Abstract
Objective:
With a period prevalence of 21.9% in the year after birth, depression is a common complication of childbearing. We assessed the impact of telephone-delivered depression care management (DCM) on symptom levels, health service utilization, and functional status 3, 6, and 12 months postpartum.
Methods:
The randomized controlled trial was conducted at the University of Pittsburgh, Pittsburgh, Pennsylvania, from March 2006 through September 2010. Women (N = 628) who screened positive for depression (a score of 10 or greater on the Edinburgh Postnatal Depression Scale) 4 to 6 weeks postpartum were evaluated with the Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen and enrolled in a randomized trial of DCM compared to enhanced usual care (EUC). Clinicians conducted telephone contacts to educate, assist with treatment decisions, monitor symptoms, facilitate access to services, and encourage links to community resources. Independent evaluators collected symptom scores, functional status, and health services use at 3, 6, and 12 months postpartum. Primary outcome was reduction of symptoms as measured by the Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement.
Results:
Mean depressive symptom and function scores significantly improved (by greater than 50%) in both groups of women but did not differ by DCM versus EUC assignment. Health services use was similar in women randomly assigned to DCM compared to EUC. Women with childhood sexual abuse responded significantly more favorably to DCM on depression and functional measures (all P values < .02).
Conclusions:
Both DCM and EUC favorably impacted depression symptom levels and function. The subgroup of women with childhood sexual abuse benefited significantly more from DCM compared to the EUC condition. Regular telephone availability of a clinician is a resource that appears to be particularly therapeutic to women with childhood sexual abuse.
Trial Registration:
ClinicalTrials.gov identifier: NCT00282776
With a period prevalence of 21.9% in the year after birth, depression is a common complication in childbearing women.1 Postpartum depression (PPD) interferes with maternal functioning,2 which is critical to support the infant’s health and development. The goals of depression treatment include both remission of the depressive symptoms and return to the woman’s optimal functional level.3,4 A comprehensive PPD identification, screening, and intervention strategy holds potential to reduce maternal disability and avert a new generation at risk.5
The term depression with perinatal onset, from the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5),6 describes an episode of major depressive disorder (MDD) that begins during pregnancy or within 4 weeks following birth. From a public health standpoint, women and their families suffer regardless of when the maternal episode begins. For the purposes of this project, we defined PPD as the presence of MDD in the 1-year period after birth. We targeted prevalent rather than incident cases, since our goals were focused on improving women’s mental health.
Pregnancy and the postpartum period are opportune times for intervention because women have contact with health care professionals and are motivated toward positive behaviors to invest in the welfare of their offspring.7 Screening for PPD has been mandated in several states.8 Recommendations include follow-up evaluations for women with positive screens coupled with interventions to target recovery.9 Although it increases detection, screening alone does not improve maternal outcomes10 due to low rates of treatment.11 Interventions for treating depression in medical settings have been framed by a chronic disease management model directed at improvement of patient, provider, and system factors that limit identification and treatment.12 A depression care manager provides education and support to patients, coordinates care, and identifies and facilitates responses to barriers. Compared to interventions in medical offices, telephone care management is flexible and can be efficiently positioned at a central location (such as a hospital), which is particularly relevant for a geographically dispersed population.
The goals of our investigation were as follow: (1) Measure the impact of telephone-delivered depression care management (DCM) on women’s symptom levels and functional status 3, 6, and 12 months after birth. Our hypothesis was that women in DCM would have more favorable symptom levels at 3, 6, and 12 months after birth compared to women in the enhanced usual care (EUC) group. (2) Evaluate the impact of DCM on the utilization of depression care. We hypothesized that women in the DCM intervention would obtain treatment more frequently than women in the EUC group. (3) Conduct a moderator analysis to identify subgroups of women who had particular benefit from DCM compared to EUC.
METHODS
The design and results of our screening study for women with PPD have been published.13 During the maternity hospitalization, women were offered screening by telephone at 4 to 6 weeks postpartum. They were contacted during the 2-week period from 4 through 6 weeks after birth. If they were not reached after 3 days, a postcard was sent with a request to call. If no contact occurred by 6 weeks, no further attempts were made. Eligible women were 18 years or older and English-speaking and had delivered at the University of Pittsburgh Women’s Hospital, Pittsburgh, Pennsylvania. Women who screened positive (score ≥ 10 on the Edinburgh Postnatal Depression Scale)14 were offered a home visit for diagnostic evaluation with the Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen (SCID-I/P w/ PSY SCREEN).15 Exclusion criteria included DSM-IV diagnoses of bipolar or psychotic disorder, a suicide attempt within 6 months of pregnancy, or substance abuse/dependence during the index pregnancy. Women who consented were enrolled in the randomized controlled trial (RCT). Ten women participated in the trial in more than 1 pregnancy. Randomization (1:1) was stratified by health coverage (private vs public) and race (white vs other).
