elderly. Neurobiology of Aging 1995;16(3):285–304. [DOI] [PubMed] [Google Scholar]
- [18].Ebly EM, Parhad IM, Hogan DB, Fung TS. Prevalence and types of dementia in the very old: results from the Canadian Study of Health and Aging. Neurology 1994;44(9):1593–600. [DOI] [PubMed] [Google Scholar]
- [19].Folstein MF, Folstein SE, and McHugh PR Mini-mental state: A practical method for grading the cognitive status of patients for the clinician. J Psychiatry Res 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- [20].Giannakopoulos P, Hof PR, Kovari E, Vallet PG, Herrmann FR, Bouras C. Distinct patterns of neuronal loss and Alzheimer’s disease lesion distribution in elderly individuals older than 90 years. J Neuropathol Exp Neurol 1996;55(12):1210–20. [DOI] [PubMed] [Google Scholar]
- [21].Giannakopoulos P, Hof PR, Vallet PG, Giannakopoulos AS, Charnay Y, Bouras C. Quantitative analysis of neuropathologic changes in the cerebral cortex of centenarians. Prog Neuropsychopharmacol Biol Psychiatry 1995;19(4):577–92. [DOI] [PubMed] [Google Scholar]
- [22].Gold G, Bouras C, Kovari E, Canuto A, Glaria BG, Malky A, Hof PR, Michel JP, Giannakopoulos P. Clinical validity of Braak neuropathological staging in the oldest-old. Acta Neuropathol (Berl) 2000;99(5):579–82; discussion 83–4. [DOI] [PubMed] [Google Scholar]
- [23].Graves AB, Larson EB, Edland SD, Bowen JD, McCormick WC, McCurry SM, Rice MM, Wenzlow A, Uomoto JM. Prevalence of dementia and its subtypes in the Japanese American population of King County, Washington state. The Kame Project. Am J Epidemiol 1996;144(8):760–71. [DOI] [PubMed] [Google Scholar]
- [24].Green MS, Kaye JA, Ball MJ. The Oregon brain aging study: neuropathology accompanying healthy aging in the oldest old. Neurology 2000;54(1):105–13. [DOI] [PubMed] [Google Scholar]
- [25].Gylys KH, Fein JA, Yang F, Wiley DJ, Miller CA, Cole GM. Synaptic changes in Alzheimer’s disease: increased amyloid-beta and gliosis in surviving terminals is accompanied by decreased PSD-95 fluorescence. Am J Pathol 2004;165(5):1809–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Head E, Starr A, Kim RC, Parachikova A, Lopez GE, Dick M, Cribbs DH. Relapsing polychondritis with features of dementia with Lewy bodies. Acta Neuropathol (Berl) 2006;112(2):217–25. [DOI] [PubMed] [Google Scholar]
- [27].Heinonen O, Soininen H, Sorvari H, Kosunen O, Paljarvi L, Koivisto E, Riekkinen PJ, Sr. Loss of synaptophysin-like immunoreactivity in the hippocampal formation is an early phenomenon in Alzheimer’s disease. Neuroscience 1995;64(2):375–84. [DOI] [PubMed] [Google Scholar]
- [28].Honer WG, Dickson DW, Gleeson J, Davies P. Regional synaptic pathology in Alzheimer’s disease. Neurobiol Aging 1992;13(3):375–82. [DOI] [PubMed] [Google Scholar]
- [29].Ingelsson M, Fukumoto H, Newell KL, Growdon JH, Hedley-Whyte ET, Frosch MP, Albert MS, Hyman BT, Irizarry MC. Early Abeta accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology 2004;62(6):925–31. [DOI] [PubMed] [Google Scholar]
- [30].Jorm AF, Korten AE, Henderson AS. The prevalence of dementia: a quantitative integration of the literature. Acta Psychiatr Scand 1987;76(5):465–79. [DOI] [PubMed] [Google Scholar]
- [31].Katzman R, Terry R, DeTeresa R, Brown T, Davis P, Fuld P, Renbing X, Peck A Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol 1988;23:138–44. [DOI] [PubMed] [Google Scholar]
- [32].Langui D, Probst A and Ulrich J Alzheimer’s changes in non-demented and demented patients: a statistical approach to their relationships. Acta Neuropathol 1995;89:57–62. [DOI] [PubMed] [Google Scholar]
- [33].Lassmann H, Weiler R, Fischer P, Bancher C, Jellinger K, Floor E, Danielczyk W, Seitelberger F, Winkler H. Synaptic pathology in Alzheimer’s disease: immunological data for markers of synaptic and large dense-core vesicles. Neuroscience 1992;46(1):1–8. [DOI] [PubMed] [Google Scholar]
- [34].Love S, Siew LK, Dawbarn D, Wilcock GK, Ben-Shlomo Y, Allen SJ. Premorbid effects of APOE on synaptic proteins in human temporal neocortex. Neurobiol Aging 2006;27(6):797–803. [DOI] [PubMed] [Google Scholar]
- [35].Lue LF, Brachova L, Civin WH, and Rogers J Inflammation, A-beta deposition, and neurofibrillary tangle formation as correlates of Alzheimer’s disease neurodegeneration. J Neuropath & Exp Neurology 1996;55:1083. [PubMed] [Google Scholar]