Consistent with epidemiologic studies, the majority of screen-positive postpartum women had mood disorders.13 Because our interest was evaluating the impact of the RCT for the group of screen-positive women to mirror the common screening scenario in clinical settings, eligible women were enrolled regardless of diagnosis other than those excluded per exclusion criteria.
Enhanced Usual Care
Women randomly assigned to EUC received more systematic evaluation and monitoring than real-world care as was required by our institutional review board for human subjects protection. Women were provided with depression education at the home visit, time to ask questions of a knowledgeable clinician, encouragement to contact their health plan to facilitate treatment, and 1 follow-up call (Table 1).
Table 1.
Overview of Enhanced Usual Care Versus Depression Telephone-Based Care Management Interventions
| Month After Birth | 1–1.5 (baseline) | 1.5–<2 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enhanced usual care | Education and insurer helpline phone number, study contact number | Letter+phone contact | None | A | A | A | |||||||
| Depression care management | Letter + phone contact | → | → | → | → | → | * | * | * | * | * | * | |
| Contact every month | A | A | A |
Symbols:
= required phone contact;
= blinded, independent evaluator assessments for both cells;
= optional contact decided by care manager and subject based on symptom resolution and subject preference.
Depression Care Management
The study team included experienced Master’s level clinicians who conducted the home visit psychiatric assessments for screen-positive women. For women randomly assigned to DCM, the clinician who conducted the visit was assigned as her care manager. Initial calls lasted 15 to 20 minutes and subsequent calls approximately 10 minutes. Educational materials were provided to the subject (Table 1). The goals for the psychoeducational DCM intervention were to (1) encourage patient self-management and provide tools; (2) provide ongoing education; (3) support shared decision-making between patient and provider(s), including assistance with decisions about medication use during breastfeeding; (4) monitor symptoms and function and evaluate progress; (5) give the subject feedback on her progress; (6) facilitate access to mental health services; (7) encourage links to community resources (workplace assistance, domestic violence services, housing, and legal services); and (8) support primary care providers and mental health practitioners through consultation to perinatal mental health specialists. The care managers reviewed cases weekly with the investigative team. At the 6-month call, the care manager and participant negotiated whether to discontinue calls if the woman was satisfied with her recovery level. All subjects could contact the study team with concerns about symptoms during the first postpartum year.
Independent Evaluator Data Collection
The objective of randomization was to create 2 equal populations that had the same average outcomes across time if no other variables intervened. Since it was not possible for the subjects to be blinded (they knew whether they received DCM or EUC), we employed independent evaluators (IEs) blind to treatment assignment. These IEs included Master’s level practitioners whose offices were located outside the site where the project was conducted. They achieved reliability on the primary outcome measure with the home visiting team by coding videotaped interviews. Subjects were reminded by the IEs to avoid mentioning any contact with other staff. Details of IE assessment are provided online in Supplementary eTable 1.
Depression Symptoms and Diagnosis
Severity of PPD was assessed with the 29-item Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement (SIGH-ADS),16 which includes atypical symptoms of depression, which are more common in women than men.17 The atypical subgroup of depressed patients has higher rates of suicide attempts, psychiatric comorbidity, disability, use of health care services, and childhood neglect and sexual abuse.17 The SCID-I/P w/ PSY SCREEN was conducted at the home visit, and the Longitudinal Interval Follow-Up Evaluation (LIFE)18 was used for collection of psychopathologic data at 3, 6, and 12 months postpartum.
Functional Status
The Global Assessment of Functioning (GAF)15 and the 12-item Short-Form Health Survey (SF-12)19 were administered at each assessment. The Gratification in the Maternal Role (GRAT)20 was used to assess mother-infant relational quality.
Child Outcomes
The Brief Infant-Toddler Social and Emotional Assessment (BITSEA)21 is a caregiver-reported measure used to assess socioemotional health at 12 months.
Maternal Chronic Conditions, Pregnancy, and Birth Outcomes
Data on pregnancy and delivery were recorded on the Peripartum Events Scale (PES).22 The number of chronic medical conditions was based on whether a doctor had ever told the woman that she had any disorder in the Medical Expenditures Panel Survey.23
Patient Beliefs and Support
We used the 30-item version of the Patient Attitudes Toward and Ratings of Care for Depression (PARC-D),24 which allows exploration of the likelihood that a patient would use specific interventions.
Service Use and Utilization Analysis
Independent evaluators interviewed the subjects at 3, 6, and 12 months after birth about the use of health care services. The data were derived from the RAND Utilization of Care25 measure and included (1) the baby’s overall health, emergency room visits, hospital admissions, and breastfeeding and duration and (2) the mother’s general and psychiatric emergency room visits, primary care, obstetrician-gynecologist, psychiatrist, other mental health professional consultations, receipt of therapy or counseling, prescriptions for medications for mental health, attendance at any self-help group, and use of complementary/alternative therapies for emotional problems or stress.
Data Analysis
Descriptive statistics are presented as means and standard deviations for continuous measures and as frequencies and percentages for categorical measures. Tests of association between measures and treatment assignment included Student t tests for normally distributed continuous measures and Mann-Whitney U tests for non-normally distributed measures. For categorical measures, tests of association included χ2 test when expected cell sizes were greater than 5 and Fisher exact test otherwise.
The effect of treatment (DCM and EUC) on outcomes was assessed using repeated measures mixed models in which outcome was regressed on treatment, time, the treatment-by-time interaction, and baseline outcome score. The model included a random intercept and assumed an unstructured covariance matrix across the assessments. Potential moderators of the association between treatment and outcomes were also assessed by a repeated measures mixed model with a random intercept and unstructured covariance matrix. The effect of the moderator was independently estimated for each treatment level including time and baseline outcome score as covariates. Another model was estimated including a moderator-by-treatment interaction in addition to the variables listed above.
RESULTS
The RCT was conducted from March 2006 through September 2010 (ClinicalTrials.gov identifier: NCT00282776).
Patient Characteristics
The CONSORT chart is presented in Figure 1. Baseline demographics, clinical characteristics, and measures of the 628 women randomly assigned to DCM versus EUC are shown in Table 2. Randomization was successful as evidenced by no significant differences at baseline. Similarly, there were no significant differences in delivery outcomes, which occurred prior to enrollment in the study but could impact maternal mental health outcomes postpartum.
Figure 1. CONSORT Flow Diagrama,b.
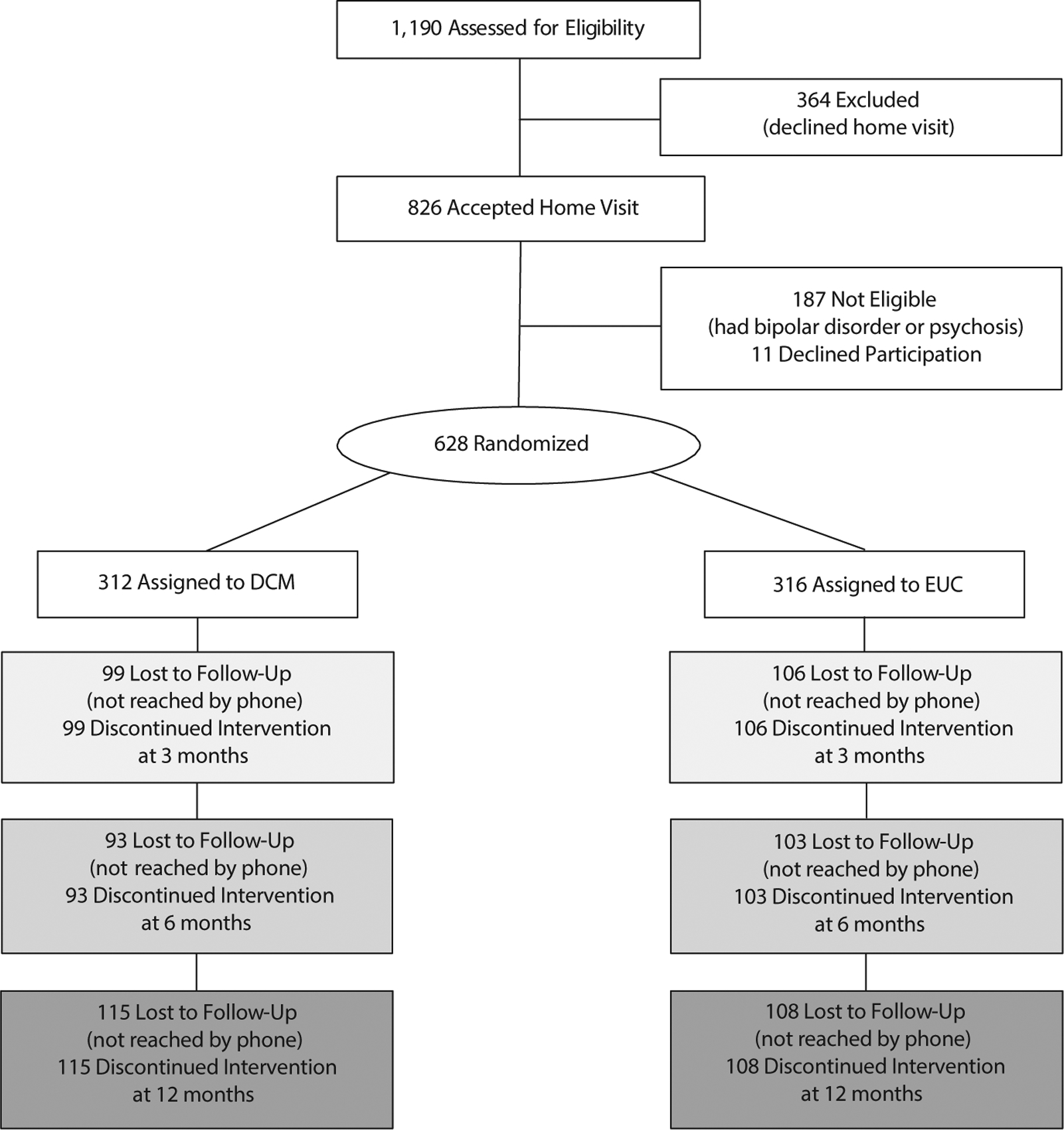
aNumbers are based on primary outcome (SIGH-ADS), and each time point (3, 6, 12 months) is independent. Because the intervention was delivered by phone, women who were not reached by phone (lost to follow-up) by definition discontinued the intervention.