- [36].Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer’s disease. American Journal of Pathology 1999;155(3):853–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Masliah E, Mallory M, Alford M, DeTeresa R, Hansen LA, McKeel DW, Jr., Morris JC. Altered expression of synaptic proteins occurs early during progression of Alzheimer’s disease. Neurology 2001;56(1):127–9. [DOI] [PubMed] [Google Scholar]
- [38].Masliah E, Mallory M, Hansen L, Alford M, Albright T, DeTeresa R, Terry R, Baudier J, Saitoh T. Patterns of aberrant sprouting in Alzheimer’s disease. Neuron 1991;6(5):729–39. [DOI] [PubMed] [Google Scholar]
- [39].Masliah E, Terry RD, Alford M, DeTeresa R, Hansen LA. Cortical and subcortical patterns of synaptophysinlike immunoreactivity in Alzheimer’s disease. Am J Pathol 1991;138(1):235–46. [PMC free article] [PubMed] [Google Scholar]
- [40].Masliah E, Terry RD, DeTeresa RM, Hansen LA. Immunohistochemical quantification of the synapse-related protein synaptophysin in Alzheimer disease. Neurosci Lett 1989;103(2):234–9. [DOI] [PubMed] [Google Scholar]
- [41].McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34:939–44. [DOI] [PubMed] [Google Scholar]
- [42].Migaud M, Charlesworth P, Dempster M, Webster LC, Watabe AM, Makhinson M, He Y, Ramsay MF, Morris RG, Morrison JH, O’Dell TJ, Grant SG. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature 1998;396(6710):433–9. [DOI] [PubMed] [Google Scholar]
- [43].Price JL, Davis, P.B., Morris, J.C. and White, D.L. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer’s disease. Neurobiology of Aging 1991;12:295–312. [DOI] [PubMed] [Google Scholar]
- [44].Reddy PH, Mani G, Park BS, Jacques J, Murdoch G, Whetsell W Jr., Kaye J, Manczak M Differential loss of synaptic proteins in Alzheimer’s disease: implications for synaptic dysfunction. J Alzheimers Dis 2005;7(2):103–17; discussion 73–80. [DOI] [PubMed] [Google Scholar]
- [45].Riley KP, Snowdon DA, Markesbery WR. Alzheimer’s neurofibrillary pathology and the spectrum of cognitive function: findings from the Nun Study. Ann Neurol 2002;51(5):567–77. [DOI] [PubMed] [Google Scholar]
- [46].Scheff SW, Price DA. Synaptic pathology in Alzheimer’s disease: a review of ultrastructural studies. Neurobiol Aging 2003;24(8):1029–46. [DOI] [PubMed] [Google Scholar]
- [47].Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging 2006;27(10):1372–84. [DOI] [PubMed] [Google Scholar]
- [48].Silver M, Newell K, Hyman B, Growdon J, Hedley-Whyte ET, Perls T. Unraveling the mystery of cognitive changes in old age: correlation of neuropsychological evaluation with neuropathological findings in the extreme old. Int Psychogeriatr 1998;10(1):25–41. [DOI] [PubMed] [Google Scholar]
- [49].Silver MH, Newell K, Brady C, Hedley-White ET, Perls TT. Distinguishing between neurodegenerative disease and disease-free aging: correlating neuropsychological evaluations and neuropathological studies in centenarians. Psychosom Med 2002;64(3):493–501. [DOI] [PubMed] [Google Scholar]
- [50].Skene H Axonal growth-associated proteins. Annu Rev Neurosci 1989;12:127–56. [DOI] [PubMed] [Google Scholar]
- [51].Spires TL, Meyer-Luehmann M, Stern EA, McLean PJ, Skoch J, Nguyen PT, Bacskai BJ, Hyman BT. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J Neurosci 2005;25(31):7278–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Sze C-I, Troncoso JC, Kawas C, Mouton P, Price DL, and Martin LJ Loss of the presynaptic vesicle protein synaptophysin in hippocampus correlates with cognitive decline in Alzheimer’s disease. Journal of Neuropathology and Experimental Neurology 1997;56(8):933–44. [DOI] [PubMed] [Google Scholar]
- [53].Terry RD, Masliah E., Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, and Katzman R Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Annals of Neurology 1991;30:572–80. [DOI] [PubMed] [Google Scholar]
- [54].von Strauss E, Viitanen M, De Ronchi D, Winblad B, Fratiglioni L. Aging and the occurrence of dementia: findings from a population-based cohort with a large sample of nonagenarians. Arch Neurol 1999;56(5):587–92. [DOI] [PubMed] [Google Scholar]
- [55].Whittle C, Corrada MM, Dick M, Ziegler R, Kahle-Wrobleski K, Paganini-Hill A, Kawas C. Neuropsychological data in nondemented oldest old: the 90+ Study. J Clin Exp Neuropsychol 2007;29(3):290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wiedenmann B, Franke WW. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell 1985;41(3):1017–28. [DOI] [PubMed] [Google Scholar]