bAll retained subjects were included in the analyses at each assessment point.
Abbreviations: DCM = telephone-based depression care management, EUC = enhanced usual care, SIGH-ADS = 29-Item Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement.
Table 2.
Measures by Treatment at Baselinea
| Measure | All (N = 628) |
DCM (n = 312) |
EUC (n = 316) |
P |
|---|---|---|---|---|
| Demographic measure | ||||
| Age, mean±SD,y | 28.7 ± 6.00 | 28.8 ± 6.09 | 28.5 ± 5.92 | .5034 |
| Race | .8461 | |||
| White | 460 (73.2) | 228 (73.1) | 232 (73.4) | |
| Black | 133 (21.2) | 68 (21.8) | 65 (20.6) | |
| Other | 35 (5.6) | 16 (5.1) | 19 (6.0) | |
| Hispanic | 9 (1.5) | 6 (2.1) | 3 (1.0) | .3339 |
| Education (level) | .8232 | |||
| < High school | 41 (6.5) | 21 (6.7) | 20 (6.3) | |
| High school | 129 (20.5) | 60 (19.2) | 69 (21.8) | |
| Some college | 199 (31.7) | 99 (31.7) | 100 (31.6) | |
| College | 154 (24.5) | 82 (26.3) | 72 (22.8) | |
| Graduate school | 105 (16.7) | 50 (16.0) | 55 (17.4) | |
| Medical insurance | .6992 | |||
| Private | 353 (56.2) | 174 (55.8) | 179 (56.6) | |
| Public | 269 (42.8) | 134 (42.9) | 135 (42.7) | |
| None | 6 (1.0) | 4 (1.3) | 2 (0.6) | |
| Marital status | .6686 | |||
| Single | 266 (42.4) | 130 (41.7) | 136 (43.0) | |
| Married/cohabiting | 349 (55.6) | 174 (55.8) | 175 (55.4) | |
| Divorced/separated | 13 (2.1) | 8 (2.6) | 5 (1.6) | |
| Parity | .0532 | |||
| 1 | 235 (37.4) | 128 (41.0) | 107 (33.9) | |
| 2 | 238 (37.9) | 111 (35.6) | 127 (40.2) | |
| 3 | 96 (15.3) | 39 (12.5) | 57 (18.0) | |
| 4 or more | 59 (9.4) | 34 (10.9) | 25 (7.9) | |
| Clinical measure | ||||
| Onset of current episode | .5702 | |||
| Before pregnancy | 213 (33.9) | 112 (35.9) | 101 (32.0) | |
| Pregnancy | 280 (44.6) | 134 (42.9) | 146 (46.2) | |
| Postpartum | 135 (21.5) | 66 (21.2) | 69 (21.8) | |
| Physically abused as a child | 102 (16.6) | 43 (14.1) | 59 (19.0) | .0964 |
| Sexually abused as a child | 129 (20.9) | 66 (21.6) | 63 (20.3) | .7039 |
| Physically abused as an adult | 186 (30.2) | 102 (33.3) | 84 (27.1) | .0918 |
| Sexually abused as an adult | 68 (11.0) | 32 (10.5) | 36 (11.6) | .6473 |
| Primary diagnosis | .2166 | |||
| Depressive | 568 (90.4) | 278 (89.1) | 290 (91.8) | |
| Anxiety disorder | 39 (6.2) | 22 (7.1) | 17 (5.4) | |
| Substance use | 3 (0.5) | 0 (0.0) | 3 (0.9) | |
| Other | 5 (0.8) | 3 (1.0) | 2 (0.6) | |
| None | 13 (2.1) | 9 (2.9) | 4 (1.3) | |
| Baseline measure, mean±SD | ||||
| SIGH-ADS | 19.6 ± 6.23 | 19.6 ± 6.35 | 19.5 ± 6.12 | .7725 |
| SF-12 Mental | 32.6 ± 10.2 | 32.9 + 10.1 | 32.4 ± 10.2 | .5343 |
| SF-12 Physical | 49.2 ± 9.44 | 49.1 ± 9.72 | 49.4 ± 9.18 | .7529 |
| Chronic medical conditions | 3.19 + 2.13 | 3.28± 2.07 | 3.10 ± 2.19 | .2005 |
| EPDS | 13.9 ± 3.61 | 13.8 ± 3.57 | 14.0 ± 3.66 | .6947 |
| GAF | 60.7 ± 6.92 | 60.7 ± 7.26 | 60.7 ± 6.57 | .9595 |
| GRAT | 3.52 ± 0.69 | 3.54 ± 0.68 | 3.51 ± 0.71 | .544 |
Values are n (%), unless otherwise stated.
Abbreviations: DCM = telephone-directed depression care management, EPDS = Edinburgh Postnatal Depression Scale, EUC = enhanced usual care, GAF = Global Assessment of Functioning, GRAT = Gratification in the Maternal Role, SF-12 = Short-Form Health Survey, SIGH-ADS = 29-Item Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement.
Preferences for Treatment
The mainstays of treatment for PPD are psychotherapy and antidepressants.3 We evaluated the subjects’ preferences and found no significant differences in women randomly assigned to DCM versus EUC. Less than half of our subjects (43.5%) were “very likely” to accept medication, and 25% were “not likely” (Supplementary eTable 2). Group counseling was an option rated “very likely” by 54.2% of mothers, as was searching the media for assistance (55.6%). Although complementary and alternative therapies are popular options in the United States,26 only 16.4% of women reported that they were “very likely” to use them.
Outcome Assessments
Approximately two-thirds of the women (66%, 67%, and 64%) provided data for the primary outcome, the SIGH-ADS, at 3, 6, and 12 months postpartum, respectively. The mean scores of the overall sample of women significantly improved across time (by more than 50%) on all outcome assessments compared to baseline (all P values < .0001) (Table 3). The number of telephone contacts (mean ± SD) were 5.46 ± 3.06 contacts for DCM (range, 0–15 contacts/person) compared to 2.08 ± 1.05 contacts for EUC (range, 0–3 contacts/person) (P < .001).
Table 3.
Repeated Measures and Parameter P Values From Mixed Models
| Month 3 | Month 6 | Month 12 | Probability Values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Measure | n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | Tx | Month | Tx × Month | Baseline |
| SIGH-ADS | ||||||||||
| DCM | 203 | 16.0 ± 8.34 | 206 | 14.5 ± 8.41 | 197 | 11.0 ± 7.12 | .0435 | <.0001 | .0204 | <.0001 |
| EUC | 210 | 15.7 ± 8.58 | 213 | 12.0 ± 7.96 | 208 | 10.4 ± 7.72 | ||||
| SF-12 | ||||||||||
| Mental | ||||||||||
| DCM | 183 | 39.7 ± 12.04 | 186 | 41.4 ± 11.93 | 168 | 45.1 ± 10.85 | .0410 | <.0001 | .0122 | <.0001 |
| EUC | 195 | 39.2 ± 11.60 | 183 | 45.0 ± 10.90 | 179 | 46.7 ± 10.36 | ||||
| Physical | ||||||||||
| DCM | 183 | 52.8 ± 7.85 | 186 | 52.9 ± 7.21 | 168 | 52.1 ± 8.00 | .0186 | .1104 | .1044 | <.0001 |
| EUC | 195 | 51.9 ± 8.89 | 183 | 50.4 ± 9.76 | 179 | 51.3 ± 8.54 | ||||
| GAF | ||||||||||
| DCM | 178 | 69.9 ± 12.84 | 185 | 72.0 ± 13.40 | 175 | 75.4 ± 11.74 | .2671 | <.0001 | .1980 | <.0001 |
| EUC | 198 | 69.3 ± 12.73 | 194 | 74.1 ± 13.03 | 195 | 76.1 ± 12.47 | ||||
| GRAT | ||||||||||
| DCM | 169 | 3.91 ± 0.61 | 175 | 4.06 ± 0.55 | 158 | 4.21 ± 0.48 | .0364 | <.0001 | .6659 | <.0001 |
| EUC | 184 | 3.99 ± 0.54 | 178 | 4.17 ± 0.57 | 174 | 4.27 ± 0.49 | ||||
Abbreviations: DCM = telephone-based depression care management, EUC = enhanced usual care, GAF = Global Assessment of Functioning, GRAT = Gratification in the Maternal Role, SF-12 = Short-Form Health Survey, SIGH-ADS = 29-Item Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement, Tx = treatment.
Depression.
In contrast to our hypothesis, significant differences in treatment outcome slightly favored the EUC group compared to the DCM group (P = .0453, Supplementary eFigure 1). However, the mean differences at 12 months were not clinically significant. For example, the SIGH-ADS score decreased from a baseline mean ± SD of 19.6 ± 6.23 to 11.0 ± 7.12 (DCM) and 10.4 ± 7.72 (EUC). Additionally, the number of urgent calls received was 49 in the DCM group and 46 in the EUC group (P = .69).
Maternal function.
The mean baseline score for all subjects on the SF-12 Mental Composite Scale (MCS) was 32.6, which is substantially less than the 25th percentile score of 41.2 for women aged 25 to 34 years.19 However, by 12 months postpartum, the mean scores were 45.1 (DCM) and 46.7 (EUC), which are below the mean for the MCS in females (47.1) aged 25 to 34 years.19 The mean SF-12 Physical Composite Scale (PCS) score at baseline was 49.2, which is at the 25th percentile.19 Across the first year, the PCS scores improved to means of 52.1 (DCM) and 51.3 (EUC), which approximate the mean score of 52.4 in 25- to 34-year old females.19
The GAF score was 60.7 at baseline, which designates moderate difficulty in social, occupational, or school functioning. By the end of the first postpartum year, scores were in the mid-70s (75.4 ± 11.9 in DCM; 76.1 ± 12.2 in EUC), which signifies symptoms being transient and expectable reactions to psychosocial stressors with no more than slight impairment in function.
Maternal role gratification.
The GRAT scores improved from 3.5 at baseline to 4.2 in the DCM group and 4.3 in the EUC group, which demonstrates successful evolution of the maternal role.20 In a separate study done by our team, a comparison group of nondepressed women followed from birth through 12 months postpartum had GRAT scores of 3.9 ± 0.7 at baseline and 4.2 ± 0.6 at 1 year.4
Child assessment.
The BITSEA showed no significant differences at 12 months. The mean ± SD scores for total problems were 8.10 ± 5.46 for DCM and 7.45 ± 4.49 for EUC (P = .38).
Utilization of Health Services
Health services use was similar in women randomized to DCM compared to EUC, with few exceptions (Supplementary eTable 3). More infants of women in the DCM group were admitted to the hospital compared to the EUC arm (8.5% vs 3.7% at 6 months; overall P value = .04). However, mothers had a lower mean ± SD number of emergency room visits if they were in the DCM group compared to the EUC condition (0.06 ± 0.23 vs 0.21 ± 1.44 at month 3; overall P value = .02).
Moderator Analysis
Variables hypothesized to be related to differential responsivity to DCM compared to EUC were explored as potential moderators. Demographic variables included age, race, education, insurance type, marital status, and parity. Clinical variables were the number of chronic medical illnesses, onset timing of index episode relative to pregnancy, primary diagnosis of MDD, physical and/or sexual abuse in childhood and in adulthood, previous contact with mental health services, history of psychiatric hospitalization, concern about health care costs, and stigma. The moderator analysis was performed for the SIGH-ADS, GAF, GRAT, SF-12 MCS, and SF-12 PCS.
A consistent finding across outcomes was that women with childhood sexual abuse responded more favorably in the DCM arm compared to EUC condition (Table 4). For example, the SIGH-ADS scores of women with childhood sexual abuse declined by about 1 point across the follow-up period in the DCM arm while women with childhood sexual abuse in the EUC group experienced nearly a 1.7-point increase (P < .01). Similarly, on the SF-12 MCS, women with childhood sexual abuse experienced a favorable 1.4-point increase if assigned to DCM but a 3.6-point decline if assigned to EUC (P = .005). On the SF-12 PCS, scores declined by 4.1 points for women with childhood sexual abuse assigned to EUC but by fewer (1.6) points if they received DCM (P < .02).
Table 4.
Moderator Analysesa
| DCM (Main Effect) |
EUC (Main Effect) |
Interaction | ||||
|---|---|---|---|---|---|---|
| Outcome | Moderator | β | P | β | P | P |
| SIGH-ADS | Sexually abused as child | −1.066 | .2761 | 1.690 | .0841 | .0077 |
| SF-12 Mental | Sexually abused as child | 1.408 | .3309 | −3.635 | .0096 | .0050 |
| SF-12 Physical | Sexually abused as child | −1.567 | .0817 | −4.152 | .0003 | .0174 |
The effect sizes (Cohen d) for SF-12 Physical, SF-12 Mental, and SIGH-ADS at month 12 are 0.28, 0.15, and −0.20, respectively. These effect sizes are considered small to medium.
Abbreviations: DCM = telephone-based depression care management, EUC = enhanced usual care, SF-12 = Short-Form Health Survey, SIGH-ADS = 29-Item Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement.
DISCUSSION
We did not confirm our hypotheses that telephone-based DCM would favorably impact depression symptom levels and function compared to EUC in postpartum women. The mean scores for all outcome assessments significantly improved from baseline across the first postpartum year independent of treatment. Similarly, the DCM intervention did not increase utilization of depression care compared to EUC. The telephone-based model of care management may not have been sufficiently robust to promote depressive symptom reduction. However, these results should not be construed as evidence that all depressed women improve with standard care, because the EUC condition that we implemented in this study may have functioned as an intervention. As is common in RCTs focused on depression, the comparator condition (here, the EUC group) is not the same as “no intervention.” The initial home visit psychiatric interview, participation in an RCT, availability of crisis call number, and interviews with a clinician at 3, 6, and 12 months may have contributed to improvement in depressive symptoms. Although infrequent contact occurred in the first year postpartum in the EUC group, these women had a connection to an expert team. Additionally, the number of crisis calls to the team was similar in the EUC and the DCM groups, which implies that the study team was perceived as a clinical resource by women in both treatment arms.
Additional evidence that the EUC arm had a positive impact (similar to DCM) is that the symptom levels in both groups improved similarly across the first year. This observation contrasts with other studies that demonstrate that symptoms of depressed women remain the same across the postpartum period. In a population-based study of over 14,000 women in the United Kingdom, 9.2% and 9.1% reported depressive symptoms at the clinical cut point of the Edinburgh Postnatal Depression Scale at 8 weeks and 8 months, respectively.27 Dipietro et al28 found significant intraindividual stability of maternal depression scores both during the second half of pregnancy and from pregnancy to 6 weeks and 2 years postpartum.
The subgroup of women with childhood sexual abuse benefited more if they were randomized to DCM compared to EUC. This subgroup represents about 20% of our subjects, which is consistent with rates from other studies.29 Childhood maltreatment confers life-long risk for general and mental health disorders and affects the development of stress-responsive neuropsychiatric systems.30 Why might monthly telephone contact with a mental health clinician benefit women with childhood sexual abuse significantly more than nonabused depressed postpartum women? Women with childhood sexual abuse may have difficulty coping with pregnancy due to the need for intimate examinations and birth, which may trigger traumatic memories.29 The clinicians conducting DCM were experienced with interventions for abused women. Care management included validation, which assists women to recognize that they did not deserve to experience the abuse.31 The approach to traumatized women included respect for autonomy, information provision, danger assessment, and safety planning.31 The regular (and crisis) telephone availability of a supportive clinician is a comforting and empowering resource that appears to be particularly therapeutic to women with childhood sexual abuse.
Finally, this investigation has many strengths, including the sample derived from a general obstetrical population and the randomized design with careful attention to blinding. A moderator analysis was performed and revealed that subjects with childhood sexual abuse were particularly responsive to DCM, which may be a relatively inexpensive and efficient intervention for this sizable group of postpartum women. Although the sample size is large (N = 628), a weakness is the incomplete follow-up of depressed women across the first year after birth (about two-thirds of the original sample).
Supplementary Material
Although perinatal depression screening has been recommended, cost-effective interventions for screen-positive women are needed.
Telephone-based depression care management (vs enhanced usual care) was used to support women’s access to mental health care in their community. Mean depressive symptom and function scores significantly improved by > 50% in both groups.
Women who experienced childhood sexual abuse had greater benefit from care management.
Acknowledgments:
The authors thank Emily Pinheiro, BA; Kelly O’Shea, MPH; and Barbara Sutcliffe, all from Northwestern University Feinberg School of Medicine, for preparing the manuscript for submission. They have no conflicts to disclose.
Funding/support: Funded by National Institutes of Health, Bethesda, Maryland, Identification and Therapy of Postpartum Depression, MH 071825 to Dr Wisner.
Role of the sponsor: The National Institute of Mental Health had no role in the conduct of the study other than funding.
Footnotes
Potential conflicts of interest: The Department of Psychiatry at Northwestern University received contractual fees for Dr Wisner’s consultation to Quinn Emanuel Urquhart & Sullivan, LLP (New York City), who represent Pfizer Pharmaceutical Company. Drs Sit, Moses-Kolko, and Wisniewski; Mss McShea and Eng; and Messrs Luther and Dills have no conflicts to disclose.
Supplementary material: Available at PSYCHIATRIST.COM.
REFERENCES
- 1.Gaynes BN, Gavin N, Meltzer-Brody S, et al. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess (Summ). 2005; (119):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller LJ. Postpartum depression. JAMA. 2002;287(6):762–765. [DOI] [PubMed] [Google Scholar]
- 3.Wisner KL, Parry BL, Piontek CM. Clinical practice: postpartum depression. N Engl J Med. 2002;347(3):194–199. [DOI] [PubMed] [Google Scholar]
- 4.Logsdon MC, Wisner K, Sit D, et al. Depression treatment and maternal functioning. Depress Anxiety. 2011;28(11):1020–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milgrom J, Gemmill AW. Screening for perinatal depression. Best Pract Res Clin Obstet Gynaecol. 2014;28(1):13–23. [DOI] [PubMed] [Google Scholar]
- 6.American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fifth Edition Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 7.Misra DP, Guyer B, Allston A. Integrated perinatal health framework: a multiple determinants model with a life span approach. Am J Prev Med. 2003;25(1):65–75. [DOI] [PubMed] [Google Scholar]
- 8.Rhodes AM, Segre LS. Perinatal depression: a review of US legislation and law. Arch Women Ment Health. 2013;16(4):259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Committee on Obstetric Practice. The American College of Obstetricians and Gynecologists Committee opinion no. 630: screening for perinatal depression. Obstet Gynecol. 2015;125(5):1268–1271. [DOI] [PubMed] [Google Scholar]
- 10.Thombs BD, Arthurs E, Coronado-Montoya S, et al. Depression screening and patient outcomes in pregnancy or postpartum: a systematic review. J Psychosom Res. 2014;76(6):433–446. [DOI] [PubMed] [Google Scholar]
- 11.Melville JL, Reed SD, Russo J, et al. Improving care for depression in obstetrics and gynecology: a randomized controlled trial. Obstet Gynecol. 2014;123(6):1237–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wisner KL, Scholle SH, Stein B. Perinatal disorders: advancing public health opportunities. J Clin Psychiatry. 2008;69(10):1602–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wisner KL, Sit DK, McShea MC, et al. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. JAMA Psychiatry. 2013;70(5):490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. [DOI] [PubMed] [Google Scholar]
- 15.First M, Spitzer R, Williams J, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen (SCID-I/P W/ PSY SCREEN). New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1994. [Google Scholar]
- 16.Williams JBW, Terman M. Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement (SIGH-ADS). New York, NY: New York State Psychiatric Institute; 2003. [Google Scholar]
- 17.Matza LS, Revicki DA, Davidson JR, et al. Depression with atypical features in the National Comorbidity Survey: classification, description, and consequences. Arch Gen Psychiatry. 2003;60(8):817–826. [DOI] [PubMed] [Google Scholar]
- 18.Keller MB, Lavori PW, Friedman B, et al. The Longitudinal Interval Follow-up Evaluation: a comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44(6):540–548. [DOI] [PubMed] [Google Scholar]
- 19.Ware JE, Kosinski M, Turner-Bowker DM, et al. User’s Manual for the SF-12v2 Health Survey (With a Supplement Documenting SF-12 Health Survey). Lincoln, RI: QualityMetric Incorporated; 2002. [Google Scholar]
- 20.Mercer RT. The process of maternal role attainment over the first year. Nurs Res. 1985;34(4):198–204. [PubMed] [Google Scholar]
- 21.Briggs-Gowan MJ, Carter AS, Irwin JR, et al. The Brief Infant-Toddler Social and Emotional Assessment: screening for social-emotional problems and delays in competence. J Pediatr Psychol. 2004;29(2):143–155. [DOI] [PubMed] [Google Scholar]
- 22.O’Hara M, Varner M, Johnson S. Assessing stressful life events associated with childbearing: the Peripartum Events Scale. J Reprod Infant Psychol. 1986;4(1–2):85–98. doi: 10.1080/02646838608408668 [DOI] [Google Scholar]
- 23.Medical Expenditures Panel survey. US Department of Health & Human Services website; https://meps.ahrq.gov/data_stats/MEPS_topics.jsp?topicid=32Z-1. 2014. [Google Scholar]
- 24.Cooper LA, Brown C, Vu HT, et al. Primary care patients’ opinions regarding the importance of various aspects of care for depression. Gen Hosp Psychiatry. 2000;22(3):163–173. [DOI] [PubMed] [Google Scholar]
- 25.HCSUS Baseline Questionnaire Table of Contents. Rand Health website; http://www.rand.org/health/projects/hcsus/Base.html. 2015. [Google Scholar]
- 26.Freeman MP, Fava M, Lake J, et al. Complementary and alternative medicine in major depressive disorder: the American Psychiatric Association Task Force report. J Clin Psychiatry. 2010;71(6):669–681. [DOI] [PubMed] [Google Scholar]
- 27.Evans J, Heron J, Francomb H, et al. Cohort study of depressed mood during pregnancy and after childbirth. BMJ. 2001;323(7307):257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dipietro JA, Costigan KA, Sipsma HL. Continuity in self-report measures of maternal anxiety, stress, and depressive symptoms from pregnancy through two years postpartum. J Psychosom Obstet Gynaecol. 2008;29(2):115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leeners B, Richter-Appelt H, Imthurn B, et al. Influence of childhood sexual abuse on pregnancy, delivery, and the early postpartum period in adult women. J Psychosom Res. 2006;61(2):139–151. [DOI] [PubMed] [Google Scholar]
- 30.Dube SR Sr, Anda RF, Whitfield CL, et al. Long-term consequences of childhood sexual abuse by gender of victim. Am J Prev Med. 2005;28(5):430–438. [DOI] [PubMed] [Google Scholar]
- 31.Chang JC. Intimate partner violence: how you can help female survivors. Cleve Clin J Med. 2014;81(7):439–446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


